Utility and Clinical Application of Circulating Tumor DNA (ctDNA) in Advanced Prostate Cancer
Abstract
:1. Background
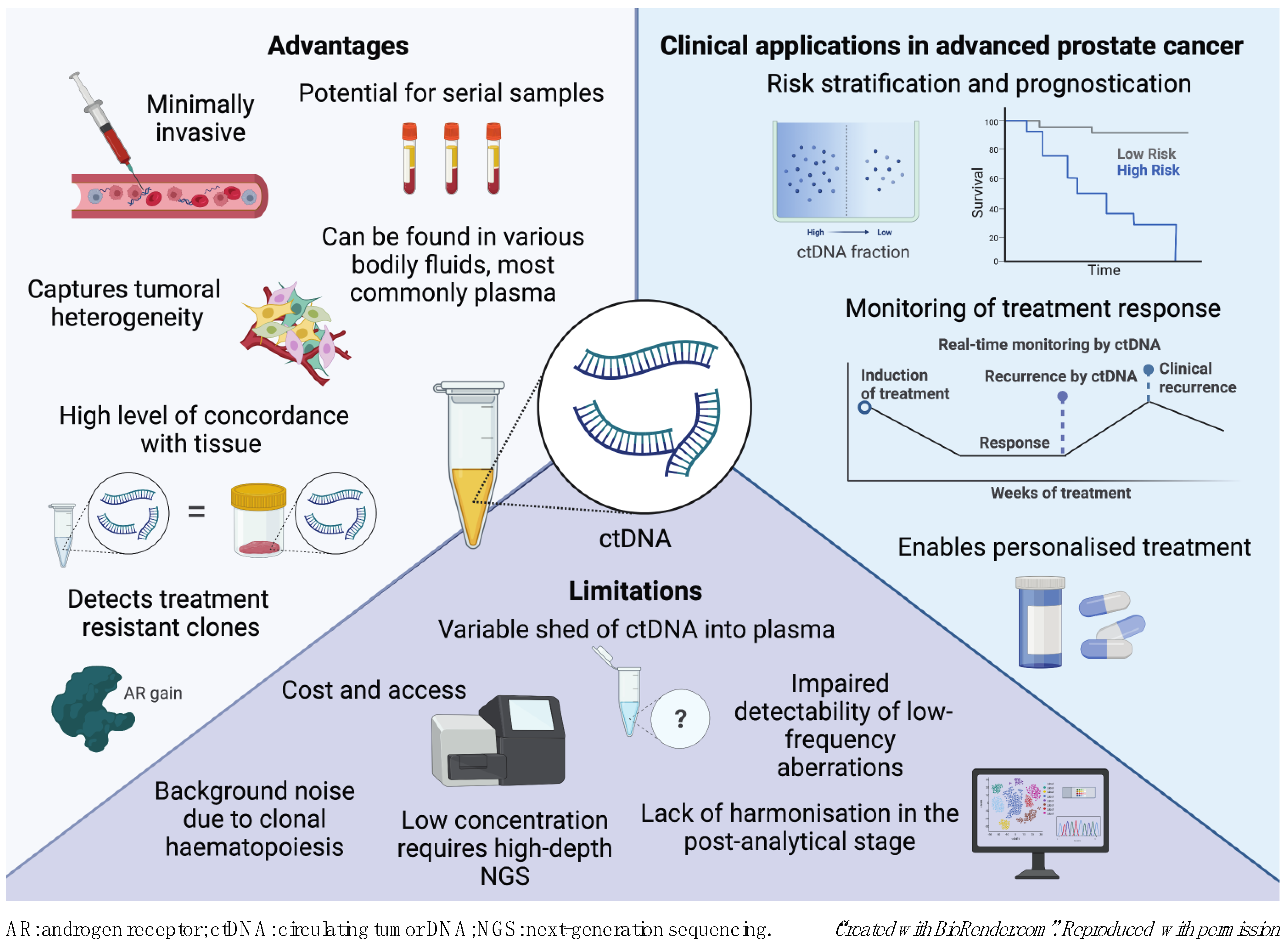
2. The Current Landscape of ctDNA in Prostate Cancer
3. Technical Considerations for ctDNA Analysis
4. Application of ctDNA in Metastatic Castration-Resistant Prostate Cancer
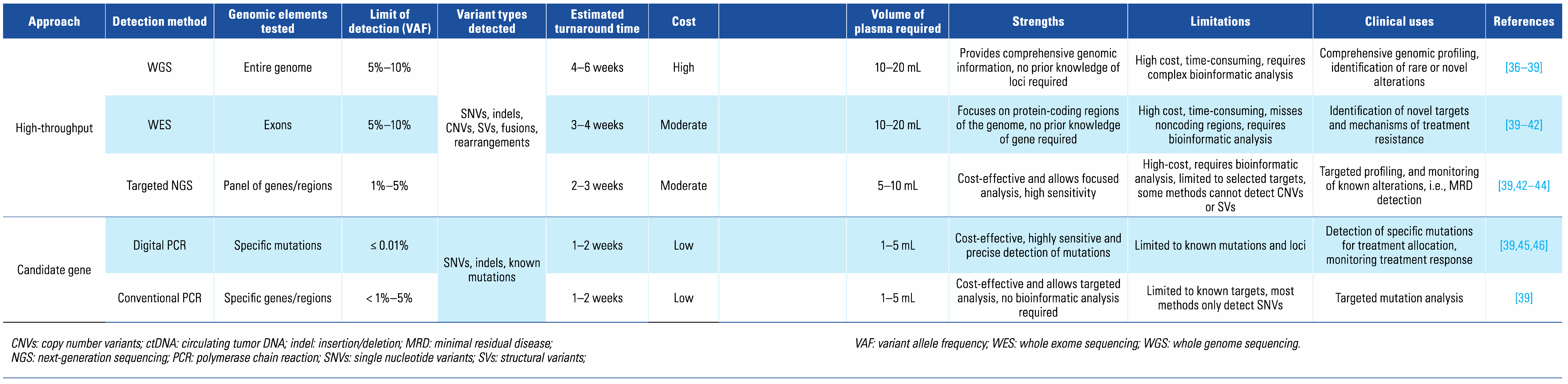 |
4.1. Pretreatment ctDNA Fraction and Profile for Prognostication
4.2. Longitudinal Monitoring of Treatment Response
4.3. Early Detection of Treatment Resistance
4.4. Facilitating Selection of Personalized Treatment
 |
5. Metastatic Hormone-Sensitive Prostate Cancer
5.1. ctDNA as a Prognostic Tool to Guide Upfront Treatment Intensification
5.2. Genomic Aberrations to Guide the Choice of Systemic Therapy in Metastatic Hormone-Sensitive Prostate Cancer
6. Challenges and Limitations of ctDNA Profiling
7. Conclusion
Conflicts of Interest
Abbreviations
| ADT | androgen deprivation therapy |
| AR | androgen receptor |
| ARPI | androgen receptor pathway inhibitor |
| cfDNA | cell-free DNA |
| CNVs | copy number variants |
| ctDNA | circulating tumor DNA |
| DDR | DNA damage response and repair |
| HRR | homologous recombination repair |
| ICI | immune checkpoint inhibitor |
| IHC | Immunohistochemistry |
| mCRPC | metastatic castration-resistant prostate cancer |
| mHSPC | metastatic hormone-sensitive prostate cancer |
| mPC | metastatic prostate cancer |
| MSI | microsatellite instability |
| NGS | next-generation sequencing |
| OS | overall survival |
| PARPi | poly (ADP-ribose) polymerase inhibitors |
| PCR | polymerase chain reaction |
| PFS | progression-free survival |
| PSA | prostate-specific antigen |
| SNVs | single nucleotide variants |
| SVs | structural variants |
| TMB | tumor mutational burden |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer, J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; et al. Prostate cancer. Nat Rev Dis Primers. 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; et al. ARASENS Trial Investigators. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; et al. PEACE-1 investigators. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022, 399, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Finzel, A.; Sadik, H.; Ghitti, G.; Laes, J.F. The combined analysis of solid and liquid biopsies provides additional clinical information to improve patient care. J Cancer Metastasis Treat. 2018, 4, 21. [Google Scholar] [CrossRef]
- Hussain, M.; Corcoran, C.; Sibilla, C.; Fizazi, K.; Saad, F.; Shore, N.; et al. Tumor genomic testing for >4,000 men with metastatic castration-resistant prostate cancer in the phase III trial PROfound (Olaparib). Clin Cancer Res. 2022, 28, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.G.; Foss, E.; Joseph, G.; Foye, A.; Beckett, B.; Motamedi, D.; et al. CT-guided bone biopsies in metastatic castration-resistant prostate cancer: factors predictive of maximum tumor yield. J Vasc Interv Radiol. 2017, 28, 1073–81e1. [Google Scholar] [CrossRef] [PubMed]
- Lorente, D.; Omlin, A.; Zafeiriou, Z.; Nava-Rodrigues, D.; Pérez-López, R.; Pezaro, C.; et al. Castration-resistant prostate cancer tissue acquisition from bone metastases for molecular analyses. Clin Genitourin Cancer. 2016, 14, 485–493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wyatt, A.W.; Annala, M.; Aggarwal, R.; Beja, K.; Feng, F.; Youngren, J.; et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J Natl Cancer Inst. 2017, 109, djx118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haffner, M.C.; Mosbruger, T.; Esopi, D.M.; Fedor, H.; Heaphy, C.M.; Walker, D.A.; et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013, 123, 4918–4922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soda, N.; Rehm, B.H.A.; Sonar, P.; Nguyen, N.T.; Shiddiky, M.J.A. Advanced liquid biopsy technologies for circulating biomarker detection. J Mater Chem, B. 2019, 7, 6670–6704. [Google Scholar] [CrossRef] [PubMed]
- Fettke, H.; Kwan, E.M.; Azad, A.A. Cell-free DNA in cancer: current insights. Cell Oncol (Dordr). 2019, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herberts, C.; Annala, M.; Sipola, J.; Ng, S.W.S.; Chen, X.E.; Nurminen, A.; et al. Deep whole-genome ctDNA chronology of treatment-resistant prostate cancer. Nature. 2022, 608, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, G.D.; Pribish, D.M.; Valone, F.H.; Memoli, V.A.; Bzik, D.J.; Yao, S.L. Soluble normal and mutated DNA sequences from single-copy genes in human blood. Cancer Epidemiol Biomarkers Prev. 1994, 3, 67–71. [Google Scholar] [PubMed]
- Bando, H.; Nakamura, Y.; Taniguchi, H.; Shiozawa, M.; Yasui, H.; Esaki, T.; et al. Effects of metastatic sites on circulating tumor DNA in patients with metastatic colorectal cancer. JCO Precis Oncol. 2022, e2100535. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.A.; Volik, S.V.; Wyatt, A.W.; Haegert, A.; Le Bihan, S.; Bell, R.H.; et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015, 21, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Barnicle, A.; Sibilla, C.; Lai, Z.; Corcoran, C.; Williams, J.A.; et al. Concordance of BRCA1, BRCA2 (BRCA), and ATM mutations identified in matched tumor tissue and circulating tumor DNA (ctDNA) in men with metastatic castration-resistant prostate cancer (mCRPC) screened in the PROfound study. J Clin Oncol. 2021, 39 (Suppl. 6), 26. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, T.; Zhang, M.; Dai, C.; Wang, L.; Wang, L.; et al. Circulating cell-free DNA-based detection of tumor suppressor gene copy number loss and its clinical implication in metastatic prostate cancer. Front Oncol. 2021, 11, 720727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tukachinsky, H.; Madison, R.W.; Chung, J.H.; Gjoerup, O.V.; Severson, E.A.; Dennis, L.; et al. Genomic analysis of circulating tumor DNA in 3,334 patients with advanced prostate cancer identifies targetable BRCA alterations and AR resistance mechanisms. Clin Cancer Res. 2021, 27, 3094–3105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geeurickx, E.; Hendrix, A. Targets, pitfalls and reference materials for liquid biopsy tests in cancer diagnostics. Mol Aspects Med. 2020, 72, 100828. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Russo, G.; Di Zazzo, E.; Pisapia, P.; Mirto, B.F.; Palmieri, A.; et al. Liquid biopsy in prostate cancer management-current challenges and future perspectives. Cancers (Basel). 2022, 14, 3272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhiantravan, N.; Emmett, L.; Joshua, A.M.; Pattison, D.A.; Francis, R.J.; Williams, S.; et al. UpFrontPSMA: a randomized phase 2 study of sequential (177) Lu-PSMA-617 and docetaxel vs docetaxel in metastatic hormone-naïve prostate cancer (clinical trial protocol). BJU Int. 2021, 128, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Maughan, B.L.; Nussenzveig, R.; Swami, U.; Gupta, S.; Agarwal, N. Prospective trial of nivolumab (Nivo) plus radium-223 (RA) in metastatic castration-resistant prostate cancer (mCRPC) evaluating circulating tumor DNA (ctDNA) levels as a biomarker of response. J Clin Oncol. 2020, 38 (Suppl. 6), TPS267. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Fizazi, K.; Mateo, J.; Matsubara, N.; Shore, N.D.; et al. for the TALAPRO-3 investigational group. Talapro-3: a phase 3, double-blind, randomized study of enzalutamide (ENZA) plus talazoparib (TALA) versus placebo plus enza in patients with DDR gene mutated metastatic castration-sensitive prostate cancer (mCSPC). J Clin Oncol. 2022, 40 (Suppl. 6), TPS221. [Google Scholar] [CrossRef]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.; Loredo, E.; et al. for the PROpel Investigators. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence. 2022, 1, EVIDoa2200043. [Google Scholar] [CrossRef] [PubMed]
- Fettke, H.; Kwan, E.M.; Bukczynska, P.; Steen, J.A.; Docanto, M.; Ng, N.; et al. Independent prognostic impact of plasma NCOA2 alterations in metastatic castration-resistant prostate cancer. Prostate. 2021, 81, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Hussung, S.; Follo, M.; Klar, R.F.U.; Michalczyk, S.; Fritsch, K.; Nollmann, F.; et al. Development and clinical validation of discriminatory multitarget digital droplet PCR assays for the detection of hot spot KRAS and NRAS mutations in cell-free DNA. J Mol Diagn. 2020, 22, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Vincent, J.J.; Mortimer, S.; Vowles, J.V.; Ulrich, B.C.; Banks, K.C.; et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res. 2018, 24, 3539–3549. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.T.; Chin, Y.M.; Low, S.K. Circulating tumor DNA-based genomic profiling assays in adult solid tumors for precision oncology: recent advancements and future challenges. Cancers (Basel). 2022, 14, 3275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fettke, H.; Steen, J.A.; Kwan, E.M.; Bukczynska, P.; Keerthikumar, S.; Goode, D.; et al. Analytical validation of an error-corrected ultra-sensitive ctDNA next-generation sequencing assay. BioTechniques. 2020, 69, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ulz, P.; Belic, J.; Graf, R.; Auer, M.; Lafer, I.; Fischereder, K.; et al. Whole-genome plasma sequencing reveals focal amplifications as a driving force in metastatic prostate cancer. Nat Comm. 2016, 7, 12008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baca, S.C.; Garraway, L.A. The genomic landscape of prostate cancer. Front Endocrinol (Lausanne). 2012, 3, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kohli, M.; Tan, W.; Zheng, T.; Wang, A.; Montesinos, C.; Wong, C.; et al. Clinical and genomic insights into circulating tumor DNA-based alterations across the spectrum of metastatic hormone-sensitive and castrate-resistant prostate cancer. EBioMedicine. 2020, 54, 102728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Dessel, L.F.; van Riet, J.; Smits, M.; Zhu, Y.; Hamberg, P.; van der Heijden, M.S.; et al. The genomic landscape of metastatic castration-resistant prostate cancers reveals multiple distinct genotypes with potential clinical impact. Nat Commun. 2019, 10, 5251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crumbaker, M.; Chan, E.K.F.; Gong, T.; Corcoran, N.; Jaratlerdsiri, W.; Lyons, R.J.; et al. The impact of whole genome data on therapeutic decision-making in metastatic prostate cancer: a retrospective analysis. Cancers (Basel). 2020, 12, 1178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quigley, D.A.; Dang, H.X.; Zhao, S.G.; Lloyd, P.; Aggarwal, R.; Alumkal, J.J.; et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell. 2018, 174, 758–769e9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vnencak-Jones, C.; Berger, M.; Pao, W.; Types of Molecular Tumor Testing. My Cancer Genome. 2016. Available online: https://www.mycancergenome.org/content/molecular-medicine/types-of-molecular-tumor-testing/ (accessed on 25 June 2023).
- Beltran, H.; Eng, K.; Mosquera, J.M.; Sigaras, A.; Romanel, A.; Rennert, H.; et al. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol. 2015, 1, 466–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramesh, N.; Sei, E.; Tsai, P.C.; Bai, S.; Zhao, Y.; Troncoso, P.; et al. Decoding the evolutionary response to prostate cancer therapy by plasma genome sequencing. Genome Biol. 2020, 21, 162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, X.; Wang, J.; Sun, Y. Circulating tumor DNA as biomarkers for cancer detection. Genomics Proteomics Bioinformatics. 2017, 15, 59–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beltran, H.; Yelensky, R.; Frampton, G.M.; Park, K.; Downing, S.R.; MacDonald, T.Y.; et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. 2013, 63, 920–926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Newman, A.M.; Bratman, S.V.; To, J.; Wynne, J.F.; Eclov, N.C.; Modlin, L.A.; et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014, 20, 548–554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, M.; Huang, C.C.; Tan, W.; Kohli, M.; Wang, L. Multiplex digital PCR to detect amplifications of specific androgen receptor loci in cell-free DNA for prognosis of metastatic castration-resistant prostate cancer. Cancers (Basel). 2020, 12, 2139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Y.; Luk, A.; Young, F.P.; Lynch, D.; Chua, W.; Balakrishnar, B.; et al. Droplet digital PCR based androgen receptor variant 7 (AR-V7) detection from prostate cancer patient blood biopsies. Int J Mol Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jayaram, A.; Wingate, A.; Wetterskog, D.; Wheeler, G.; Sternberg, C.N.; Jones, R.; et al. Plasma tumor gene conversions after one cycle abiraterone acetate for metastatic castration-resistant prostate cancer: a biomarker analysis of a multicenter international trial. Ann Oncol. 2021, 32, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018, 8, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.; Dolling, D.; Sumanasuriya, S.; Christova, R.; Pope, L.; Carreira, S.; et al. Plasma Cell-free DNA concentration and outcomes from taxane therapy in metastatic castration-resistant prostate cancer from two phase III trials (FIRSTANA and PROSELICA). Eur Urol. 2018, 74, 283–291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wyatt, A.W.; Azad, A.A.; Volik, S.V.; Annala, M.; Beja, K.; McConeghy, B.; et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. 2016, 2, 1598–1606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torquato, S.; Pallavajjala, A.; Goldstein, A.; Toro, P.V.; Silberstein, J.L.; Lee, J.; et al. Genetic alterations detected in cell-free DNA are associated with enzalutamide and abiraterone resistance in castration-resistant prostate cancer. JCO Precis Oncol. 2019, 3, PO1800227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beltran, H.; Hruszkewycz, A.; Scher, H.I.; Hildesheim, J.; Isaacs, J.; Yu, E.Y.; et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin Cancer Res. 2019, 25, 6916–6924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bryce, A.H.; Chen, Y.H.; Liu, G.; Carducci, M.A.; Jarrard, D.M.; Garcia, J.A.; et al. Patterns of cancer progression of metastatic hormone-sensitive prostate cancer in the ECOG3805 CHAARTED trial. Eur Urol Oncol. 2020, 3, 717–724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumanasuriya, S.; Seed, G.; Parr, H.; Christova, R.; Pope, L.; Bertan, C.; et al. Elucidating prostate cancer behaviour during treatment via low-pass whole-genome sequencing of circulating tumour DNA. Eur Urol. 2021, 80, 243–253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goodall, J.; Assaf, Z.J.; Shi, Z.; Seed, G.; Zhang, L.; Lauffer, B.; et al. Circulating tumor DNA (ctDNA) dynamics associate with treatment response and radiological progression-free survival (rPFS): analyses from a randomized phase II trial in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020, 38 (Suppl. 15), 5508. [Google Scholar] [CrossRef]
- Goodall, J.; Mateo, J.; Yuan, W.; Mossop, H.; Porta, N.; Miranda, S.; et al. TOPARP-A investigators. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. 2017, 7, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Annala, M.; Fu, S.; Bacon, J.V.W.; Sipola, J.; Iqbal, N.; Ferrario, C.; et al. Cabazitaxel versus abiraterone or enzalutamide in poor prognosis metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase II trial. Ann Oncol. 2021, 32, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Bluemn, E.G.; Coleman, I.M.; Lucas, J.M.; Coleman, R.T.; Hernandez-Lopez, S.; Tharakan, R.; et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell. 2017; 32, 474–489.e6. [Google Scholar] [CrossRef] [PubMed]
- Fettke, H.; Kwan, E.M.; Docanto, M.M.; Bukczynska, P.; Ng, N.; Graham, L.K.; et al. Combined cell-free DNA and RNA profiling of the androgen receptor: clinical utility of a novel multianalyte liquid biopsy assay for metastatic prostate cancer. Eur Urol. 2020, 78, 173–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jayaram, A.; Wingate, A.; Wetterskog, D.; Conteduca, V.; Khalaf, D.; Sharabiani, M.T.A.; et al. Plasma androgen receptor copy number status at emergence of metastatic castration-resistant prostate cancer: a pooled multicohort analysis. JCO Precis Oncol. 2019; 3, PO.19.00123. [Google Scholar] [CrossRef] [PubMed]
- Tolmeijer, S.H.; Boerrigter, E.; Schalken, J.A.; Geerlings, M.J.; van Oort, I.M.; van Erp, N.P.; et al. A systematic review and meta-analysis on the predictive value of cell-free DNA-based androgen receptor copy number gain in patients with castration-resistant prostate cancer. JCO Precis Oncol. 2020, 4, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Coleman, I.; Morrissey, C.; Zhang, X.; True, L.D.; Gulati, R.; et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016, 22, 369–378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quigley, D.; Alumkal, J.J.; Wyatt, A.W.; Kothari, V.; Foye, A.; Lloyd, P.; et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 2017, 7, 999–1005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loehr, A.; Hussain, A.; Patnaik, A.; Bryce, A.H.; Castellano, D.; Font, A.; et al. Emergence of BRCA reversion mutations in patients with metastatic castration-resistant prostate cancer after treatment with rucaparib. Eur Urol. 2022, 83, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Tolmeijer, S.H.; Boerrigter, E.; Sumiyoshi, T.; Ng, S.; Kwan, E.M.; Annala, M.; et al. On-treatment plasma ctDNA fraction and treatment outcomes in metastatic castration-resistant prostate cancer. J Clin Oncol. 2022, 40 (Suppl. 16), 5051. [Google Scholar] [CrossRef]
- Conteduca, V.; Casadei, C.; Scarpi, E.; Brighi, N.; Schepisi, G.; Lolli, C.; et al. Baseline plasma tumor DNA (ctDNA) correlates with PSA kinetics in metastatic castration-resistant prostate cancer (mCRPC) treated with abiraterone or enzalutamide. Cancers (Basel). 2022, 14, 2219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conteduca, V.; Jayaram, A.; Romero-Laorden, N.; Wetterskog, D.; Salvi, S.; Gurioli, G.; et al. Plasma androgen receptor and docetaxel for metastatic castration-resistant prostate cancer. Eur Urol. 2019, 75, 368–373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; et al. TRITON2 investigators. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020, 38(3763) 3772. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Prostate Cancer V1.2023. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 25 June 2023).
- Mota, J.M.; Barnett, E.; Nauseef, J.T.; Nguyen, B.; Stopsack, K.H.; Wibmer, A.; et al. Platinum-based chemotherapy in metastatic prostate cancer with DNA repair gene alterations. JCO Precis Oncol. 2020, 4, 355–366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensen, K.; Konnick, E.Q.; Schweizer, M.T.; Sokolova, A.O.; Grivas, P.; Cheng, H.H.; et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference. JAMA Oncol. 2021, 7, 107–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.; et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021, 398, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Ferraldeschi, R.; Nava Rodrigues, D.; Riisnaes, R.; Miranda, S.; Figueiredo, I.; Rescigno, P.; et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol. 2015, 67, 795–802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rescigno, P.; Lorente, D.; Dolling, D.; Ferraldeschi, R.; Rodrigues, D.N.; Riisnaes, R.; et al. Docetaxel treatment in PTEN- and ERG-aberrant metastatic prostate cancers. Eur Urol Oncol. 2018, 1, 71–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwan, E.M.; Dai, C.; Fettke, H.; Hauser, C.; Docanto, M.M.; Bukczynska, P.; et al. Plasma cell–free DNA profiling of PTEN-PI3K-AKT pathway aberrations in metastatic castration-resistant prostate cancer. JCO Precis Oncol. 2021, PO.20.00424. [Google Scholar] [CrossRef] [PubMed]
- May, K.F., Jr.; Gulley, J.L.; Drake, C.G.; Dranoff, G.; Kantoff, P.W. Prostate cancer immunotherapy. Clin Cancer Res. 2011, 17, 5233–5238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell. 2020, 38, 489–499.e3. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.P.; Fisher, V.; Weberpals, J.; Gjoerup, O.; Tierno, M.B.; Huang, R.S.P.; et al. Comparative effectiveness of immune checkpoint inhibitors vs chemotherapy by tumor mutational burden in metastatic castration-resistant prostate cancer. JAMA Netw Open. 2022, 5, e225394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gongora, A.B.L.; Marshall, C.H.; Velho, P.I.; Lopes, C.D.H.; Marin, J.F.; Camargo, A.A.; et al. Extreme responses to a combination of DNA-damaging therapy and immunotherapy in CDK12-altered metastatic castration-resistant prostate cancer: a potential therapeutic vulnerability. Clin Genitourin Cancer. 2022, 20, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Cieślik, M.; Lonigro, R.J.; Vats, P.; Reimers, M.A.; Cao, X.; et al. PCF/SU2C International Prostate Cancer Dream Team. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018, 173, 1770–1782.e14. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willis, J.; Lefterova, M.I.; Artyomenko, A.; Kasi, P.M.; Nakamura, Y.; Mody, K.; et al. Validation of microsatellite instability detection using a comprehensive plasma-based genotyping panel. Clin Cancer Res. 2019, 25, 7035–7045. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.; Herberts, C.; Fu, S.; Yip, S.; Wong, A.; Wang, G.; et al. BRCA2, A.T.M.; and CDK12 defects differentially shape prostate tumor driver genomics and clinical aggression. Clin Cancer Res. 2021, 27, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Isaacsson Velho, P.; Fu, W.; Wang, H.; Mirkheshti, N.; Qazi, F.; Lima, F.A.S.; et al. Wnt-pathway activating mutations are associated with resistance to first-line abiraterone and enzalutamide in castration-resistant prostate cancer. Eur Urol. 2020, 77, 14–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Cheng, L.; Li, J.; Farah, E.; Atallah, N.M.; Pascuzzi, P.E.; et al. Inhibition of the Wnt/β-catenin pathway overcomes resistance to enzalutamide in castration-resistant prostate cancer. Cancer Res. 2018, 78, 3147–3162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016, 22, 298–305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conteduca, V.; Ku, S.Y.; Fernandez, L.; Dago-Rodriquez, A.; Lee, J.; Jendrisak, A.; et al. Circulating tumor cell heterogeneity in neuroendocrine prostate cancer by single cell copy number analysis. NPJ Precis Oncol. 2021, 5, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chi, K.N.; Mukherjee, S.; Saad, F.; Winquist, E.; Ong, M.; Kolinsky, M.P.; et al. Prostate cancer biomarker enrichment and treatment selection (PC-BETS) study: a Canadian cancer trials group phase II umbrella trial for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020, 38 (Suppl. 15), 5551. [Google Scholar] [CrossRef]
- De Laere, B.; Crippa, A.; Discacciati, A.; Larsson, B.; Oldenburg, J.; Mortezavi, A.; et al. ProBio Investigators. Clinical trial protocol for ProBio: an outcome-adaptive and randomised multiarm biomarker-driven study in patients with metastatic prostate cancer. Eur Urol Focus. 2022, 8, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Crippa, A.; De Laere, B.; Discacciati, A.; Larsson, B.; Connor, J.T.; Gabriel, E.E.; et al. The ProBio trial: molecular biomarkers for advancing personalized treatment decision in patients with metastatic castration-resistant prostate cancer. Trials. 2020, 21, 579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vandekerkhove, G.; Struss, W.J.; Annala, M.; Kallio, H.M.L.; Khalaf, D.; Warner, E.W.; et al. Circulating tumor DNA abundance and potential utility in de novo metastatic prostate cancer. Eur Urol. 2019, 75, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.A.; Gray, K.P.; Shaw, G.; MacConaill, L.E.; Evan, C.; Bernard, B.; et al. Compound genomic alterations of TP53, PTEN, and RB1 tumor suppressors in localized and metastatic prostate cancer. Eur Urol. 2019, 76, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Ha, G.; Gulati, R.; Brown, L.C.; McKay, R.R.; Dorff, T.; et al. CDK12-mutated prostate cancer: clinical outcomes with standard therapies and immune checkpoint blockade. JCO Precis Oncol. 2020, 4, 382–392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velez, M.G.; Kosiorek, H.E.; Egan, J.B.; McNatty, A.L.; Riaz, I.B.; Hwang, S.R.; et al. Differential impact of tumor suppressor gene (TP53, PTEN, RB1) alterations and treatment outcomes in metastatic, hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2022, 25, 479–483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, Y.; Wu, J.; Gu, W.; Wang, J.; Lin, G.; Qin, X.; et al. Prognostic value of germline DNA repair gene mutations in de novo metastatic and castration-sensitive prostate cancer. Oncologist. 2020, 25, e1042–e1050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Annala, M.; Struss, W.J.; Warner, E.W.; Beja, K.; Vandekerkhove, G.; Wong, A.; et al. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair-deficient prostate cancer. Eur Urol. 2017, 72, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Lucas, J.; Aguilar-Bonavides, C.; Thomas, S.; Gormley, M.; Chowdhury, S.; et al. Genomic aberrations associated with overall survival (OS) in metastatic castration-sensitive prostate cancer (mCSPC) treated with apalutamide (APA) or placebo (PBO) plus androgen deprivation therapy (ADT) in TITAN. J Clin Oncol. 2022, 40 (Suppl. 16), 5066. [Google Scholar] [CrossRef]
- Nizialek, E.; Lim, S.J.; Wang, H.; Isaacsson Velho, P.; Yegnasubramanian, S.; Antonarakis, E.S. Genomic profiles and clinical outcomes in primary versus secondary metastatic hormone-sensitive prostate cancer. Prostate. 2021, 81, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Swami, U.; Graf, R.P.; Nussenzveig, R.H.; Fisher, V.; Tukachinsky, H.; Schrock, A.B.; et al. SPOP mutations as a predictive biomarker for androgen receptor axis–targeted therapy in de novo metastatic castration-sensitive prostate cancer. Clin Cancer Res. 2022, 28, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Tolmeijer, S.H.; Boerrigter, E.; Sumiyoshi, T.; Kwan, E.M.; Ng, S.; Annala, M.; et al. Early on-treatment changes in circulating tumor DNA fraction and response to enzalutamide or abiraterone in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2023; CCR-22-2998. [Google Scholar] [CrossRef] [PubMed]
- Loehr, A.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; et al. Response to rucaparib in BRCA-mutant metastatic castration-resistant prostate cancer identified by genomic testing in the TRITON2 study. Clin Cancer Res. 2021, 27, 6677–6686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chi, K.N.; Barnicle, A.; Sibilla, C.; Lai, Z.; Corcoran, C.; Barrett, J.C.; et al. Detection of BRCA1, BRCA2, and ATM alterations in matched tumor tissue and circulating tumor DNA in patients with prostate cancer screened in PROfound. Clin Cancer Res. 2023, 29, 81–91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taavitsainen, S.; Annala, M.; Ledet, E.; Beja, K.; Miller, P.J.; Moses, M.; et al. Evaluation of commercial circulating tumor DNA test in metastatic prostate cancer. JCO Precis Oncol. 2019, 3, PO.19.00014. [Google Scholar] [CrossRef] [PubMed]
- Kwan, E.M.; Wyatt, A.W.; Chi, K.N. Towards clinical implementation of circulating tumor DNA in metastatic prostate cancer: opportunities for integration and pitfalls to interpretation. Front Oncol. 2022, 12, 1054497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2023 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Kostos, L.; Fettke, H.; Kwan, E.M.; Azad, A.A. Utility and Clinical Application of Circulating Tumor DNA (ctDNA) in Advanced Prostate Cancer. Soc. Int. Urol. J. 2023, 4, 273-286. https://doi.org/10.48083/RFSH8912
Kostos L, Fettke H, Kwan EM, Azad AA. Utility and Clinical Application of Circulating Tumor DNA (ctDNA) in Advanced Prostate Cancer. Société Internationale d’Urologie Journal. 2023; 4(4):273-286. https://doi.org/10.48083/RFSH8912
Chicago/Turabian StyleKostos, Louise, Heidi Fettke, Edmond M. Kwan, and Arun A. Azad. 2023. "Utility and Clinical Application of Circulating Tumor DNA (ctDNA) in Advanced Prostate Cancer" Société Internationale d’Urologie Journal 4, no. 4: 273-286. https://doi.org/10.48083/RFSH8912
APA StyleKostos, L., Fettke, H., Kwan, E. M., & Azad, A. A. (2023). Utility and Clinical Application of Circulating Tumor DNA (ctDNA) in Advanced Prostate Cancer. Société Internationale d’Urologie Journal, 4(4), 273-286. https://doi.org/10.48083/RFSH8912




