It’s Getting Hot in There: In Vitro Study on Ureteral Tissue Thermal Profiles During Laser Ureteral Lithotripsy
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
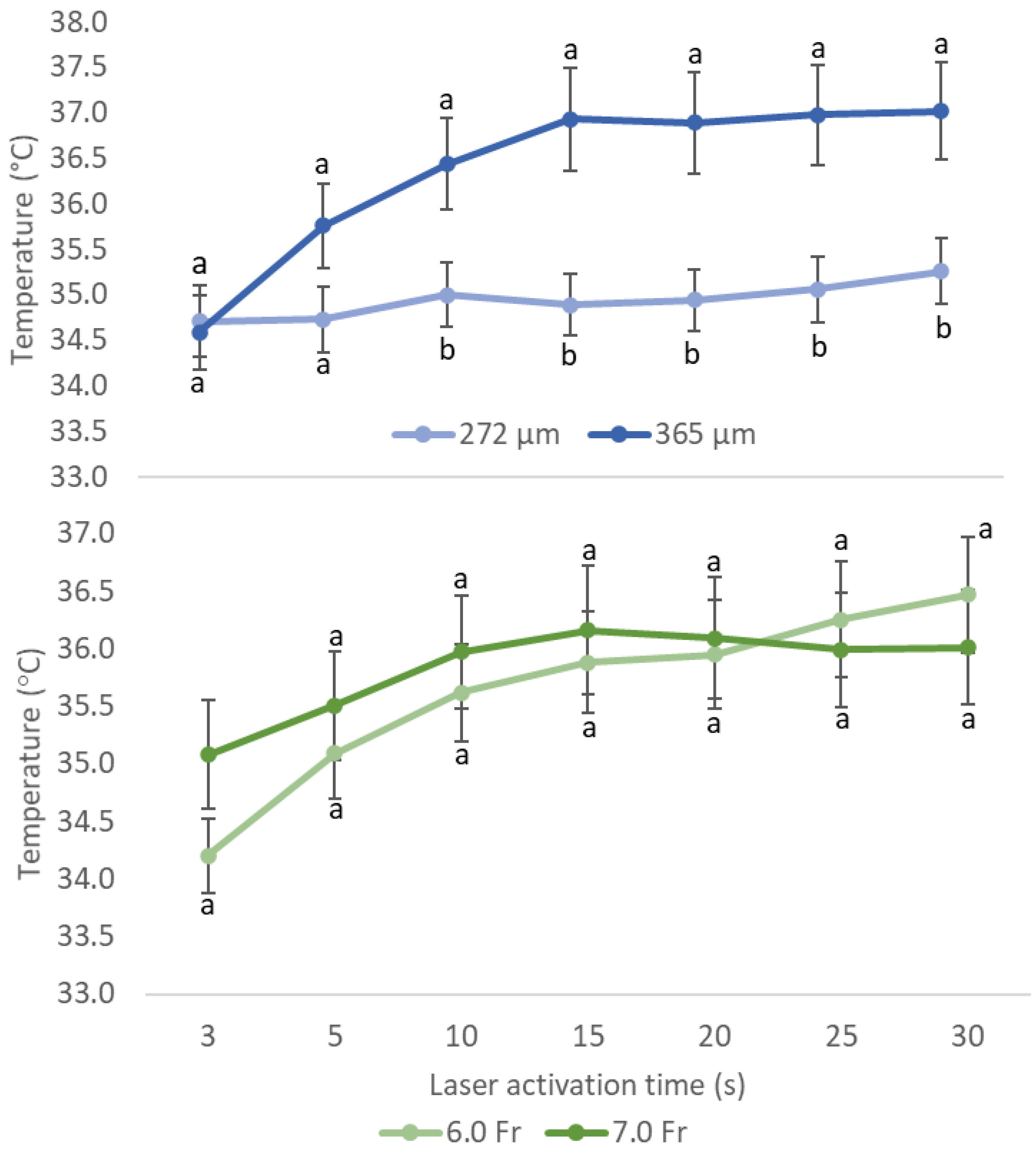
3.1. Effects of Different Sizes of Ureteroscope and Laser Fiber on Temperature
3.2. Possible Outcomes of Multiple Variables for Temperature Rise
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skolarikos, A.; Jung, H.U.; Somani, B.; Davis, N.D.; Tzelves, L.; Neisius, A.; Tailly, T.; Geraghty, R.; Gambaro, G. Guidelines Urolithiasis; EAU Guidelines Office: Arnhem, The Netherlands, 2022. [Google Scholar]
- Chung, J.H.; Baek, M.; Park, S.S.; Han, D.H. The feasibility of pop-dusting using high-power laser (2 J_50 Hz) in retrograde intrarenal surgery for renal stones: Retrospective single-center experience. J. Endourol. 2020, 35, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Adamou, C.; Farsari, E.; Ntasiotis, P.; Vagionis, A.; Gerakaris, A.; Gkialas, K.; Callas, G.; Pagonis, K.; Liatsikos, E.; Kallidonis, P. Is the use of a ureteral access sheath necessary for maintaining safe irrigation temperatures during flexible ureteroscopy when a high power Holmium:YAG laser is used? Clues from an in-vivo experimental study. Eur. Urol. Open Sci. 2020, 9, 28–30. [Google Scholar] [CrossRef]
- Molina, W.R.; Silva, I.N.; Da Silva, R.D.; Gustafson, D.; Sehrt, D.; Kim, F.J. Influence of saline on temperature profile of laser lithotripsy activation. J. Endourol. 2015, 29, 235–239. [Google Scholar] [CrossRef] [PubMed]
- El-Abd, A.S.; Suliman, M.G.; Abo Farha, M.O.; Ramadan, A.R.; El-Tatawy, H.H.; El-Gamal, O.M.; El-Gamal, S.A.; Figenshau, R.; El Abd, S.A. The development of ureteric strictures after ureteroscopic treatment for ureteric calculi: A long-term study at two academic centres. Arab. J. Urol. 2014, 12, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Perez Castro, E.; Osther, P.J.; Jinga, V.; Razvi, H.; Stravodimos, K.G.; Parikh, K.; Kural, A.R.; de la Rosette, J.J. Differences in ureteroscopic stone treatment and outcomes for distal, mid-, proximal, or multiple ureteral locations: The clinical research office of the endourological society ureteroscopy global study. Eur. Urol. 2014, 66, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.E.; Gadelmoula, M.M.; Abdelkawi, I.F.; Abdellatif, A.M.; Abdel-Moneim, A.M.; Hammouda, H.M. Ureteral stricture after ureteroscopy for stones: A prospective study for the incidence and risk factors. Urol. Ann. 2019, 11, 276–281. [Google Scholar] [PubMed]
- Srougi, V.; Padovani, G.P.; Marchini, G.S.; Vicentini, F.C.; Mazzucchi, E.; Srougi, M. Outcomes of surgical treatment of ureteral strictures after laser uretero lithotripsy for impacted stones. Can. J. Urol. 2015, 22, 8079–8084. [Google Scholar] [PubMed]
- Joshi, H.N.; Singh, A.K.; Koirala, N.P.; Karmacharya, R.M. Outcome of uretero renoscopic lithotripsy (URSL) with holmium laser vs. pneumatic lithotripter for lower ureteric stones, experience from University Hospital of Nepal. Kathmandu. Univ. Med. J. 2020, 18, 49–53. [Google Scholar] [CrossRef]
- Liang, H.; Liang, L.J.; Yu, Y.; Huang, B.; Chen, J.N.; Wang, C.G.; Zhu, Z.G.; Liang, X.Z. Thermal effect of holmium laser during ureteroscopic lithotripsy. BMC Urol. 2020, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, V.; Keller, E.X.; Rodriuez-Monsalve, M.; Audouin, M.; Doizi, S.; Traxer, O. Systemic review of ureteral access sheaths: Facts and myths. BJU Int. 2018, 22, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Van de Berg, N.J.; Van den Dobbelsteen, J.J.; Jansen, F.W.; Grimbergen, C.A.; Dankelman, J. Energetic soft-tissue treatment technologies: An overview of procedural fundamentals and safety factors. Surg. Endosc. 2013, 27, 3085–3099. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.J.; Gemert Martin, J.C. Optical-Thermal Response of Laser-Irradiated Tissue; Plenum Press: New York, NY, USA, 2011. [Google Scholar]
- Tonyali, S.; von Bargen, M.F.; Ozkan, A.; Gratzke, C.; Miernik, A. The heat is on: The impact of excessive temperature increments on complications of laser treatment for ureteral and renal stones. World J. Urol. 2023, 41, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- Butticè, S.; Sener, T.E.; Proietti, S.; Dragos, L.; Tefik, T.; Doizi, S.; Traxer, O. Temperature changes inside the kidney: What happens during holmium:yttrium-aluminium-garnet laser usage? J. Endourol. 2016, 30, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, P.; Traxer, O. Update on lasers in urology 2014: Current assessment on holmium:yttriumaluminum-garnet (Ho:YAG) laser lithotripter settings and laser fibers. World J. Urol. 2015, 33, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.W.; Evan, C.C.; Westin, R.T.; WNeal, S.; Glenn, M.P.; Michael, E.L. Effect of laser settings and irrigation rates on ureteral temperature during holmium laser lithotripsy, an in vitro model. J. Endourol. 2018, 32, 59–63. [Google Scholar]
- Gallegos, H.; Bravo, J.C.; Sepulveda, F.; Astroza, G.M. Intrarenal temperature measurement associated with holmium laser intracorporeal lithotripsy in an ex vivo model. Centr. Eur. J. Urol. 2021, 74, 588–594. [Google Scholar]
- Aldoukhi AHall, T.; Ghani, K.; Roberts, W. Strike rate: Analysis of laser fiber to stone distance during different modes of laser lithotripsy. J. Urol. 2020, 32, 724–729. [Google Scholar] [CrossRef]
- Winship, B.; Terry, R.; Boydston, K.; Carlos, E.; Wollin, D.; Peters, C.; Li, J.Q.; Preminger, G.; Lipkin, M. Holmium:yttrium-aluminum-garnet laser pulse type affects irrigation temperatures in a benchtop ureteral model. J. Endourol. 2019, 33, 896–901. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tee, Z.Y.; Yong, C.H.; Goh, Y.K.; Lim, M.S. It’s Getting Hot in There: In Vitro Study on Ureteral Tissue Thermal Profiles During Laser Ureteral Lithotripsy. Soc. Int. Urol. J. 2024, 5, 826-834. https://doi.org/10.3390/siuj5060062
Tee ZY, Yong CH, Goh YK, Lim MS. It’s Getting Hot in There: In Vitro Study on Ureteral Tissue Thermal Profiles During Laser Ureteral Lithotripsy. Société Internationale d’Urologie Journal. 2024; 5(6):826-834. https://doi.org/10.3390/siuj5060062
Chicago/Turabian StyleTee, Zhou Yin, Chun Hou Yong, Yue Keng Goh, and Meng Shi Lim. 2024. "It’s Getting Hot in There: In Vitro Study on Ureteral Tissue Thermal Profiles During Laser Ureteral Lithotripsy" Société Internationale d’Urologie Journal 5, no. 6: 826-834. https://doi.org/10.3390/siuj5060062
APA StyleTee, Z. Y., Yong, C. H., Goh, Y. K., & Lim, M. S. (2024). It’s Getting Hot in There: In Vitro Study on Ureteral Tissue Thermal Profiles During Laser Ureteral Lithotripsy. Société Internationale d’Urologie Journal, 5(6), 826-834. https://doi.org/10.3390/siuj5060062






