Abstract
Background/Objectives: Randomised trials show that screening with prostate-specific antigen (PSA) and systematic prostate biopsies can reduce prostate cancer mortality but leads to high rates of overdiagnosis. Today, improved diagnostic methods more selectively detect potentially lethal, high-grade prostate cancer. Methods: This is a narrative review of modern diagnostic methods, ongoing trials, national policies and knowledge gaps related to screening and early detection of prostate cancer. Results: Screening intervals can be prolonged in men with PSA values below around 1 ng/mL as these men are at very low long-term risk of prostate cancer death. Overdiagnosis can be reduced by magnetic resonance imaging (MRI) and lesion-targeted prostate biopsies. Risk calculators and ancillary biomarkers can select men for further investigation and thereby reduce resource needs. These new methods are evaluated in large, randomised screening trials. The remaining knowledge gaps include optimal PSA cut-offs, screening intervals, start and stop ages, and the long-term balance between benefits and harm. Until recently, almost no national healthcare authority recommended population-based screening for prostate cancer. Now, the European Union Council recommends an evaluation of the feasibility of organised, risk-stratified screening. This has led to several pilot projects. In some other parts of the world, such as sub-Saharan Africa and the Caribbean, such initiatives are lacking despite high prostate cancer mortality rates. Conclusions: Risk-stratified prostate cancer screening including MRI and targeted biopsy reduces overdiagnosis. Results from ongoing research are needed to optimise screening protocols and to define long-term benefits and harms. Initiatives for early detection and screening are emerging across the world but are still lacking in many countries with high prostate cancer mortality.
1. Introduction
Prostate cancer is usually incurable when symptoms occur. This necessitates measures to detect and treat the disease before it causes symptoms. When serum prostate-specific antigen (PSA) testing was introduced in the early 1990s, early detection of prostate cancer became feasible. In the following decade, randomised screening trials were started in Europe and the USA. The European Randomized Study of Screening for Prostate Cancer (ERSPC) showed that prostate cancer mortality can be reduced by screening [1]. The diagnostic method used in the ERSPC, a systematic prostate biopsy in all men with a PSA above 3–4 ng/mL, did, however, lead to much harm in the form of overdiagnosis and overtreatment [2]. With few exceptions, national healthcare authorities, therefore, have not recommended population-based screening for prostate cancer.
In parallel with the randomised screening trials, unorganised PSA testing became common in many countries. Widespread PSA testing led to a dramatic rise in the incidence of localised prostate cancer in Europe, Northern America, and Oceania [3,4]. This unorganised PSA testing is costly, ineffective, and leads to a more unfavourable balance between benefits and harms than organised screening [5].
In the past two decades, the introduction of magnetic resonance imaging (MRI) of the prostate and other refined methods has made it possible to more selectively diagnose potentially lethal, high-grade prostate cancer. New screening trials that incorporate these methods are ongoing.
In this narrative review, we summarise the diagnostic advances, results from the completed screening trials, design of and preliminary results from the ongoing trials, considerations about information to men, and prostate cancer screening policies, and we provide future perspectives.
2. Individual Versus Population Perspective
Individual men want to know their own chances of benefit and harm if they decide to obtain testing for early diagnosis of prostate cancer. These are inherently difficult to establish because of various forms of selection bias that affect non-randomised comparisons between tested and untested individuals. The best estimates are derived from per protocol analysis of screening trials. The healthcare authorities’ perspective is different: their primary concern is the health of the population. Their decisions are based on the effects of screening in an invited population, assessed by intention-to-screen analysis of randomised trials. The differences between these two perspectives are summarised in Table 1.

Table 1.
Characteristics of the different perspectives of individual-based testing and population-based screening for prostate cancer. MRI = magnetic resonance imaging.
3. Completed Screening Trials
The randomised prostate cancer screening trials that have reported on prostate cancer mortality, with a few small exceptions, used serum PSA as the primary screening test and a systematic prostate biopsy for men with a PSA value over 3–4 ng/mL. The design and results of the 4 large trials are summarised below.
3.1. The ERSPC Trial
ERSPC started in the mid-1990s in seven countries and randomised 162,242 men aged 55–69 years. The screening interval was 4 years in most centres. After a median follow-up of 16 years, the relative prostate cancer mortality reduction in the group invited to screening compared with the control group was 20%; the absolute reduction was 1.8 deaths per 1000 men [1]. The excess incidence of prostate cancer in the screening versus the control group was 30 diagnoses per 1000 men (risk ratio 1.34). These figures mean that for each prevented death from prostate cancer, 18 more men were diagnosed with prostate cancer in the screening group than in the control group. This is called the number needed to diagnose (NND).
An analysis of the Dutch part of ERSPC, adjusted for non-compliance and PSA testing in the control group, suggested a 51% net mortality reduction among screening participants (intention-to-screen analysis: 32%) [6].
3.2. The Gothenburg-1 Trial
The Gothenburg-1 trial started in 1995 as an independent trial but joined the ERSPC for the core age group 55–69 years. A population-based sample of 20,000 men aged 50–64 years was randomised 1:1 to either biennial screening or a control group. The 14-year relative prostate cancer mortality reduction was 44%, the absolute reduction 0.4%, and the NND 12 [7]. After 22 years, the relative reduction was 29%, the absolute reduction 0.6%, and the NND 9 [8]. The mortality reduction was confined to men who started screening before 60 years [9].
3.3. The Prostate, Lung, Colorectal and Ovarian (PLCO) Trial
The PLCO cancer screening trial recruited 76,693 US men aged 55–74 years from 1993. Men in the screening group were invited to annual PSA testing for 6 years and annual digital rectal examination for 4 years. After 15 years, there was no mortality benefit in the screening group compared with the control group [10]. The PLCO results cannot be used for evaluating the effect of screening versus no screening, as almost half of the men had been PSA tested before entering the study, 90% of the control men were PSA tested during the study, and less than half of the men with raised PSA underwent a prostate biopsy. All this diluted any potential screening benefit [10].
3.4. The Cluster Randomised Trial of PSA Testing for Prostate Cancer (CAP) Trial
The CAP trial invited 75,707 men in the UK aged 50–69 years for a single PSA test. Of these, 36% participated. In the control group of almost 350,000 men, 25% were PSA-tested at least once. After 10 years, there was no difference in prostate cancer mortality, but after 15 years a small prostate cancer-specific mortality difference emerged: 0.69% in the invited men versus 0.78% in the control group, rate ratio 0.92, 95% CI 0.85–0.99. The excess incidence in the screening group was then 1.4 per 1000 men [11].
4. Diagnostic Improvements
The use of a systematic prostate biopsy for all men with a PSA value over a fixed cut-off level leads to unacceptable overdiagnosis. Over the past couple of decades, diagnostic methods that more selectively detect potentially lethal high-grade prostate cancer have been developed. These are briefly outlined below.
4.1. Use of PSA for Risk Stratifying Testing Intervals
PSA levels lower than the commonly used cut-off of 3 ng/mL in middle-aged men are strongly associated with the long-term risk of metastatic and lethal prostate cancer [12,13]. The PSA level can therefore be used to risk stratify screening intervals [14,15]. Men with a PSA below 0.5–1.0 ng/mL are typically re-invited after 5–8 years (Figure 1). Men over 60 years with a PSA < 1 ng/mL have a sufficiently low risk of dying from prostate cancer to stop screening [12,13].
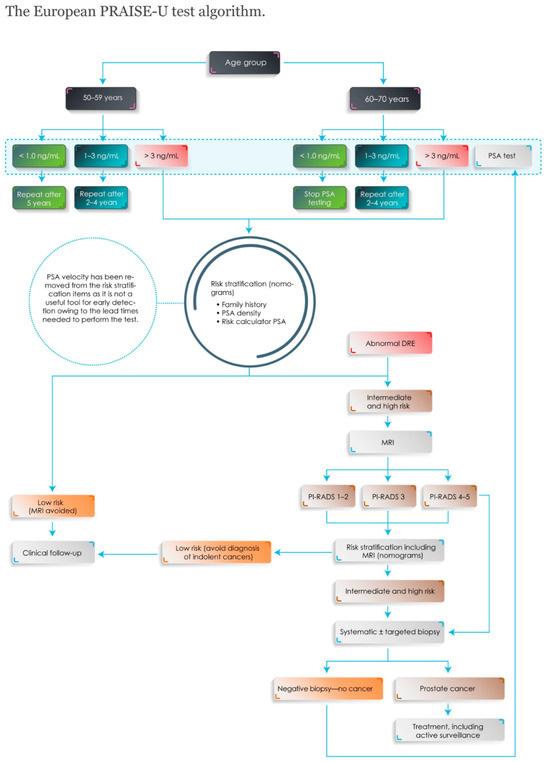
Figure 1.
The European PRAISE-U test algorithm. Abbreviations: DRE = digital rectal examination. MRI = magnetic resonance imaging. PI-RADS = Prostate Imaging–Reporting and Data System. PRAISE-U = Prostate cancer Awareness and Initiative for Screening in the European Union. PSA = prostate-specific antigen. Reprinted with permission from 3rd WUOF/SIU ICUD on Localized Prostate Cancer. World Urologic Oncology Federation 2024.
4.2. PSA Density
PSA density is a better marker than PSA alone for Gleason grade group (GG) ≥ 2 cancer. In men with PSA 3.0–10 ng/mL and PSA density < 0.10 ng/mL/cm3, fewer than 10% have GG ≥ 2 cancer on systematic biopsy [16]. Many screening trials and programmes use PSA density for selecting men with a non-suspicious or equivocal prostate MRI for biopsy.
4.3. MRI and Lesion-Targeted Biopsy
Prostate MRI, with or without contrast enhancement, has a high negative predictive value for GG ≥ 2 cancer [17,18]. This means that most men with PSA ≥ 3 ng/mL and a negative MRI (Prostate Imaging–Reporting and Data System [PI-RADS] 1–2) do not require a prostate biopsy. In a screening setting, this proportion is 55–65% [19,20,21]. Replacing systematic biopsies with biopsies targeting MRI lesions reduces the detection of GG 1 cancer by more than half in a screening setting, with a similar or slightly lower detection of GG ≥ 2 cancer [17,21]. Combining targeted and systematic biopsy cores slightly increases the detection of GG ≥ 2 cancer compared with targeted cores only, but also increases the risk of overdiagnosis [22]. The optimal biopsy protocol is yet to be defined.
Biopsy compliance is higher in men with a positive MRI than in men with only a positive PSA [21,23].
MRI has also been evaluated as the primary screening test in some small, prospective trials: Imperial Prostate 1 Prostate Cancer Screening Trial Using Imaging (IP1-PROSTAGRAM) [24], Prostate MRI versus PSA screening for prostate cancer detection (MVP) [23], and ReIMAGINE [25]. Although MRI detects GG ≥ 2 cancer lesions in men with low PSA values, the use of PSA for selecting men for MRI is necessary for screening to be cost-effective [24].
4.4. Risk Calculators
Prostate cancer risk calculators can be used to select men with a raised PSA for further evaluation with either an MRI or a systematic biopsy. They include a set of clinical variables, typically age, family history, previous biopsy, PSA, findings on digital rectal examination, prostate volume, and MRI results. Some also include ethnicity and genetic high-risk variants. Including prostate volume and MRI findings improves the identification of men with GG ≥ 2 cancer [26]. The most validated calculators are those from the Prostate Biopsy Collaborative Group, the Rotterdam section of the ERSPC, and the Prostate Cancer Prevention Trial [26].
4.5. Biomarkers
The three best studied serum biomarkers are the 4KScore, PHI (Prostate Health Index), and Stockholm-3 [27]. They all include total and free PSA, plus either PSA isoforms or other serum proteins. Stockholm-3 includes also a polygenic risk score. In some studies, the serum analysis has been combined with clinical information.
These tests identify men who despite PSA ≥ 3 ng/mL have a low risk of GG ≥ 2 cancer. Their use reduces the need for MRI scans by around one-third and the detection of GG 1 cancer by one-fourth, at the cost of delaying diagnosis of GG 2 cancer in a few per cent of men [28,29,30].
The 4Kscore is being evaluated for screening in the Finnish ProScreen trial [31], Stockholm-3 in the Swedish Sthlm3-MRI trial [32], and the PHI test in Czechia (NCT05603351) and China [33].
5. Ongoing Screening Trials
Four ongoing, large, randomised prostate cancer screening trials are described below and in Table 2. A fifth large trial, TRANSFORM, will be launched in the United Kingdom in 2025 with the aim of defining optimal screening test combinations.

Table 2.
Summary of the ongoing large, randomised prostate cancer screening trials. Abbreviation: GG = Grade group. PROBASE = Prostate Cancer Early Detection Study Based on a Baseline PSA Value in Young Men. MRI = magnetic resonance imaging.
5.1. The ProScreen Trial
The Finnish ProScreen trial randomly allocates men aged 50–63 years to either screening invitation or a control group. The trial started recruiting in 2018 and aims to enrol 115,000 men. The primary outcome is prostate cancer mortality at 10 and 15 years.
In the first screening round, 9.7% of 7744 participating men in the screening group had PSA ≥ 3 ng/mL [31]. Of these, 70% had a 4Kscore ≥ 7.5% and were referred for MRI. Cancer detection in the screening group, including 49% non-participants, was 0.3% GG 1 and 1.1% GG ≥ 2. In the control group, cancer detection was 0.1% GG 1 and 0.6% GG ≥ 2 after a median follow-up of 3.2 years.
5.2. The Gothenburg-2 Trial
The Swedish Gothenburg-2 screening trial investigates whether MRI and targeted biopsies reduce overdiagnosis compared with systematic biopsies. A PSA cut-off of 1.8 ng/mL is also investigated. Over 58,000 men aged 50–60 years were randomised in 2015–2020.
After a median of 4 years, the relative risk of detecting GG 1 cancer in the MRI-targeted versus the systematic biopsy group was 0.43 (95% CI 0.32 to 0.57) [21]. This risk was lower in the repeated screening rounds than in the first round. The relative risk of detecting GG ≥ 2 cancer was 0.84 (95% CI, 0.66 to 1.07) [21]. In the first screening round, 25% of the men with PSA 1.8–2.9 ng/mL had an MRI lesion and 4.6% were diagnosed with GG ≥ 2 cancer [34].
5.3. The Prostate Cancer Early Detection Study Based on a Baseline PSA Value in Young Men (PROBASE) Trial
The German PROBASE trial compares PSA-based screening starting at age 45 versus 50 years. The primary endpoint is metastatic prostate cancer by age 60 years. PROBASE recruited 46,495 men in 2014–2019 with an 11% participation rate. Of the 23,301 men who were randomised to start screening at age 45 years, 1.5% had an initial PSA ≥ 3 ng/mL. Of these, almost half had a confirmatory PSA < 3 ng/mL and were not further investigated. GG ≥ 2 cancer was diagnosed in 0.14% of the PSA-tested men [35].
Of men with an initial PSA ≤ 1.5 ng/mL, only 0.4% had PSA ≥ 3 ng/mL on repeat testing after 5 years; of those with PSA < 0.5 ng/mL, this proportion was 0.03% [15].
5.4. The Stockholm3-MRI Trial
The Swedish Stockholm3-MRI trial was designed as a diagnostic study in men aged 50–74 years. Men with PSA ≥ 1.5 ng/mL and no detected cancer were re-invited after 2–3 years (n = 2078 of whom 1500 participated). Among men with PSA ≥ 3.0 ng/mL in the second screening round who had an MRI in the first round, only 2.4% had PI-RADS 4–5 and 5.4% had PI-RADS 3 on their second MRI [32]. GG ≥ 2 cancer was detected in 3.8% of these men, and in 13% of the men who had their first MRI scan in the second screening round.
5.5. Screening Trials in High-Risk Populations
Family history, ethnicity, polygenic risk scores and germline mutation analysis can identify men at high risk of developing prostate cancer [36]. Although more intensive screening is usually recommended for these men, the optimal screening protocol is not known. Several prospective studies are ongoing [36].
6. Prostate Cancer Screening Policies
With few exceptions, national healthcare authorities recommend against population-based screening for prostate cancer. In most countries, men may have a PSA test after appropriate counselling about potential benefits and harms.
6.1. Africa
Prostate cancer mortality rates in Sub-Saharan Africa are among the highest in the world [3]. Owing to competing public health problems and resource issues, there is no population-based prostate cancer screening programme. Some African countries have guidelines for prostate cancer screening [37], but the understanding of prostate cancer early detection in the general population is poor [38].
6.2. The Americas
Mexico is the only country in the Americas where the healthcare authorities recommend screening for prostate cancer, but there is no organised screening programme [39]. In the USA, national authorities advise that PSA testing may be offered to individual men aged 55–69 “based on professional judgement and patient preferences” [40]. Canada has a weak recommendation against screening men aged 55–69 and a strong recommendation against screening for other ages.
Although the Caribbean has the highest prostate cancer mortality in the world [3], no Caribbean nation has an early detection programme for prostate cancer [41].
6.3. Asia
PSA testing on an individual basis can be obtained in most Asian countries. Most Japanese towns offer screening with PSA [42]. In China, some hospitals offer screening [43]. Kazakhstan started a government-funded screening programme in 2013, but it was closed in 2017 because of poor efficiency.
6.4. Europe
Since 2022, the European Union (EU) Council has encouraged the member states to evaluate the feasibility and effectiveness of organised prostate cancer screening programmes [44]. One motive is that risk-stratified screening and the use of MRI plus targeted biopsy reduce overdiagnosis, and another is that unorganised PSA testing is widespread but inefficient.
Lithuania initiated a primary care-based, early prostate cancer detection programme in 2006 [45]. In Sweden, regional population-based screening was launched in 2020 [20]. The EU-funded Prostate cancer Awareness and Initiative for Screening in the European Union (PRAISE-U) project initiated screening pilot projects in Spain, Poland, Ireland, and Lithuania in 2023 [46]. In Czechia and Estonia, nationwide pilots of primary care-based screening were introduced in 2024. Many other European countries plan to start screening pilots within a few years.
6.5. Oceania
In Australia and New Zealand, current guidelines advocate general practitioners to discuss PSA testing with men of appropriate ages in a shared decision-making process. Educational initiatives by the Urologic Society of Australia and New Zealand and the Prostate Cancer Foundation of Australia promote risk-stratified screening awareness. Several strategies are in place to mitigate overdiagnosis and related harms. Both countries predominantly use prebiopsy MRI and encourage active surveillance. The early detection activity is low in the other Oceanian nations.
7. Information to Men Invited to Screening
Recent systematic reviews conclude that decision aids for PSA testing are underused and have shortcomings in design [47,48]. It is unclear how much they contribute to shared decision-making [47]. Developing better decision aids adapted to various cultural contexts is therefore of high priority. Explaining overdiagnosis is particularly challenging [49]. The optimal amount and form of information for men who are invited to a screening programme may be different from that for men who actively ask for testing.
8. Conclusions and Future Directions
The use of MRI and lesion-targeted biopsies reduces the detection of low-grade cancer, and we can now better select men for active surveillance. Although this suggests that screening now results in less overdiagnosis and overtreatment than before, more results from the ongoing randomised screening trials are needed to estimate how much the benefit-to-harm ratio is improved. Some important knowledge gaps are summarised in Box 1.
Box 1. Important remaining knowledge gaps about prostate cancer screening.
- How are men best informed about the potential advantages and disadvantages?
- What are the optimal PSA cut-off values in different age groups?
- Which are the optimal start and stop ages?
- What is the outcome of repeated screening rounds with modern diagnostics?
- What are the optimal screening intervals for men with a raised PSA and negative further investigations?
- What is the value of an ancillary test to select men for MRI or biopsy in repeated screening?
- What are the cost-effectiveness and health economics of different screening algorithms?
- What are the long-term mortality and overdiagnosis outcomes after modern screening?
- How transferable are results from ongoing screening trials to other populations and ethnic groups?
Very few GG ≥ 2 cancers are detected in 45-year-old men [35], so starting population screening at age 45 appears too early. Modelling based on the Gothenburg-1 trial suggests that screening should start before the age of 60 years [9], but this finding was not consistent across ERSPC centres [50]. Although there is no evidence for starting to screen men over 70 years, continued screening of men aged 70–75 years with long life expectancy may be beneficial [51].
Serum PSA will most likely remain the primary screening test. The optimal PSA cut-off for a subsequent MRI may, however, be lower than the currently used 3 ng/mL [24,34]. MRI resources will be a bottleneck for implementing large-scale screening, so using the resources wisely is key. An MRI protocol without contrast enhancement is clearly advantageous to save resources. Fortunately, only a minority of men with a persistent PSA ≥ 3.0 ng/mL require a repeat MRI scan within 2–3 years [32,52], but more research is needed to define those who do and when to repeat scanning in those who do not. Various ancillary tests to select men for MRI are being evaluated [27]. High-quality prostate MRI readings and targeted biopsies require structured training, audits, and feedback on biopsy results.
Further aspects that need to be considered before initiating population-based screening for prostate cancer include competing healthcare priorities, healthcare structure, available resources for diagnostic investigations and treatment, and various country- and cultural-specific organisational aspects [53]. Late diagnosis of prostate cancer is still the norm in low- and middle-income countries. With growing populations and longer life expectancy, the number of prostate cancer deaths in these countries will dramatically increase [53], so there is an obvious need to find cost-effective measures for earlier diagnosis. In healthcare systems with insufficient MRI capacity, the use of ultrasound to measure the prostate volume for calculating PSA density and a risk calculator may select men with a high PSA value for MRI scanning or a systematic biopsy [54,55]. Large-scale evaluations of multiparametric ultrasound and micro-ultrasound are awaited.
The ongoing screening trials will fill many of the remaining knowledge gaps and provide high-level evidence for the benefits and harms of modern prostate cancer screening. We have much to look forward to in the coming years.
Author Contributions
Original draft preparation: O.B.; Review and editing: all authors: O.B., M.J., A.R.P., P.F.P., H.V.P., W.R., K.Z.-S., K.Z. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This review article was based on a chapter in the book “3rd WUOF/SIU ICUD On Localized Prostate Cancer”, published in October 2024. We wish to express our gratitude to Société Internationale d’Urologie (SIU) and the book editors Scott Eggener, Mack Roach 3rd and Laurence Klotz, who made the book and this review article possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hugosson, J.; Roobol, M.J.; Mansson, M.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-yr follow-up of the European randomized study of screening for prostate cancer. Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef]
- Tsodikov, A.; Gulati, R.; Heijnsdijk, E.A.M.; Pinsky, P.F.; Moss, S.M.; Qiu, S.; de Carvalho, T.M.; Hugosson, J.; Berg, C.D.; Auvinen, A.; et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann. Intern. Med. 2017, 167, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 update on prostate cancer epidemiology and risk factors—A systematic review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Li, M.; Bray, F.; Kvale, R.; Serraino, D.; Lorenzoni, V.; Auvinen, A.; Dal Maso, L. Prostate cancer incidence and mortality in europe and implications for screening activities: Population based study. BMJ 2024, 386, e077738. [Google Scholar] [CrossRef]
- Arnsrud Godtman, R.; Holmberg, E.; Lilja, H.; Stranne, J.; Hugosson, J. Opportunistic testing versus organized prostate-specific antigen screening: Outcome after 18 years in the Göteborg randomized population-based prostate cancer screening trial. Eur. Urol. 2015, 68, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Bokhorst, L.P.; Bangma, C.H.; van Leenders, G.J.; Lous, J.J.; Moss, S.M.; Schroder, F.H.; Roobol, M.J. Prostate-specific antigen-based prostate cancer screening: Reduction of prostate cancer mortality after correction for nonattendance and contamination in the rotterdam section of the European randomized study of screening for prostate cancer. Eur. Urol. 2014, 65, 329–336. [Google Scholar] [CrossRef]
- Hugosson, J.; Carlsson, S.; Aus, G.; Bergdahl, S.; Khatami, A.; Lodding, P.; Pihl, C.G.; Stranne, J.; Holmberg, E.; Lilja, H. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Franlund, M.; Mansson, M.; Godtman, R.A.; Aus, G.; Holmberg, E.; Kollberg, K.S.; Lodding, P.; Pihl, C.G.; Stranne, J.; Lilja, H.; et al. Results from 22 years of followup in the Göteborg randomized population-based prostate cancer screening trial. J. Urol. 2022, 208, 292–300. [Google Scholar] [CrossRef]
- Carlsson, S.V.; Arnsrud Godtman, R.; Pihl, C.G.; Vickers, A.; Lilja, H.; Hugosson, J.; Mansson, M. Young age on starting prostate-specific antigen testing is associated with a greater reduction in prostate cancer mortality: 24-year follow-up of the Göteborg randomized population-based prostate cancer screening trial. Eur. Urol. 2023, 83, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.F.; Prorok, P.C.; Yu, K.; Kramer, B.S.; Black, A.; Gohagan, J.K.; Crawford, E.D.; Grubb, R.L.; Andriole, G.L. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer 2017, 123, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Turner, E.L.; Young, G.J.; Metcalfe, C.; Walsh, E.I.; Lane, J.A.; Sterne, J.A.C.; Noble, S.; Holding, P.; Ben-Shlomo, Y.; et al. Prostate-specific antigen screening and 15-year prostate cancer mortality: A secondary analysis of the cap randomized clinical trial. JAMA 2024, 331, 1460–1470. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Bjork, T.; Manjer, J.; Nilsson, P.M.; Dahlin, A.; Bjartell, A.; Scardino, P.T.; Ulmert, D.; Lilja, H. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: Case-control study. BMJ 2010, 341, c4521. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Assel, M.; Sjoberg, D.; Ulmert, D.; Hugosson, J.; Lilja, H.; Vickers, A. Influence of blood prostate specific antigen levels at age 60 on benefits and harms of prostate cancer screening: Population based cohort study. BMJ 2014, 348, g2296. [Google Scholar] [CrossRef]
- Ola, I.O.; Talala, K.; Tammela, T.; Taari, K.; Murtola, T.; Kujala, P.; Raitanen, J.; Auvinen, A. Raitanen and A. Auvinen. Prostate cancer incidence in men with prostate-specific antigen below 3 ng/ml: The Finnish randomized study of screening for prostate cancer. Int. J. Cancer 2023, 152, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Krilaviciute, A.; Kaaks, R.; Seibold, P.; de Vrieze, M.; Lakes, J.; Radtke, J.P.; Kuczyk, M.; Harke, N.N.; Debus, J.; Fink, C.A.; et al. Risk-adjusted screening for prostate cancer—Defining the low-risk group by data from the PROBASE trial. Eur. Urol. 2024, 86, 493–500. [Google Scholar] [CrossRef]
- Nordstrom, T.; Akre, O.; Aly, M.; Gronberg, H.; Eklund, M. Prostate-specific antigen (PSA) density in the diagnostic algorithm of prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 57–63. [Google Scholar] [CrossRef]
- Fazekas, T.; Shim, S.R.; Basile, G.; Baboudjian, M.; Koi, T.; Przydacz, M.; Abufaraj, M.; Ploussard, G.; Kasivisvanathan, V.; Rivas, J.G.; et al. Magnetic resonance imaging in prostate cancer screening: A systematic review and meta-analysis. JAMA Oncol. 2024, 10, 745–754. [Google Scholar] [CrossRef]
- Bass, E.J.; Pantovic, A.; Connor, M.; Gabe, R.; Padhani, A.R.; Rockall, A.; Sokhi, H.; Tam, H.; Winkler, M.; Ahmed, H.U. A systematic review and meta-analysis of the diagnostic accuracy of biparametric prostate MRI for prostate cancer in men at risk. Prostate Cancer Prostatic Dis. 2021, 24, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Eklund, M.; Jaderling, F.; Discacciati, A.; Bergman, M.; Annerstedt, M.; Aly, M.; Glaessgen, A.; Carlsson, S.; Gronberg, H.; Nordstrom, T.; et al. MRI-targeted or standard biopsy in prostate cancer screening. N. Engl. J. Med. 2021, 385, 908–920. [Google Scholar] [CrossRef]
- Bratt, O.; Godtman, R.A.; Jiborn, T.; Wallstrom, J.; Akre, O.; Carlsson, S.; Nordstrom, T.; Thimansson, E.; Alterbeck, M.; Zackrisson, S.; et al. Population-based organised prostate cancer testing: Results from the first invitation of 50-year-old men. Eur. Urol. 2023, 85, 207–214. [Google Scholar] [CrossRef]
- Hugosson, J.; Godtman, R.A.; Wallstrom, J.; Axcrona, U.; Bergh, A.; Egevad, L.; Geterud, K.; Khatami, A.; Socratous, A.; Spyratou, V.; et al. Results after four years of screening for prostate cancer with PSA and MRI. N. Engl. J. Med. 2024, 391, 1083–1095. [Google Scholar] [CrossRef]
- Zattoni, F.; Fasulo, V.; Kasivisvanathan, V.; Kesch, C.; Marra, G.; Martini, A.; Falagario, U.; Soeterik, T.; van den Bergh, R.; Rajwa, P.; et al. Enhancing prostate cancer detection accuracy in magnetic resonance imaging-targeted prostate biopsy: Optimizing the number of cores taken. Eur. Urol. Open Sci. 2024, 66, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Nam, R.; Patel, C.; Milot, L.; Hird, A.; Wallis, C.; Macinnis, P.; Singh, M.; Emmenegger, U.; Sherman, C.; Haider, M.A. Prostate MRI versus PSA screening for prostate cancer detection (the MVP study): A randomised clinical trial. BMJ Open 2022, 12, e059482. [Google Scholar] [CrossRef] [PubMed]
- Eldred-Evans, D.; Tam, H.; Sokhi, H.; Padhani, A.R.; Connor, M.; Price, D.; Gammon, M.; Klimowska-Nassar, N.; Burak, P.; Day, E.; et al. An evaluation of screening pathways using a combination of magnetic resonance imaging and prostate-specific antigen: Results from the IP1-PROSTAGRAM study. Eur. Urol. Oncol. 2023, 6, 295–302. [Google Scholar] [CrossRef]
- Marsden, T.; McCartan, N.; Brown, L.; Rodriguez-Justo, M.; Syer, T.; Brembilla, G.; Van Hemelrijck, M.; Coolen, T.; Attard, G.; Punwani, S.; et al. The reimagine prostate cancer risk study protocol: A prospective cohort study in men with a suspicion of prostate cancer who are referred onto an MRI-based diagnostic pathway with donation of tissue, blood and urine for biomarker analyses. PLoS ONE 2022, 17, e0259672. [Google Scholar] [CrossRef]
- Denijs, F.B.; van Harten, M.J.; Meenderink, J.J.L.; Leenen, R.C.A.; Remmers, S.; Venderbos, L.D.F.; van den Bergh, R.C.N.; Beyer, K.; Roobol, M.J. Risk calculators for the detection of prostate cancer: A systematic review. Prostate Cancer Prostatic Dis. 2024, 27, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Shim, S.R.; Quhal, F.; Rajwa, P.; Pradere, B.; Yanagisawa, T.; Bekku, K.; Laukhtina, E.; von Deimling, M.; Teoh, J.Y.; et al. Diagnostic accuracy of liquid biomarkers for clinically significant prostate cancer detection: A systematic review and diagnostic meta-analysis of multiple thresholds. Eur. Urol. Oncol. 2024, 7, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, T.; Discacciati, A.; Bergman, M.; Clements, M.; Aly, M.; Annerstedt, M.; Glaessgen, A.; Carlsson, S.; Jaderling, F.; Eklund, M.; et al. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): A prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol. 2021, 22, 1240–1249. [Google Scholar] [CrossRef]
- Agnello, L.; Vidali, M.; Giglio, R.V.; Gambino, C.M.; Ciaccio, A.M.; Lo Sasso, B.; Ciaccio, M. Prostate health index (PHI) as a reliable biomarker for prostate cancer: A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2022, 60, 1261–1277. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, A.; Mansson, M.; Kohestani, K.; Spyratou, V.; Wallstrom, J.; Hellstrom, M.; Lilja, H.; Vickers, A.; Carlsson, S.V.; Godtman, R.; et al. Performance of 4Kscore as a reflex test to prostate-specific antigen in the GÖTEBORG-2 prostate cancer screening trial. Eur. Urol. 2024, 86, 223–229. [Google Scholar] [CrossRef]
- Auvinen, A.; Tammela, T.L.J.; Mirtti, T.; Lilja, H.; Tolonen, T.; Kenttamies, A.; Rinta-Kiikka, I.; Lehtimaki, T.; Natunen, K.; Nevalainen, J.; et al. Prostate cancer screening with PSA, Kallikrein Panel, and MRI The ProScreen randomized trial. JAMA 2024, 331, 1452–1459. [Google Scholar] [CrossRef]
- Nordstrom, T.; Annerstedt, M.; Glaessgen, A.; Carlsson, S.; Clements, M.; Abbadi, A.; Gronberg, H.; Jaderling, F.; Eklund, M.; Discacciati, A. Repeated prostate cancer screening using prostate-specific antigen testing and magnetic resonance imaging: A secondary analysis of the STHLM3-MRI randomized clinical trial. JAMA Netw. Open 2024, 7, e2354577. [Google Scholar] [CrossRef]
- Chiu, P.K.; Liu, A.Q.; Lau, S.Y.; Teoh, J.Y.; Ho, C.C.; Yee, C.H.; Hou, S.M.; Chan, C.K.; Tang, W.L.; Bangma, C.H.; et al. A 2-year prospective evaluation of the Prostate Health Index in guiding biopsy decisions in a large cohort. BJU Int. 2024, 135, 71–77. [Google Scholar] [CrossRef]
- Moller, F.; Mansson, M.; Wallstrom, J.; Hellstrom, M.; Hugosson, J.; Arnsrud Godtman, R. Prostate cancers in the prostate-specific antigen interval of 1.8-3 ng/ml: Results from the Göteborg-2 prostate cancer screening trial. Eur. Urol. 2024, 86, 95–100. [Google Scholar] [CrossRef]
- Arsov, C.; Albers, P.; Herkommer, K.; Gschwend, J.; Imkamp, F.; Peters, I.; Kuczyk, M.; Hadaschik, B.; Kristiansen, G.; Schimmoller, L.; et al. A randomized trial of risk-adapted screening for prostate cancer in young men-results of the first screening round of the PROBASE trial. Int. J. Cancer 2022, 150, 1861–1869. [Google Scholar] [CrossRef]
- Graham, N.J.; Souter, L.H.; Salami, S.S. A systematic review of family history, race/ethnicity, and genetic risk on prostate cancer detection and outcomes: Considerations in PSA-based screening. Urol. Oncol. 2025, 43, 29–40. [Google Scholar] [CrossRef]
- Makau-Barasa, L.K.; Manirakiza, A.; Carvalho, A.L.; Rebbeck, T.R. Prostate cancer screening, diagnostic, treatment procedures and costs in sub-Saharan Africa: A situational analysis. Cancer Control 2022, 29, 10732748221084932. [Google Scholar] [CrossRef]
- Baratedi, W.M.; Tshiamo, W.B.; Mogobe, K.D.; McFarland, D.M. Barriers to prostate cancer screening by men in Sub-Saharan Africa: An integrated review. J. Nurs. Scholarsh. 2020, 52, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Tourinho-Barbosa, R.R.; Pompeo, A.C.; Glina, S. Prostate cancer in Brazil and Latin America: Epidemiology and screening. Int. Braz. J. Urol. 2016, 42, 1081–1090. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T.; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; et al. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [CrossRef]
- Ramesar, N.S.; Hosein, A.; Samaroo, K.; Ali, J. Prostate cancer in the Caribbean. Cureus 2023, 15, e50150. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Okinaka, Y.; Nishizawa, K.; Yoshida, T.; Ishitoya, S.; Shichiri, Y.; Kim, C.J.; Iwata, T.; Yokokawa, R.; Arai, Y.; et al. Population-based prostate-specific antigen screening for prostate cancer may have an indirect effect on early detection through opportunistic testing in Kusatsu City, Shiga, Japan. Mol. Clin. Oncol. 2023, 18, 3. [Google Scholar] [CrossRef]
- Ruan, X.; Zhang, N.; Wang, D.; Huang, J.; Huang, J.; Huang, D.; Chun, T.T.S.; Ho, B.S.H.; Ng, A.T.; Tsu, J.H.; et al. The impact of prostate-specific antigen screening on prostate cancer incidence and mortality in china: 13-year prospective population-based cohort study. JMIR Public Health Surveill. 2024, 10, e47161. [Google Scholar] [CrossRef]
- Council of the European Union. Council recommendation of 9 December 2022 on strengthening prevention through early detection: A new EU approach on cancer screening replacing council recommendation 2003/878/EC 2022/C 473/01. Off. J. Eur. Union 2022. Available online: https://eu.vlex.com/vid/raccomandazione-consiglio-9dicembre-2022-916324729 (accessed on 19 November 2024).
- Patasius, A.; Krilaviciute, A.; Smailyte, G. Prostate cancer screening with PSA: Ten years’ experience of population based early prostate cancer detection programme in lithuania. J. Clin. Med. 2020, 9, 3826. [Google Scholar] [CrossRef]
- Van Poppel, H.; Roobol, M.J.; Chandran, A. Early detection of prostate cancer in the European Union: Combining forces with PRAISE-U. Eur. Urol. 2023, 84, 519–522. [Google Scholar] [CrossRef]
- Pekala, K.R.; Shill, D.K.; Austria, M.; Langford, A.T.; Loeb, S.; Carlsson, S.V. Shared decision-making before prostate cancer screening decisions. Nat. Rev. Urol. 2024, 21, 329–338. [Google Scholar] [CrossRef]
- Riikonen, J.M.; Guyatt, G.H.; Kilpelainen, T.P.; Craigie, S.; Agarwal, A.; Agoritsas, T.; Couban, R.; Dahm, P.; Jarvinen, P.; Montori, V.; et al. Decision aids for prostate cancer screening choice: A systematic review and meta-analysis. JAMA Intern. Med. 2019, 179, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, T.I.; Pickles, K.; Riikonen, J.M.; Tikkinen, K.A.O.; Bell, K.J.L.; Glasziou, P. Including information on overdiagnosis in shared decision making: A review of prostate cancer screening decision aids. MDM Policy Pract. 2022, 7, 23814683221129875. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, N.; Talala, K.; Remmers, S.; Tammela, T.L.J.; Hugosson, J.; Roobol, M.J.; Taari, K.; Arnsrud Godtman, R.; Bangma, C.; Auvinen, A. Which men benefit from prostate cancer screening? Prostate cancer mortality by subgroup in the European randomised study of screening for prostate cancer. BJU Int. 2024, 134, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.R.; Schoots, I.G.; Bokhorst, L.P.; Drost, F.H.; van Leenders, G.J.; Krestin, G.P.; Dwarkasing, R.S.; Barentsz, J.O.; Schroder, F.H.; Bangma, C.H.; et al. Characteristics of prostate cancer found at fifth screening in the European randomized study of screening for prostate cancer Rotterdam: Can we selectively detect high-grade prostate cancer with upfront multivariable risk stratification and magnetic resonance imaging? Eur. Urol. 2018, 73, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wallstrom, J.; Geterud, K.; Kohestani, K.; Maier, S.E.; Pihl, C.G.; Socratous, A.; Stranne, J.; Arnsrud-Godtman, R.; Mansson, M.; Hellstrom, M.; et al. Prostate cancer screening with magnetic resonance imaging: Results from the second round of the Göteborg prostate cancer screening 2 trial. Eur. Urol. Oncol. 2022, 5, 54–60. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef]
- Straat, K.R.V.; Hagens, M.J.; Cools Paulino Pereira, L.J.; van den Bergh, R.C.N.; Mazel, J.W.; Noordzij, M.A.; Rynja, S.P. Risk calculator strategy before magnetic resonance imaging stratification for biopsy-naive men with suspicion for prostate cancer: A cost-effectiveness analysis. Eur. Urol. Open Sci. 2024, 70, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wagensveld, I.M.; Osses, D.F.; Groenendijk, P.M.; Zijta, F.M.; Busstra, M.B.; Rociu, E.; Barentsz, J.O.; Michiel Sedelaar, J.P.; Arbeel, B.; Roeleveld, T.; et al. A prospective multicenter comparison study of risk-adapted ultrasound-directed and magnetic resonance imaging-directed diagnostic pathways for suspected prostate cancer in biopsy-naive men. Eur. Urol. 2022, 82, 318–326. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).