Rectal Spacer Reduces Gastrointestinal Side Effects of Radiation Post Radical Prostatectomy
Abstract
1. Introduction
2. Methods
2.1. Study Population
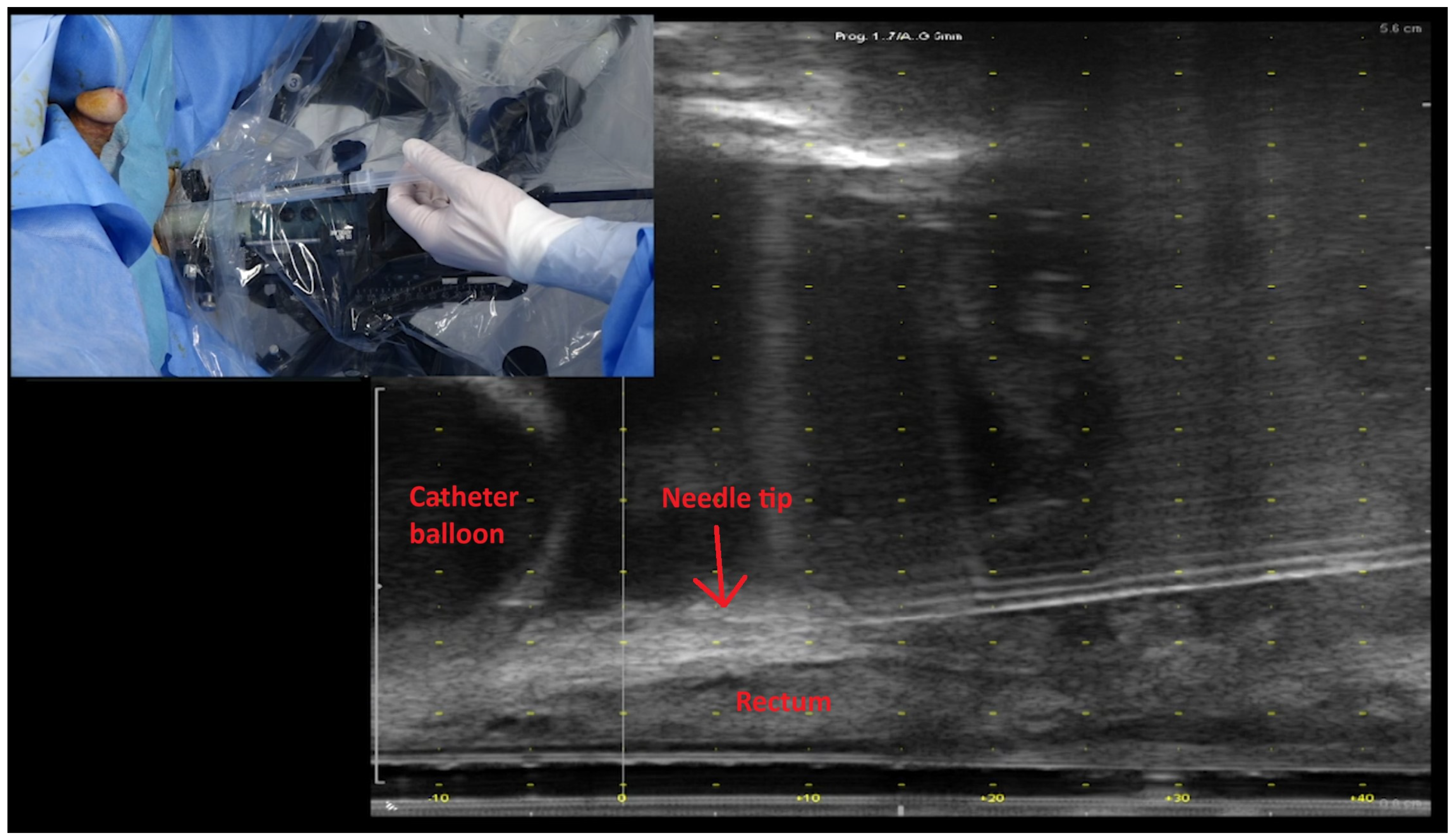
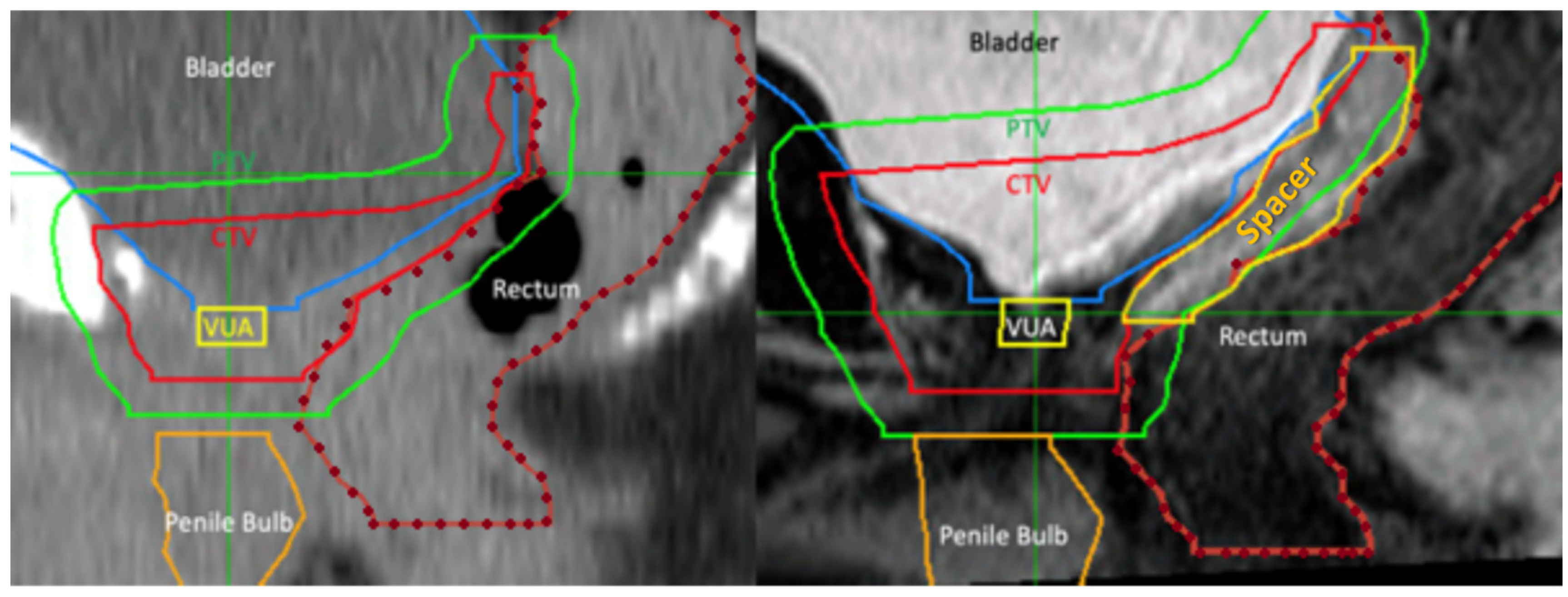
2.2. Rectal Spacer and Technique
2.3. Radiation Therapy
2.4. Radiation Toxicities
2.5. Study Endpoints
2.6. Data Collection and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaorsky, N.G.; Calais, J.; Fanti, S.; Tilki, D.; Dorff, T.; Spratt, D.E.; Kishan, A.U. Salvage therapy for prostate cancer after radical prostatectomy. Nat. Rev. Urol. 2021, 18, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Leiker, A.J.; Desai, N.B.; Folkert, M.R. Rectal radiation dose-reduction techniques in prostate cancer: A focus on the rectal spacer. Future Oncol. 2018, 14, 2773–2788. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; Clarke, N.W.; Cook, A.D.; Kynaston, H.G.; Petersen, P.M.; Catton, C.; Cross, W.; Logue, J.; Parulekar, W.; Payne, H.; et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomised, controlled phase 3 trial. Lancet 2020, 396, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Sargos, P.; Chabaud, S.; Latorzeff, I.; Magné, N.; Benyoucef, A.; Supiot, S.; Pasquier, D.; Abdiche, M.S.; Gilliot, O.; Graff-Cailleaud, P.; et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Kneebone, A.; Fraser-Browne, C.; Duchesne, G.M.; Fisher, R.; Frydenberg, M.; Herschtal, A.; Williams, S.G.; Brown, C.; Delprado, W.; Haworth, A.; et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): A randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Ghadjar, P.; Hayoz, S.; Bernhard, J.; Zwahlen, D.R.; Hölscher, T.; Gut, P.; Polat, B.; Hildebrandt, G.; Müller, A.C.; Plasswilm, L.; et al. Dose-intensified Versus Conventional-dose Salvage Radiotherapy for Biochemically Recurrent Prostate Cancer after Prostatectomy: The SAKK 09/10 Randomized Phase 3 Trial. Eur. Urol. 2021, 80, 306–315. [Google Scholar] [CrossRef]
- Pinkawa, M. Spacer application for prostate cancer radiation therapy. Future Oncol. 2014, 10, 851–864. [Google Scholar] [CrossRef]
- Rocco, B.; Cozzi, G. Denonvilliers’ Fascia: Anatomy, Surgical Planes, Use in Reconstruction. In Robot-Assisted Radical Prostatectomy: Beyond the Learning Curve; Davis, J.W., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 113–118. [Google Scholar]
- Connolly, J.A.; Shinohara, K.; Presti, J.C., Jr.; Carroll, P.R. Local recurrence after radical prostatectomy: Characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology 1996, 47, 225–231. [Google Scholar] [CrossRef]
- Lehrich, B.; Moyses, H.; Ravera, J.; Yoshida, J.; Torrey, R.; Baghdassarian, R.; Gazzaniga, M.; Weinberg, A.; Phan, C.; Chalfin, S.; et al. Five-year results of post-prostatectomy patients administered a hydrogel rectal spacer implant in conjunction with dose escalated external beam radiation therapy. J. Radiat. Oncol. 2019, 8, 31–38. [Google Scholar] [CrossRef]
- Sidhom, M.A.; Kneebone, A.B.; Lehman, M.; Wiltshire, K.L.; Millar, J.L.; Mukherjee, R.K.; Shakespeare, T.P.; Tai, K.-H. Post-prostatectomy radiation therapy: Consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother. Oncol. 2008, 88, 10–19. [Google Scholar] [CrossRef]
- Chao, M.; Ho, H.; Joon, D.L.; Chan, Y.; Spencer, S.; Ng, M.; Wasiak, J.; Lawrentschuk, N.; McMillan, K.; Sengupta, S.; et al. The use of tissue fiducial markers in improving the accuracy of post-prostatectomy radiotherapy. Radiat. Oncol. J. 2019, 37, 43–50. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services NIoH, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), v4.03; EORTC: Brussels, Belgium, 2010.
- Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; Kos, M.; et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 971–977. [Google Scholar] [CrossRef]
- Mariados, N.F.; Orio, P.F., 3rd; Schiffman, Z.; Van, T.J.; Engelman, A.; Nurani, R.; Kurtzman, S.M.; Lopez, E.; Chao, M.; Boike, T.P.; et al. Hyaluronic Acid Spacer for Hypofractionated Prostate Radiation Therapy: A Randomized Clinical Trial. JAMA Oncol. 2023, 9, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Valuck, B.W.; Chundury, A.; Gay, H.; Bosch, W.; Michalski, J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: Impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract. Radiat. Oncol. 2017, 7, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Karsh, L.I.; Gross, E.T.; Pieczonka, C.M.; Aliotta, P.J.; Skomra, C.J.; Ponsky, L.E.; Nieh, P.T.; Han, M.; Hamstra, D.A.; Shore, N.D. Absorbable Hydrogel Spacer Use in Prostate Radiotherapy: A Comprehensive Review of Phase 3 Clinical Trial Published Data. Urology 2018, 115, 39–44. [Google Scholar] [CrossRef]
- Miller, L.E.; Efstathiou, J.A.; Bhattacharyya, S.K.; Payne, H.A.; Woodward, E.; Pinkawa, M. Association of the Placement of a Perirectal Hydrogel Spacer with the Clinical Outcomes of Men Receiving Radiotherapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e208221. [Google Scholar] [CrossRef] [PubMed]
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; et al. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Gross, E.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase 3 trial. Pract. Radiat. Oncol. 2018, 8, e7–e15. [Google Scholar] [CrossRef] [PubMed]
- Pinkawa, M.; Schubert, C.; Escobar-Corral, N.; Holy, R.; Eble, M.J. Application of a hydrogel spacer for postoperative salvage radiotherapy of prostate cancer. Strahlenther. Onkol. 2015, 191, 375–379. [Google Scholar] [CrossRef]
- Yeh, J.; Tokita, K.; Chien, J.; Ravera, J. (P141) rectal spacer injection in postprostatectomy patients undergoing high-dose salvage external beam radiation. Oncology 2015, 29 (Suppl. S1). [Google Scholar]
- Yang, D.X.; Verma, V.; An, Y.; Yu, J.B.; Sprenkle, P.C.; Leapman, M.S.; Park, H.S. Radiation Dose to the Rectum with Definitive Radiation Therapy and Hydrogel Spacer Versus Postprostatectomy Radiation Therapy. Adv. Radiat. Oncol. 2020, 5, 1225–1231. [Google Scholar] [CrossRef]
- Zaine, H.; Vandendorpe, B.; Bataille, B.; Lacornerie, T.; Wallet, J.; Mirabel, X.; Lartigau, E.; Pasquier, D. Salvage Radiotherapy for Macroscopic Local Recurrence Following Radical Prostatectomy. Front. Oncol. 2021, 11, 1258. [Google Scholar] [CrossRef]
- Wu, W.-C.; Lai, Y.-L.; Liang, J.-A. Salvage radiotherapy for biochemical recurrence after radical prostatectomy: Experience of a single center. Ther. Radiol. Oncol. 2018, 2, 3. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Scardino, P.T.; Kattan, M.W.; Pisansky, T.M.; Slawin, K.M.; Klein, E.A.; Anscher, M.S.; Michalski, J.M.; Sandler, H.M.; Lin, D.W.; et al. Predicting the Outcome of Salvage Radiation Therapy for Recurrent Prostate Cancer after Radical Prostatectomy. J. Clin. Oncol. 2007, 25, 2035–2041. [Google Scholar] [CrossRef]
- De Bruycker, A.; Lambert, B.; Claeys, T.; Delrue, L.; Mbah, C.; De Meerleer, G.; Villeirs, G.; De Vos, F.; De Man, K.; Decaestecker, K.; et al. Prevalence and prognosis of low-volume, oligorecurrent, hormone-sensitive prostate cancer amenable to lesion ablative therapy. BJU Int. 2017, 120, 815–821. [Google Scholar] [CrossRef]

| Characteristic | ||
|---|---|---|
| N | 67 | |
| Age (years), mean (SD) | 64.4 (5.7) | |
| Pre-prostatectomy PSA, median (IQR) | 6.60 (4.50, 9.40) | |
| Clinical TNM staging, n (%) | T1 | 17 (25%) |
| T2 | 49 (73%) | |
| T3 | 1 (1%) | |
| Pathological TNM staging, n (%) | T2 | 18 (27%) |
| T3 | 49 (73%) | |
| T3a | 38 | |
| T3b | 11 | |
| Positive margin rate, n (%) | 43 (64%) | |
| ISUP Grade, n (%) | 1 | 3 (4%) |
| 2 | 31 (46%) | |
| 3 | 16 (24%) | |
| 4 | 10 (15%) | |
| 5 | 7 (10%) |
| Pre-Spacer Insertion Factors | ||
|---|---|---|
| Pre-radiation PSA, median (IQR) | 0.20 (0.14, 0.36) | |
| Space between bladder and rectum pre-spacer insertion (mm), n (%) | 0 | 61 (91%) |
| 1 | 5 (7%) | |
| 2 | 1 (1%) | |
| Neoadjuvant therapy, n (%) | None | 17 (25%) |
| Triptorelin | 9 (13%) | |
| Leuprorelin | 33 (49%) | |
| Goserelin | 8 (12%) | |
| Proposed duration of ADT, n (%) | 24 months | 1 (2%) |
| 6 months | 49 (98%) | |
| Post-rectal spacer insertion factors | ||
| Type of rectal spacer, n (%) | Hyaluronic Acid | 44 (66%) |
| Hydrogel | 20 (30%) | |
| No spacer inserted | 3 (4%) | |
| Vesico-rectal separation in x-axis post spacer insertion (mm), median (IQR) | 11.0 (10.0, 13.0) | |
| Distance in y-axis post spacer insertion (mm), median (IQR) | 42.0 (38.0, 48.0) | |
| Radiation therapy duration (days), median (IQR) | 56.0 (55.0, 57.0) | |
| Ease of spacer insertion, n (%) | 1—very difficult | 3 (4%) |
| 2—difficult | 4 (6%) | |
| 3—moderate | 22 (33%) | |
| 4—easy | 33 (49%) | |
| 5—very easy | 5 (7%) |
| Volume of Rectum Receiving Radiation Dose | Pre-Spacer Insertion (cm3), Median (SD) | Post-Spacer Insertion (cm3), Median (SD) | Reduction, % | p-Value |
|---|---|---|---|---|
| Rectal volume receiving 60% of radiation dose | 39.95 (6.95) | 29.61 (7.35) | 25.9 | <0.001 |
| Rectal volume receiving 70% of radiation dose | 35.39 (6.23) | 24.32 (6.76) | 34.2 | <0.001 |
| Rectal volume receiving 80% of radiation dose | 29.56 (6.16) | 19.11 (6.01) | 35.4 | <0.001 |
| Rectal volume receiving 90% of radiation dose | 23.65 (5.23) | 13.32 (4.83) | 43.7 | <0.001 |
| Rectal volume receiving 100% of radiation dose | 8.54 (3.72) | 3.27 (2.57) | 61.7 | <0.001 |
| Toxicity | Severity | Definition of Toxicity Severity | Acute Toxicity | Late Toxicity |
|---|---|---|---|---|
| Any GI Toxicity | 0 | 48 (72%) | 59 (88%) | |
| 1 | 18 (27%) | 7 (10%) | ||
| 2 | 1 (1%) | 1 (1%) | ||
| Anal Haemorrhage | 0 | No anal haemorrhage | 67 (100%) | 67 (100%) |
| Anal Pain | 0 | No pain | 65 (97%) | 67 (100%) |
| 1 | Mild pain | 2 (3%) | 0 | |
| Faecal Incontinence | 0 | No faecal incontinence | 67 (100%) | 67 (100%) |
| Diarrhoea | 0 | No diarrhoea | 56 (84%) | 66 (99%) |
| 1 | Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline | 10 (15%) | 0 (0%) | |
| 2 | Increase of 4–6 stools per day over baseline; moderate increase in ostomy output compared to baseline | 1 (1%) | 1 (1%) | |
| Proctitis | 0 | No proctitis | 60 (90%) | 61 (91%) |
| 1 | Rectal discomfort, intervention not indicated | 7 (10%) | 6 (9%) | |
| Rectal Haemorrhage | 0 | No rectal haemorrhage | 67 (100%) | 66 (99%) |
| 1 | Mild; intervention not indicated | 0 (0%) | 1 (1%) | |
| Rectal Pain | 0 | No rectal pain | 67 (100%) | 67 (100%) |
| Rectal Fistula | 0 | No rectal fistula | 67 (100%) | 67 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, A.; Bolton, D.; Pham, T.; Angus, D.; Pan, D.; Joon, D.L.; Tan, A.; McMillan, K.; Chan, Y.; Manohar, P.; et al. Rectal Spacer Reduces Gastrointestinal Side Effects of Radiation Post Radical Prostatectomy. Soc. Int. Urol. J. 2024, 5, 111-121. https://doi.org/10.3390/siuj5020020
Hong A, Bolton D, Pham T, Angus D, Pan D, Joon DL, Tan A, McMillan K, Chan Y, Manohar P, et al. Rectal Spacer Reduces Gastrointestinal Side Effects of Radiation Post Radical Prostatectomy. Société Internationale d’Urologie Journal. 2024; 5(2):111-121. https://doi.org/10.3390/siuj5020020
Chicago/Turabian StyleHong, Anne, Damien Bolton, Trung Pham, David Angus, David Pan, Daryl Lim Joon, Alwin Tan, Kevin McMillan, Yee Chan, Paul Manohar, and et al. 2024. "Rectal Spacer Reduces Gastrointestinal Side Effects of Radiation Post Radical Prostatectomy" Société Internationale d’Urologie Journal 5, no. 2: 111-121. https://doi.org/10.3390/siuj5020020
APA StyleHong, A., Bolton, D., Pham, T., Angus, D., Pan, D., Joon, D. L., Tan, A., McMillan, K., Chan, Y., Manohar, P., Thomas, J., Ho, H., Orio, P., Holt, E., Cokelek, M., Lawrentschuk, N., Foroudi, F., & Chao, M. (2024). Rectal Spacer Reduces Gastrointestinal Side Effects of Radiation Post Radical Prostatectomy. Société Internationale d’Urologie Journal, 5(2), 111-121. https://doi.org/10.3390/siuj5020020





