Is the Bulbar Urethral Stricture a Single and Uniform Disease?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Patient Classification
2.3. Surgical Technique
2.4. Patient Follow-Up and Definition of Recurrence
2.5. Statistical Analysis
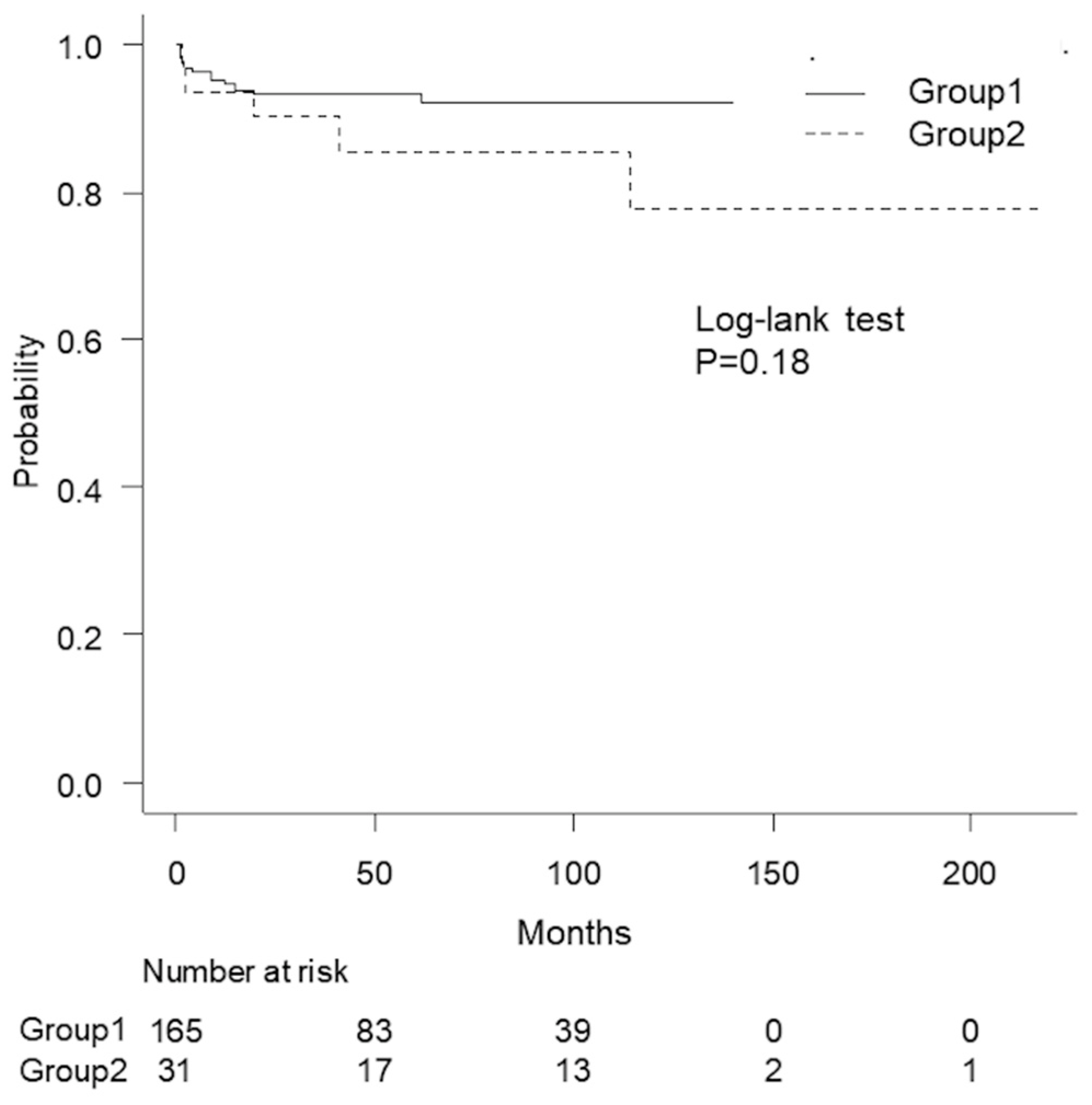
3. Results
3.1. Comparison of Stricture Characteristics
3.2. Comparison of Required Surgical Techniques and Outcomes
3.3. Comparison of Stricture Lengths in Patients Treated with EPA or NTAU
3.4. Predictive Factors for Substitution Urethroplasty
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LSE | Length, segment, and etiology |
| BUS | bulbar urethral strictures |
| NTAU | non-transecting anastomotic urethroplasty |
| EPA | excision and primary anastomosis |
| OA | onlay augmentation urethroplasty |
| SU | staged urethroplasty |
| BMI | body mass index |
| IQR | interquartile range |
| OR | odds ratio |
| 95% CI | 95% confidence interval |
| TAP | tunica albuginea plication |
References
- Palminteri, E.; Berdondini, E.; Verze, P.; De Nunzio, C.; Vitarelli, A.; Carmignani, L. Contemporary urethral stricture characteristics in the developed world. Urology 2013, 81, 191–196. [Google Scholar] [CrossRef]
- Lumen, N.; Hoebeke, P.; Willemsen, P.; De Troyer, B.; Pieters, R.; Oosterlinck, W. Etiology of urethral stricture disease in the 21st century. J. Urol. 2009, 182, 983–987. [Google Scholar] [CrossRef]
- Cotter, K.J.; Hahn, A.E.; Voelzke, B.B.; Myers, J.B.; Smith, T.G., 3rd; Elliott, S.P.; Alsikafi, N.F.; Breyer, B.N.; Vanni, A.J.; Buckley, J.C.; et al. Trends in Urethral Stricture Disease Etiology and Urethroplasty Technique from a Multi-institutional Surgical Outcomes Research Group. Urology 2019, 130, 167–174. [Google Scholar] [CrossRef]
- Stein, D.M.; Thum, D.J.; Barbagli, G.; Kulkarni, S.; Sansalone, S.; Pardeshi, A.; Gonzalez, C.M. A geographic analysis of male urethral stricture aetiology and location. BJU Int. 2013, 112, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.G.; Mundy, T.; Dubey, D.; El-Kassaby, A.W.; Firdaoessaleh; Kodama, R.; Santucci, R. SIU/ICUD consultation on urethral strictures: Pelvic fracture urethral injuries. Urology 2014, 83 (Suppl. S3), S48–S58. [Google Scholar] [CrossRef] [PubMed]
- Wessells, H.; Angermeier, K.W.; Elliott, S.; Gonzalez, C.M.; Kodama, R.; Peterson, A.C.; Reston, J.; Rourke, K.; Stoffel, J.T.; Vanni, A.J.; et al. Male Urethral Stricture: American Uro-logical Association Guideline. J. Urol. 2017, 197, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Lumen, N.; Campos-Juanatey, F.; Greenwell, T.; Martins, F.E.; Osman, N.I.; Riechardt, S.; Waterloos, M.; Barratt, R.; Chan, G.; Esperto, F.; et al. European Association of Urology Guidelines on Urethral Stricture Disease (Part 1): Management of Male Urethral Stricture Disease. Eur. Urol. 2021, 80, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Rourke, K.F.; Welk, B.; Kodama, R.; Bailly, G.; Davies, T.; Santesso, N.; Violette, P.D. Canadian Urological Association guideline on male urethral stricture. Can. Urol. Assoc. J. 2020, 14, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Morey, A.F.; Kizer, W.S. Proximal bulbar urethroplasty via extended anastomotic approach—What are the limits? J. Urol. 2006, 175, 2145–2149; discussion 2149. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.A.; Flynn, K.J.; Hahn, A.E.; Cotter, K.; Alsikafi, N.F.; Breyer, B.N.; Broghammer, J.A.; Buckley, J.C.; Elliott, S.P.; Myers, J.B.; et al. Development and Validation of A Male Anterior Urethral Stricture Classification System. Urology 2020, 143, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Andrich, D.E.; Mundy, A.R. Non-transecting anastomotic bulbar urethroplasty: A preliminary report. BJU Int. 2012, 109, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Ojima, K.; Shinchi, M.; Hirano, Y.; Hamamoto, K.; Ito, K.; Asano, T.; Takahashi, E.; Kimura, F.; Azuma, R. Single-surgeon experience of excision and primary anastomosis for bulbar urethral stricture: Analysis of surgical and patient-reported outcomes. World J. Urol. 2021, 39, 3063–3069. [Google Scholar] [CrossRef] [PubMed]
- Mundy, A.R. Anastomotic urethroplasty. BJU Int. 2005, 96, 921–944. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Shinchi, M.; Ojima, K.; Masunaga, A.; Ito, K.; Asano, T.; Takahashi, E.; Kimura, F.; Azuma, R. Evaluation of the effect of urethroplasty for anterior urethral strictures by a validated disease-specific patient-reported outcome measure. World J. Urol. 2019, 37, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Heinke, T.; Gerharz, E.W.; Bonfig, R.; Riedmiller, H. Ventral onlay urethroplasty using buccal mucosa for complex stricture repair. Urology 2003, 61, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Jezior, J.R.; Schlossberg, S.M. Excision and primary anastomosis for anterior urethral stricture. Urol. Clin. N. Am. 2002, 29, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Iselin, C.E.; Webster, G.D. Dorsal onlay urethroplasty for urethral stricture repair. World J. Urol. 1998, 16, 181–185. [Google Scholar] [CrossRef]
- Terlecki, R.P.; Steele, M.C.; Valadez, C.; Morey, A.F. Grafts are Unnecessary for Proximal Bulbar Reconstruction. J. Urol. 2010, 184, 2395–2399. [Google Scholar] [CrossRef]
- VanDyke, M.E.; Baumgarten, A.S.; Ortiz, N.M.; Dropkin, B.M.; Joice, G.A.; Khouri, R.K.; Filho, J.E.P.; Ward, E.E.; Hudak, S.J.; Morey, A.F. Extended Primary Anastomosis with Penile Plication (EPAPP): A Promising New Alternative to Perineal Urethrostomy for Reconstruction of Long Urethral Strictures. Urology 2021, 149, 245–250. [Google Scholar] [CrossRef]
| Segment | Group 1 | Group 2 | p | |||
|---|---|---|---|---|---|---|
| Factors | Unit/Category | Median/Number | IQR/% | Median/Number | IQR/% | |
| N (%) | 165 (84) | 31 (16) | ||||
| Age | y.o. | 53 | 39–64 | 55 | 43–70 | 0.05 |
| Smoke | none | 60 | 36 | 11 | 35 | 0.99 |
| DM | present | 10 | 6.1 | 1 | 3.2 | 0.83 |
| BMI | kg/m2 | 23 | 22–25 | 23 | 21–26 | 0.53 |
| Prior urethroplasty | ≥1 | 19 | 12 | 7 | 23 | 0.16 |
| Stricture length | mm | 10 | 7–15 | 23 | 10–32 | <0.001 |
| Lumen | non-obliterated | 121 | 73 | 29 | 94 | 0.020 |
| Prior transurethral procedure | ≥2 | 71 | 43 | 18 | 58 | 0.17 |
| Etiology | trauma | 78 | 47 | 8 | 26 | 0.010 |
| idiopathic | 51 | 31 | 11 | 35 | ||
| iatrogenic | 34 | 21 | 9 | 29 | ||
| others | 2 | 1.0 | 3 | 10 | ||
| Group 1 | Group 2 | p | ||||
|---|---|---|---|---|---|---|
| Factor | Unit/Category | Median/Number | IQR/% | Median/Number | IQR/% | |
| Surgical technique | EPA | 125 | 76 | 6 | 19 | <0.001 a |
| NTAU | 16 | 10 | 0 | 0 | ||
| OA | 20 | 12 | 18 | 58 | ||
| SU | 4 | 2.4 | 7 | 23 | ||
| Corporal splitting | Present | 62 | 62 | 1 | 3.2 | <0.001 |
| Operation time | minute | 157 | 138–187 | 186 | 135–213 | 0.19 |
| Blood loss | mL | 59 | 32–115 | 68 | 21–156 | 0.67 |
| Initial Result | Final Result | |||||||
|---|---|---|---|---|---|---|---|---|
| VIF | OR | 95% CI | p | OR | 95% CI | p | ||
| Segment | group 2 | 1.1 | 18 | 5.1–62 | <0.001 | 19 | 5.6–66 | <0.001 |
| Prior urethroplasty | ≥1 | 1.0 | 2.0 | 0.58–7.0 | 0.27 | |||
| Lumen | non-obliterated | 1.1 | 13 | 1.2–100 | 0.030 | 13 | 1.5–100 | 0.020 |
| Prior transurethral procedure | ≥2 | 1.2 | 2.5 | 0.85–7.2 | 0.10 | 3.1 | 1.1–8.3 | 0.030 |
| Smoking | present | 1.2 | 1.7 | 0.57–5.0 | 0.35 | |||
| Age | ≥53 y.o. | 1.1 | 0.98 | 0.36–2.7 | 0.96 | |||
| Stricture length | >20 mm | 1.1 | 16 | 5.3–49 | <0.001 | 18 | 6.1–54 | <0.001 |
| BMI | >25 | 1.2 | 1.9 | 0.65–5.4 | 0.24 | |||
| Etiology | non-traumatic | 1.3 | 1.5 | 0.45–5.2 | 0.49 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabei, T.; Horiguchi, A.; Shinchi, M.; Hirano, Y.; Ojima, K.; Ito, K.; Azuma, R. Is the Bulbar Urethral Stricture a Single and Uniform Disease? Soc. Int. Urol. J. 2024, 5, 85-92. https://doi.org/10.3390/siuj5020014
Tabei T, Horiguchi A, Shinchi M, Hirano Y, Ojima K, Ito K, Azuma R. Is the Bulbar Urethral Stricture a Single and Uniform Disease? Société Internationale d’Urologie Journal. 2024; 5(2):85-92. https://doi.org/10.3390/siuj5020014
Chicago/Turabian StyleTabei, Tadashi, Akio Horiguchi, Masayuki Shinchi, Yusuke Hirano, Kenichiro Ojima, Keiichi Ito, and Ryuichi Azuma. 2024. "Is the Bulbar Urethral Stricture a Single and Uniform Disease?" Société Internationale d’Urologie Journal 5, no. 2: 85-92. https://doi.org/10.3390/siuj5020014
APA StyleTabei, T., Horiguchi, A., Shinchi, M., Hirano, Y., Ojima, K., Ito, K., & Azuma, R. (2024). Is the Bulbar Urethral Stricture a Single and Uniform Disease? Société Internationale d’Urologie Journal, 5(2), 85-92. https://doi.org/10.3390/siuj5020014





