Abstract
Given its known prognostic role, we aimed to investigate the role of neutrophil–lymphocyte ratio (NLR) as a biomarker in metastatic castration-resistant prostate cancer (mCRPC) patients receiving ADT, either as monotherapy or in conjunction with abiraterone acetate (AA) and prednisone. This retrospective cohort study analyzed the LATITUDE study of men with high-risk mCSPC. Patients were assigned to receive either AA, prednisone, and androgen deprivation therapy (ADT) or placebo plus ADT. Using a previously established NLR threshold of 2.5, we evaluated if this could predict clinical response to abiraterone. At baseline, there were no significant differences in NLR values between the treatment groups. Of the known baseline prognostic factors, NLR was associated with albumin levels and Eastern Cooperative Oncology Group performance scores. Moreover, the number of bone metastases was higher in patients with NLR ≥ 2.5. On multivariable analysis, baseline NLR ≥ 2.5 did not predict overall survival, PSA progression-free, or metastasis-free survival. However, changes in PSA and NLR at six months indicated distinct survival patterns between the placebo and AA groups, suggesting the potential for their combined assessment as a prognostic tool. Baseline NLR was not an independent predictor factor for response to AA in the LATITUDE study, though NLR changes at 6 months may predict better survival beyond PSA values alone. Further research is required to better understand in which patients with advanced prostate cancer NLR changes may be a useful prognostic tool.
1. Introduction
Prostate cancer ranks as one of the most prevalent malignancies in North America [1,2]. Recent advancements in treatment options have improved therapeutic outcomes for patients, particularly in metastatic prostate cancer, where the morbidity and mortality are higher [3,4,5]. In particular, the combination of abiraterone acetate (AA) and prednisone with androgen deprivation therapy (ADT) demonstrated improved survival in two large studies of metastatic castrate-sensitive prostate cancer (mCSPC) [6,7]. The use of accessible, laboratory biomarkers may help refine optimal treatment selection between various options which now exist, including androgen receptor pathway inhibition and chemotherapy.
The neutrophil-to-leukocyte ratio (NLR) is a well-recognized biomarker across various cancers; it generally signifies systemic inflammation, which is acknowledged as one hallmark of cancer [8]. Derived from the complete blood count, it does not require specialized testing and is therefore highly accessible and inexpensive. An elevated NLR is believed to be related to a tumor-conducive microenvironment through the augmentation of neutrophil-dependent systemic inflammatory responses and the attenuation of lymphocyte-mediated antitumor immunity [9]. Previous research has consistently identified an elevated NLR as an adverse prognostic indicator, particularly in metastatic castration-resistant prostate cancer (mCRPC) cases [9,10,11,12]. A prior analysis of the pivotal COU302 study of AA in mCRPC suggested that an NLR ≥ 2.5 may predict poorer response to AA in patients with treatment-naïve mCRPC [13]. However, this association is not universally observed across all treatment types; for instance, NLR does not predict outcomes in taxane-based therapies [10].
Therefore, to further validate the potential of NLR as a biomarker in mCSPC patients, this study investigates its role in the LATITUDE study. Our main objective is to determine whether baseline NLR can be used as a predictive biomarker, with our analyses also evaluating the potential for use as a biomarker following 6 months of treatment. Overall, our analyses provide more information on its use as a biomarker in prostate cancer patients.
2. Material and Methods
2.1. Patients
In this retrospective cohort study, we analyzed the LATITUDE study population, which included male individuals aged 18 years and above diagnosed with high-risk mCSPC. This study, carried out under YODA Project # 2020-4311, used data obtained from the Yale University Open Data Access Project, which has an agreement with Janssen Research & Development, L.L.C. The interpretation and reporting of research using this data are solely the responsibility of the authors and do not necessarily represent the official views of the Yale University Open Data Access Project or Janssen Research & Development, L.L.C. The project was approved by YODA project data access reviewers in 2020. Briefly, the inclusion criteria specified patients with Eastern Cooperative Oncology Group (ECOG) performance status scores of 0 to 2, and at least two of the three high-risk prognostic factors (Gleason score of ≥8, presence of three or more lesions on bone scan, or presence of measurable visceral metastasis except lymph node metastasis). Notably, 98% of patients had a Gleason score ≥ 8 [6]. Pertinent to the present study, patients were required to have an adequate hematologic function (for example, exclusion of neutropenia < 1.5 × 109 cells/L). Patients were randomly assigned to receive AA (1000 mg) once daily orally plus prednisone (5 mg) once daily orally and ADT (AA plus prednisone group), or matching placebos plus ADT (placebo group), until disease progression.
For the purpose of our analysis, we stratified the study cohort based on the baseline NLR, which is calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The decision to adopt a threshold NLR value of 2.5 aligns with our previous study [13].
2.2. Statistical Analysis
We selected the following baseline factors as covariates for our survival analyses: age, ethnicity, Eastern Cooperative Oncology Group (ECOG) Performance Status, albumin, prostate-specific antigen levels, lactate dehydrogenase (LDH), presence of visceral metastases, Brief Pain Index Score, and International Society of Urological Pathology (ISUP) Gleason Grade Groups. The descriptive analysis parametric variables are represented by mean and standard deviation, non-parametric variables are represented by a median, interquartile interval, and proportion frequency as percentages, and the confidence interval adopted was 95%, with a p-value < 0.05 considered significant. The normality of the variables was tested using Kolgomorov–Smirnov and Shapiro–Wilk tests. Overall survival (OS) was estimated using the Kaplan–Meier method and stratified according to the absolute values and relative changes in NLR levels.
R version 4.3.0 was used for all statistical analyses. A Pearson’s chi-square test was used to compare groups with nominal variables and, to evaluate the differences in baseline characteristics and clinical outcomes between patient groups, we employed both t-tests and Wilcoxon tests, based on the nature of the data.
3. Results
The baseline characteristics for 1109 patients included in the study are outlined in Supplementary Table S1. The overall mean and median NLR values at screening were 2.52 and 2.25 for patients treated with AA + Prednisone + ADT, and 2.54 and 2.27 for those receiving ADT alone. No significant difference was observed between the treatment groups’ most baseline factors. However, significantly higher NLR values were found among patients with an ECOG score of 2, among Hispanic patients (versus non-Hispanic patients), and among those with below-median albumin levels.
Evaluation of baseline associations of groups with baseline NLR < 2.5 and ≥ 2.5 is shown in Table 1. Out of the total, 705 patients had an NLR < 2.5, while 456 had an NLR ≥ 2.5. The distribution of patients across treatment arms (AA + prednisone + ADT vs. ADT alone) showed no significant variation (p = 0.75). There were significant differences in albumin levels, with those having NLR < 2.5 showing a mean albumin level of 38.52 g/dL compared to 38.03 g/dL in the NLR ≥ 2.5 group (p = 0.03). Additionally, the proportion of patients with baseline bone metastases was higher (68% vs. 59%, p = 0.03) among patients with an NLR < 2.5. No statistically significant differences were noted in other parameters such as age, hemoglobin, PSA, LDH, presence of visceral metastases, BPI score, and Gleason grade.

Table 1.
Comparison of baseline clinical characteristics between groups with baseline NLR < 2.5 and ≥2.5.
We first assessed for baseline factors known to be prognostic in this cohort, with significant results included in our multivariable Cox regression analysis to assess if NLR was predictive of overall survival (Table 2). The significantly lower univariate hazard ratio (HR) for patients treated with abiraterone compared to placebo (HR = 0.46, 95% CI 0.39–0.53, p < 0.001) underscores the efficacy of this treatment. Additionally, hemoglobin levels above the median are associated with a notable improvement in survival (HR = 0.68, 95% CI 0.58–0.80, p < 0.001). In contrast, factors such as elevated lactate dehydrogenase levels above the upper limit of the normal and baseline ECOG performance status of 2, compared to 0–1, are correlated with poorer outcomes (HR = 1.20 and 1.77, respectively). In the multivariable Cox regression analysis, the treatment benefit of abiraterone remains robust and unchanged (HR = 0.46, 95% CI 0.38–0.54, p < 0.001), reaffirming its significant role in improving survival. However, the influence of albumin levels and lactate dehydrogenase becomes statistically non-significant in this multivariable context, suggesting that their univariate associations might be confounded by other factors. Notably, age emerges as a significant factor in this analysis, with patients aged 75 and above exhibiting a higher risk of decreased survival (HR = 1.52, 95% CI 1.12–2.08, p = 0.008) compared to those aged 65 or less. NLR did not demonstrate any significant associations in either analysis.

Table 2.
Results of multivariable Cox regression analyses on overall survival (OS). BPI, Brief Pain Index; NLR, neutrophil-to-lymphocyte ratio; ECOG, Eastern Cooperative Group; PSA, prostate specific antigen.
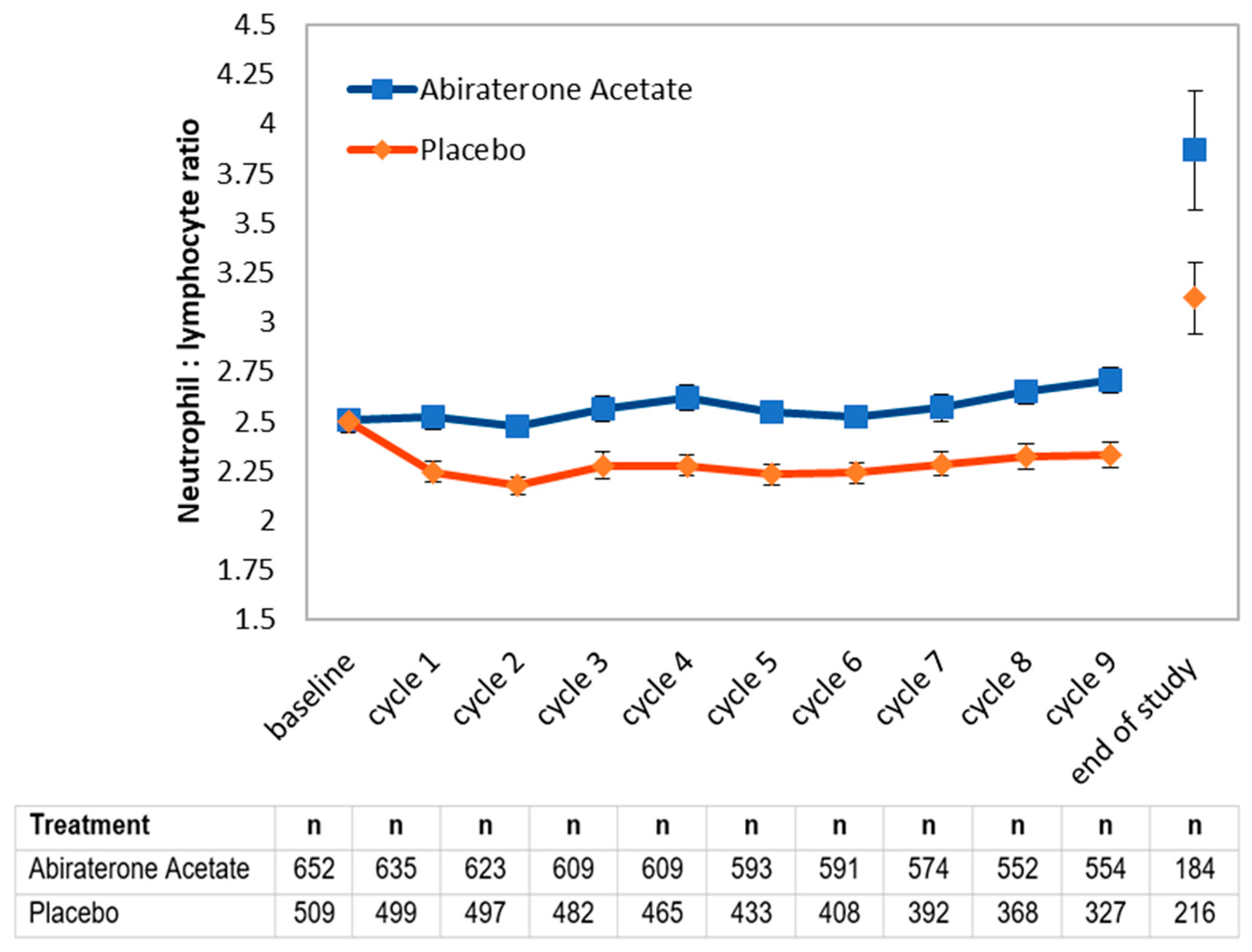
We next assessed whether NLR values during treatment may hold prognostic value (Figure 1). The use of prednisone is expected to result in higher NLR values, explaining the difference between treatment arms. An initial decline suggests a response to treatment. Therefore, we decided to evaluate whether 6-month NLR values may provide additional information beyond 6-month PSA nadir values, which are well-known to be prognostic. Notably, among patients with an excellent response (PSA < 0.03) at 6 months, a concomitant NLR value < 2.5 corresponded to better overall survival in both arms of the study (Table 3). Among patients with a good response at 6 months, a concomitant NLR value < 2.5 in the AA arm similarly corresponded to better overall survival, but this was not significant in the placebo arm.
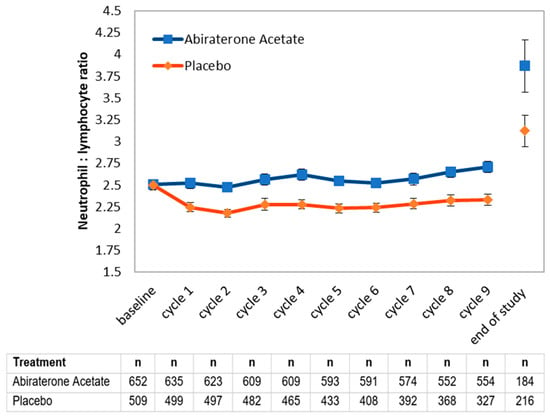
Figure 1.
Evolution of mean neutrophil-to-lymphocyte ratio (NLR) by treatment arm among available complete blood count values. Values were obtained as part of regularly blood work at the start of each monthly cycle in the LATITUDE study, or at the time of study exit for patients who progressed (end of study).

Table 3.
Relationship of 6-month PSA and NLR values with overall survival (OS).
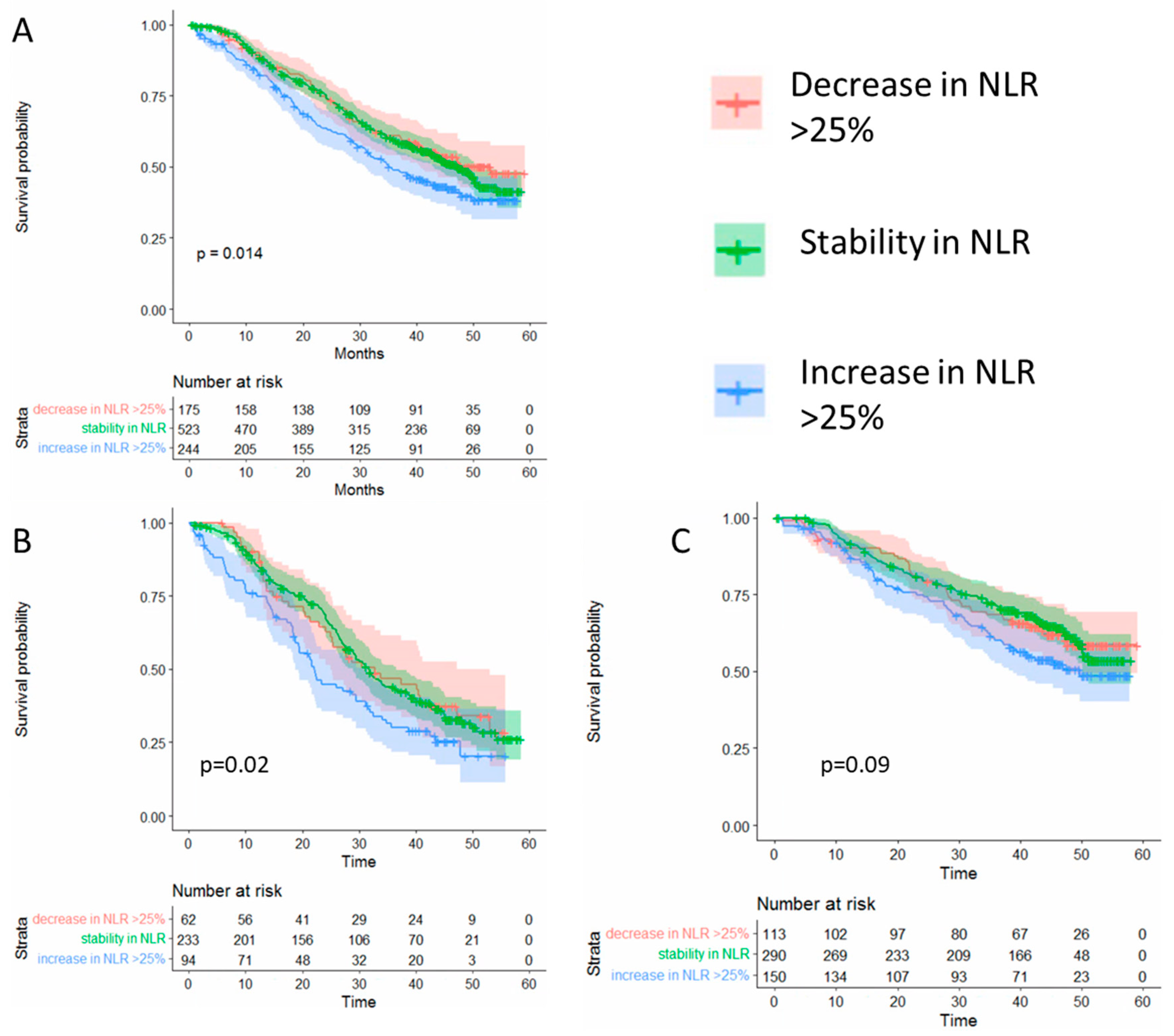
Given that using an arbitrary cut-off may not fully represent individual changes over time, we next evaluated whether individual changes at six months of >25% from baseline in NLR predict overall survival. Using this cut-off, we observed significantly worse overall survival among patients who had an NLR increase > 25% from baseline but not among those with stable or decreasing NLR values (Figure 2).
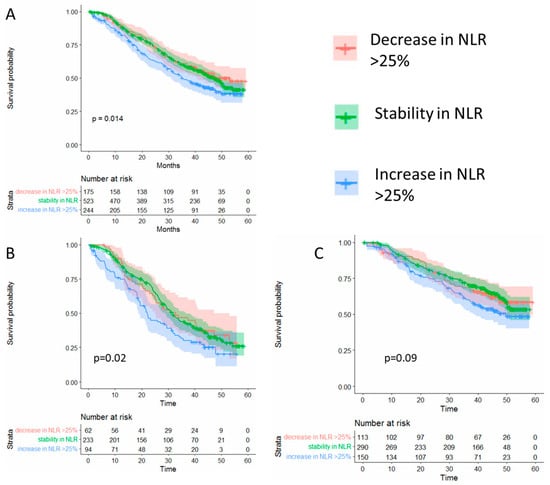
Figure 2.
(A) Overall survival according to the changes in the neutrophil to lymphocyte ratio (NLR) among all patients in the study Changes in NLR at six months after randomization compared to baseline. (B) Overall survival among patients in the placebo arm. (C) Overall survival according to patients in the abiraterone plus prednisone arm.
4. Discussion
Our study provides a comprehensive analysis of the neutrophil-to-lymphocyte ratio (NLR) as a prognostic marker in the mCSPC population treated with ADT or ADT plus abiraterone and prednisone. We demonstrate that baseline NLR is significantly associated with other clinical parameters such as ECOG performance status, ethnicity, and albumin levels. In contrast to a previous study in mCRPC, we did not demonstrate that baseline NLR predicted outcomes, which may be linked to the number of patients in the study [13]. However, we did demonstrate, as in mrCRPC, that values over time may hold prognostic value. Overall, these associations highlight the multifaceted role of systemic inflammation, as reflected by NLR, in the prognosis of cancer patients [6,11].
Our results also confirm the role of other known prognostic factors in mCRPC [14] As expected, higher hemoglobin levels, age < 75, normal LDH levels, lower baseline ECOG status, and higher albumin levels correspond to better overall survival. These factors have also been reported by other researchers in the context of metastatic prostate cancer and should be considered in clinical practice to identify patients at risk of adverse oncological outcomes [15,16,17,18].
With an ongoing study evaluating whether 6-month de-intensification can occur in certain patients with mCSPC, our analyses suggest the added utility of NLR combined with PSA as a prognostic factor [19,20]. We showed that, in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with abiraterone, those who exhibit both a lower NLR (<2.5) and PSA levels (≤0.3 or >0.3 but <4), demonstrate better survival while, in the context of AA therapy, which includes prednisone, it may be nonetheless reasonable to extrapolate this to other potent androgen receptor antagonist therapies, such as enzalutamide or apalutamide
It appears that the use of NLR needs to be evaluated specifically to each context. Previous reports have suggested that baseline NLR is not predictive in mCSPC [21,22]. Prior research describes that NLR is higher in metastatic hormone-sensitive prostate cancer compared to localized prostate cancer, suggesting a correlation between increased NLR and treatment resistance in mCRPC [23]. We found similar increases over the course of disease progression in both the pre-chemotherapy and post-chemotherapy in mCRPC in the COU-302 and COU-301 studies [13]. Similar to our findings of NLR at six months in the current study, others have similarly reported that NLR appeared to decrease concurrently with the PSA nadir [13,22]. Further, these findings appear consistent whether prednisone, which increases the NLR (Figure 2), was administered or not. Overall, these findings suggest that NLR may be indicative of the current aggressiveness of prostate cancer, offering the potential for real-time insights into disease dynamics and treatment efficacy.
While NLR may increase throughout treatment, our study does not show any clear relationship with grade. With the LATITUDE study selecting specifically for high-grade disease (98% of patients had Gleason 8–10 prostate cancer), we hypothesize this may be one of the driving factors explaining why we did not find NLR to be independently prognostic, in contrast to numerous other prostate cancer studies [23,24,25]. NLR is recognized as correlating with tumoral myeloid infiltration and, for patients with high-grade disease, it remains possible there is relatively less immune infiltration [26]. This may further explain why we found NLR nadir at six months to be prognostic, when a greater relative reduction of tumor cells could be expected.
While we use prospectively annotated data from a landmark, international randomized clinical trial, there are nonetheless limitations to our post hoc study. In addition to the specific inclusion criteria, including high-grade prostate cancer, the potential for selection bias for participation in a clinical trial exists. Prior studies describe higher NLR mean values in mCSPC outside of a clinical trial. Further, various clinical factors, such as infections, could alter the NLR value and were not controlled for in our data. Finally, our analyses are limited to the outcome of overall survival and do not evaluate other pertinent patient outcomes, including symptoms or progression-free survival [21].
In summary, our findings demonstrate that NLR was not prognostic of overall survival for patients with mCSPC in the LATITUDE study. Nonetheless, NLR may be complementary to PSA values in estimating patient prognosis over time. Future research should further elucidate the underlying mechanisms driving the associations between NLR and oncological outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/siuj5040044/s1. Table S1. Neutrophil to lymphocyte ratio (NLR) among patients in the LATITUDE study according to baseline characteristics.
Author Contributions
Conceptualization, P.T.; Formal analysis, P.T.; Data curation, P.T.; Writing—original draft, C.V.S.; Writing—review & editing, C.V.S., M.-L.R. and P.T.; Project administration, P.T. All authors have read and agreed to the published version of the manuscript.
Funding
P.T. is supported by a clinician-scientist award from the Fonds de Recherche de Québec—Santé (#354004). C.V.S. is supported by an international fellow scholarship from the Canadian Urological Association Scholarship Foundation.
Institutional Review Board Statement
Ethics approval was obtained at all participating institution and the study was conducted according to the Declaration of Helsinki.
Informed Consent Statement
All patients provided written informed consent before participation.
Data Availability Statement
Data in this study is available from the Yale University Open Data Access Project.
Conflicts of Interest
P.T. reports research funding from AstraZeneca, as well as personal fees as a consultant for TerSera, Knight, Ferring, and Janssen. Other authors have no potential conflicts of interest to report.
References
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef]
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.-M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projection du fardeau du cancer au Canada en 2022. Can. Med. Assoc. J. 2022, 194, E819–E826. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Patrikidou, A.; Loriot, Y.; Eymard, J.C.; Albiges, L.; Massard, C.; Ileana, E.; Di Palma, M.; Escudier, B.; Fizazi, K. Who dies from prostate cancer? Prostate Cancer Prostatic Dis. 2014, 17, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Finianos, A.; Gupta, K.; Clark, B.; Simmens, S.J.; Aragon-Ching, J.B. Characterization of Differences Between Prostate Cancer Patients Presenting With De Novo Versus Primary Progressive Metastatic Disease. Clin. Genitourin. Cancer 2017, 16, 85–89. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, X.; Zhao, X.; Li, X.F.; Xu, R. Neutrophil-to-Lymphocyte Ratio Predicts PSA Response and Prognosis in Prostate Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158770. [Google Scholar] [CrossRef]
- van Soest, R.J.; Templeton, A.J.; Vera-Badillo, F.E.; Mercier, F.; Sonpavde, G.; Amir, E.; Tombal, B.; Rosenthal, M.; Eisenberger, M.A.; Tannock, I.F.; et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: Data from two randomized phase III trials. Ann. Oncol. 2015, 26, 743–749. [Google Scholar] [CrossRef]
- Sonpavde, G.; Pond, G.R.; Armstrong, A.J.; Clarke, S.J.; Vardy, J.L.; Templeton, A.J.; Wang, S.-L.; Paolini, J.; Chen, I.; Chow-Maneval, E.; et al. Prognostic impact of the neutrophil-to-lymphocyte ratio in men with metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 2014, 12, 317–324. [Google Scholar] [CrossRef]
- Lolli, C.; Caffo, O.; Scarpi, E.; Aieta, M.; Conteduca, V.; Maines, F.; Bianchi, E.; Massari, F.; Veccia, A.; Chiuri, V.E.; et al. Systemic Immune-Inflammation Index Predicts the Clinical Outcome in Patients with mCRPC Treated with Abiraterone. Front. Pharmacol. 2016, 7, 376. [Google Scholar] [CrossRef]
- Loubersac, T.; Nguile-Makao, M.; Pouliot, F.; Fradet, V.; Toren, P. Neutrophil-to-lymphocyte Ratio as a Predictive Marker of Response to Abiraterone Acetate: A Retrospective Analysis of the COU302 Study. Eur. Urol. Oncol. 2020, 3, 298–305. [Google Scholar] [CrossRef]
- Onal, C.; Sedef, A.M.; Kose, F.; Oymak, E.; Guler, O.C.; Sumbul, A.T.; Aksoy, S.; Yildirim, B.A.; Besen, A.A.; Muallaoglu, S.; et al. The hematologic parameters in metastatic castration-resistant prostate cancer patients treated with abiraterone acetate. Future Oncol. 2018, 15, 1469–1479. [Google Scholar] [CrossRef]
- Halabi, S.; Small, E.J.; Kantoff, P.W.; Kattan, M.W.; Kaplan, E.B.; Dawson, N.A.; Levine, E.G.; Blumenstein, B.A.; Vogelzang, N.J. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J. Clin. Oncol. 2003, 21, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Garrett-Mayer, E.; de Wit, R.; Tannock, I.; Eisenberger, M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin. Cancer Res. 2010, 16, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Kong, D.M.; Li, L. Prognostic value of ECOG performance status and Gleason score in the survival of castration-resistant prostate cancer: A systematic review. Asian J. Androl. 2021, 23, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Alvim, C.M.; Mansinho, A.; Paiva, R.S.; Brás, R.; Semedo, P.M.; Lobo-Martins, S.; da Ponte, C.B.; Macedo, D.; Ribeiro, L.; Dos Reis, J.P.; et al. Prognostic factors for patients treated with abiraterone. Future Sci. OA 2019, 6, FSO436. [Google Scholar] [CrossRef]
- ClinicalTrials.gov [Internet]. Identifier NCT05884398, a Study of an Intermittent ADT Approach with Apalutamide Monotherapy in Participants with mCSPC (LIBERTAS); National Library of Medicine (US): Bethesda, MD, USA, 12 February 2024. Available online: https://beta.clinicaltrials.gov/study/NCT05884398 (accessed on 8 June 2024).
- Halabi, S.; Armstrong, A.J.; Sartor, O.; de Bono, J.; Kaplan, E.; Lin, C.Y.; Solomon, N.C.; Small, E.J. Prostate-specific antigen changes as surrogate for overall survival in men with metastatic castration-resistant prostate cancer treated with second-line chemotherapy. J. Clin. Oncol. 2013, 31, 3944–3950. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Shayegan, B.; Morgan, S.C.; Hamilton, R.J.; Cagiannos, I.; Basappa, N.S.; Ferrario, C.; Gotto, G.T.; Fernandes, R.; Roy, S.; et al. Prognostic Association between Common Laboratory Tests and Overall Survival in Elderly Men with De Novo Metastatic Castration Sensitive Prostate Cancer: A Population-Based Study in Canada. Cancers 2021, 13, 2844. [Google Scholar] [CrossRef]
- Salah, S.; Abu-Hijlih, R.; Abuhijla, F.; Tamimi, F.; Al-Tell, A.; Shahait, M. Pretreatment neutrophil-to-lymphocyte ratio as a potential prognostic biomarker for newly diagnosed patients with metastatic castration-sensitive prostate cancer. Cancer Rep. 2021, 4, e1392. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Yokomizo, Y.; Ito, Y.; Ito, H.; Ishiguro, H.; Teranishi, J.; Makiyama, K.; Miyoshi, Y.; Miyamoto, H.; Yao, M.; et al. Pretreatment neutrophil-to-lymphocyte ratio predicts the prognosis in patients with metastatic prostate cancer. BMC Cancer 2016, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Costanzo-Garvey, D.L.; Keeley, T.; Case, A.J.; Watson, G.F.; Alsamraae, M.; Yu, Y.; Su, K.; Heim, C.E.; Kielian, T.; Morrissey, C.; et al. Neutrophils are mediators of metastatic prostate cancer progression in bone. Cancer Immunol. Immunother. CII 2020, 69, 1113–1130. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz-Amit, R.; Templeton, A.J.; Omlin, A.; Pezaro, C.; Atenafu, E.G.; Keizman, D.; Vera-Badillo, F.; Seah, J.A.; Attard, G.; Knox, J.J.; et al. Clinical variables associated with PSA response to abiraterone acetate in patients with metastatic castration-resistant prostate cancer. Ann. Oncol. 2014, 25, 657–662. [Google Scholar] [CrossRef]
- Guo, C.; Sharp, A.; Gurel, B.; Crespo, M.; Figueiredo, I.; Jain, S.; Vogl, U.; Rekowski, J.; Rouhifard, M.; Gallagher, L.; et al. Targeting myeloid chemotaxis to reverse prostate cancer therapy resistance. Nature 2023, 623, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).