2022 WUOF/SIU International Consultation on Urological Diseases: Management of Locally Advanced Renal Cell Carcinoma
Abstract
:Introduction
Classification of VTT in Renal Cell Carcinoma
Pathophysiology of Tumor Venous Thrombosis and Clinical Manifestations of RCC with IVC Tumor Thrombus
Diagnostic Imaging and Staging
Neoadjuvant Therapy Before Radical Nephrectomy and Thrombectomy
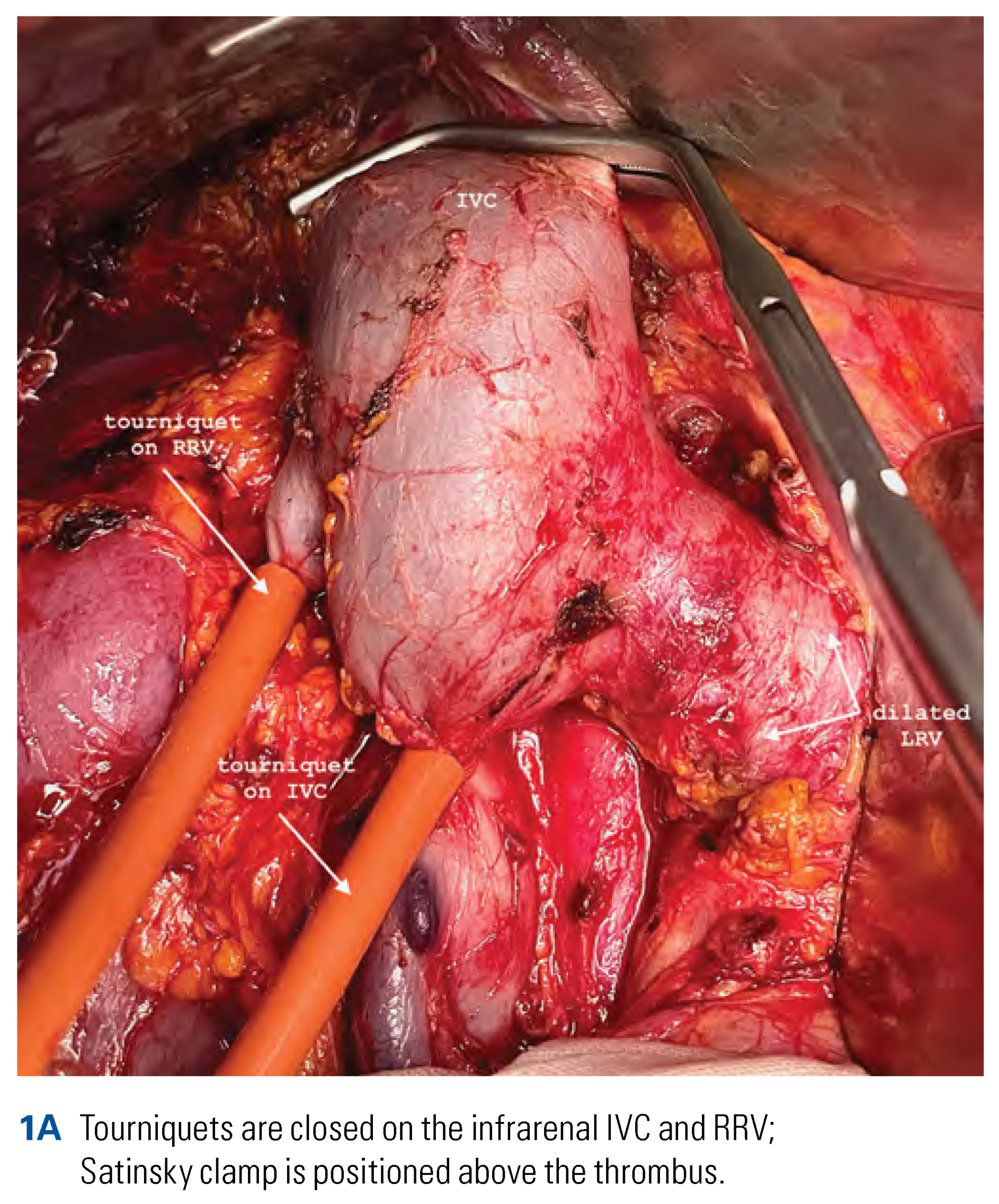
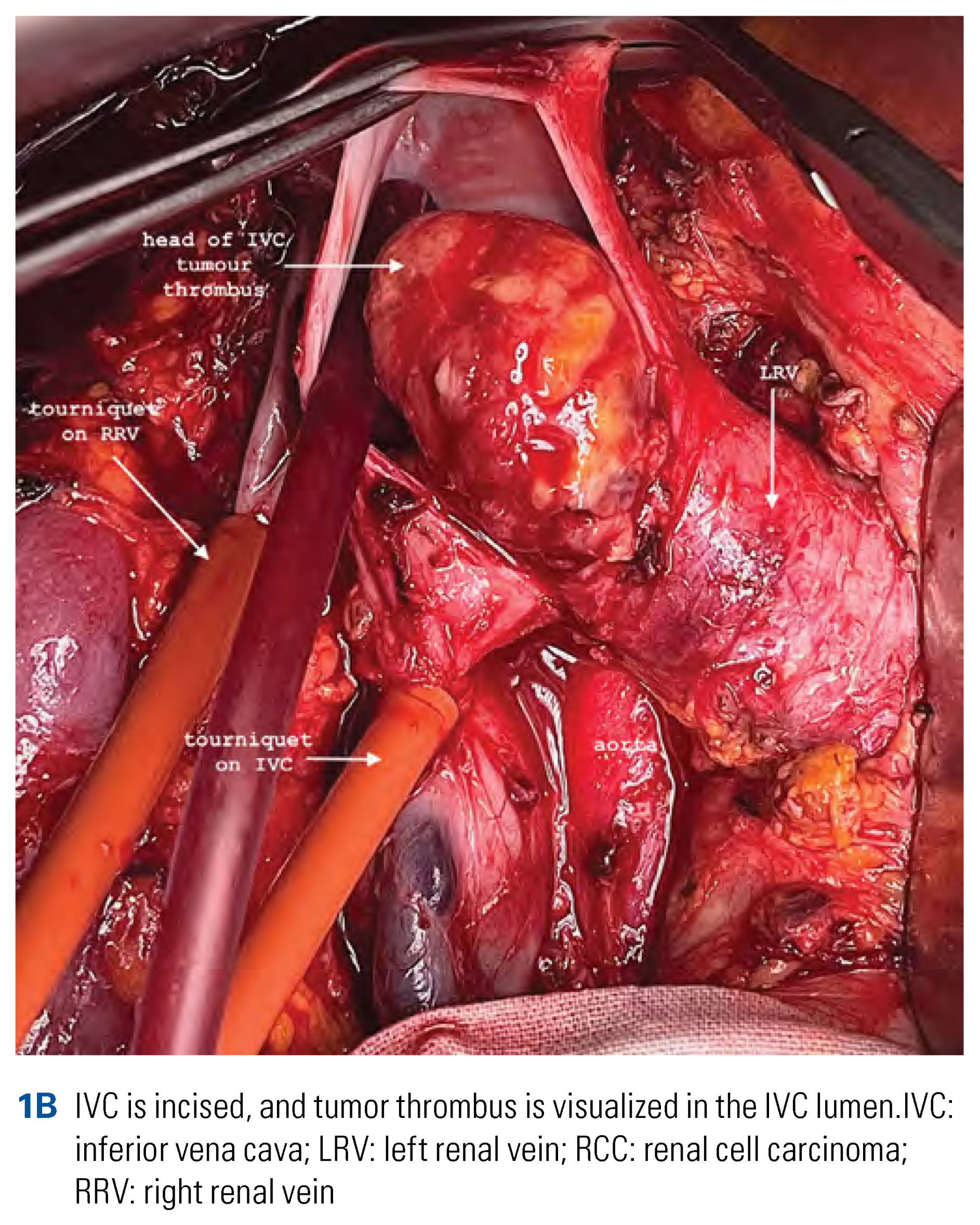
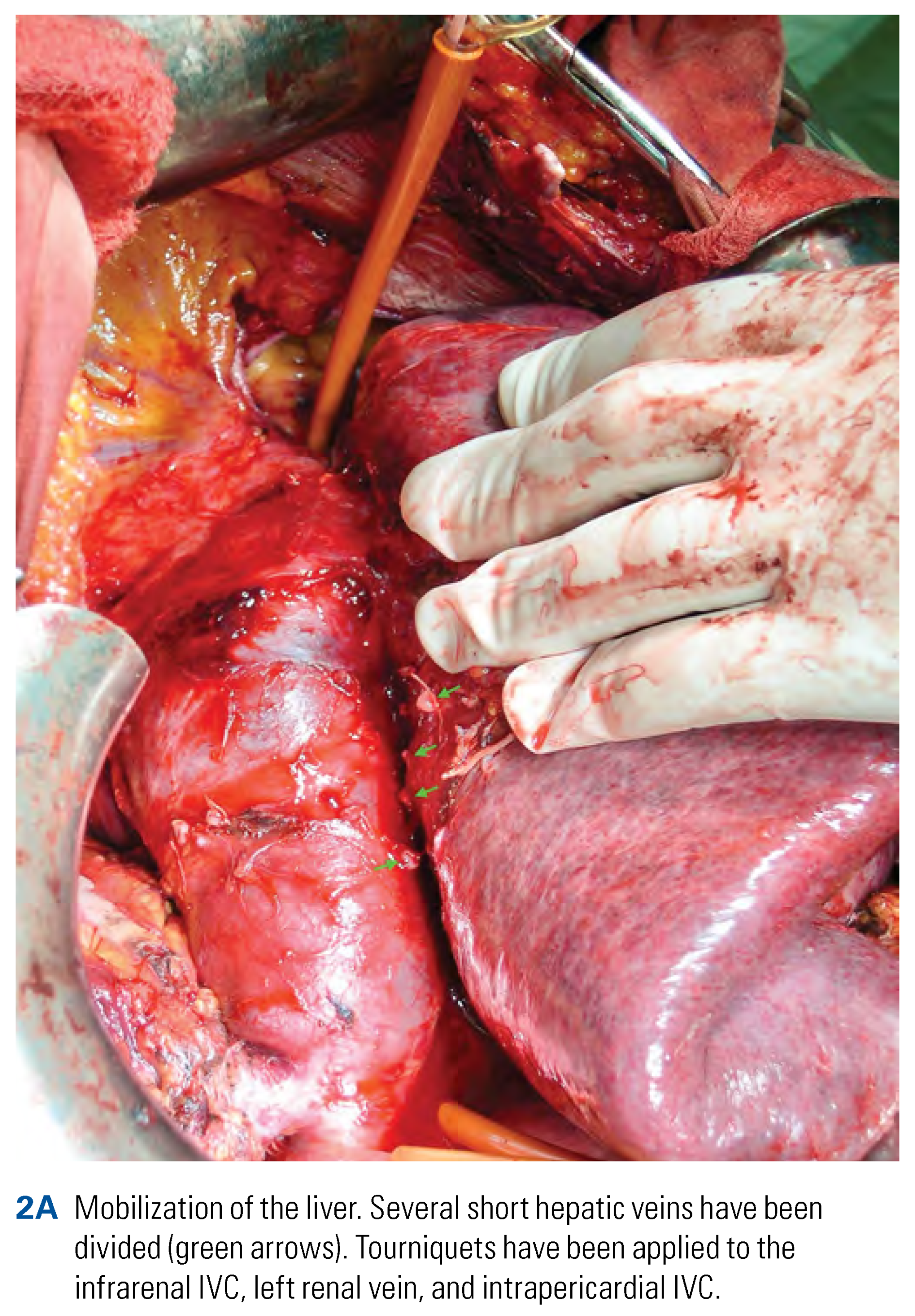
Surgical Technique of Radical Nephrectomy with IVC Thrombectomy
Thrombectomy in Patients with Level I-III Tumor Thrombus
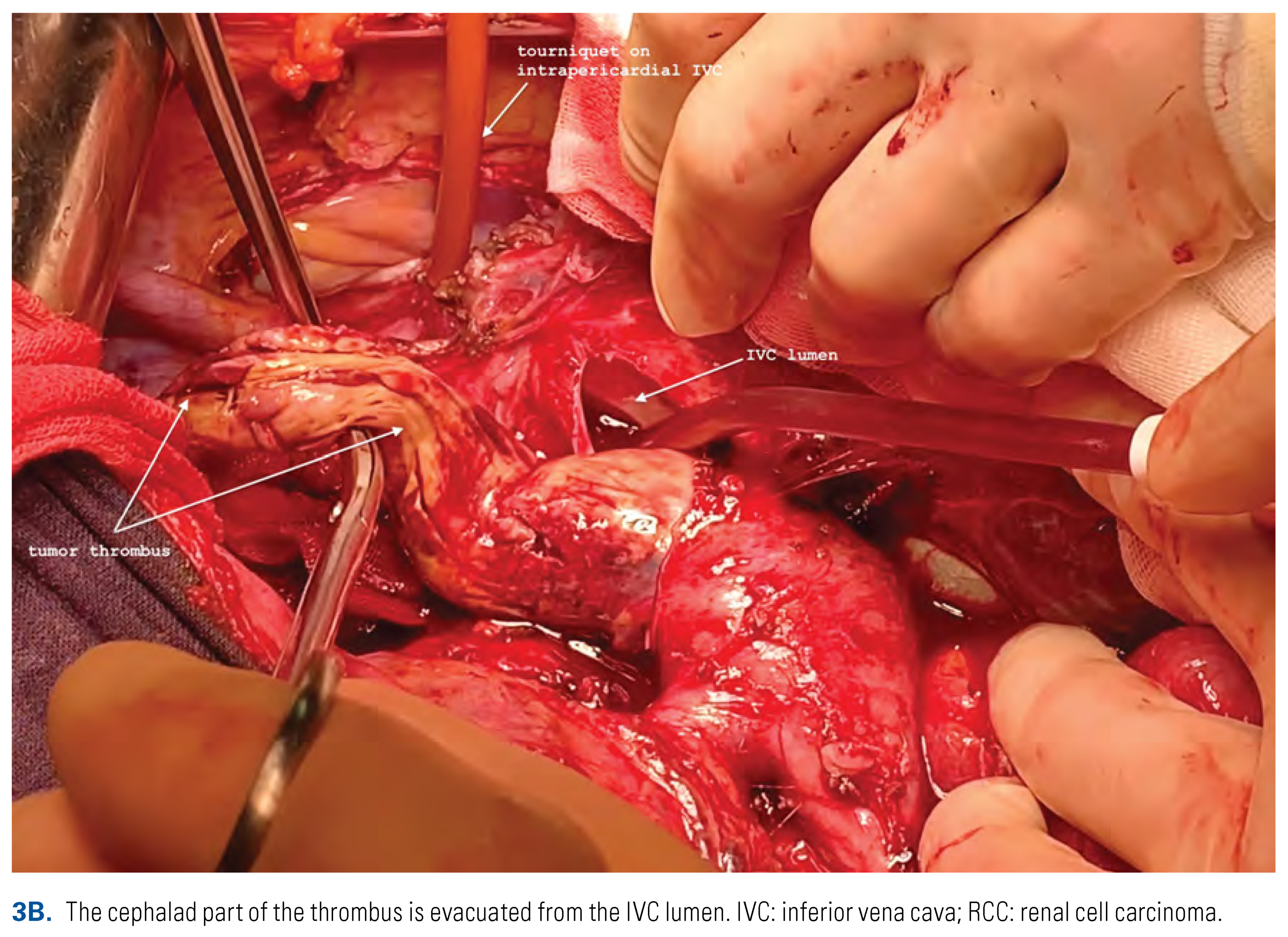
Thrombectomy in Patients with Supradiaphragmatic (Level IV) Tumor Thrombus
Thrombectomy in Patients with Tumor IVC Wall Invasion and/or Descending Bland Thrombosis
Minimally Invasive Radical Nephrectomy and IVC Thrombectomy
Minimally Invasive Level 0-I-II Thrombectomy
Minimally Invasive Level III Thrombectomy
Results of Surgical Management of RCC with VTT
Perioperative Complications
Oncologic Outcomes
Conclusion
Competing Interests
Abbreviations
| IO | Immune-oncology therapy |
| RCC | renal cell carcinoma |
| VTT | venous tumor thrombus |
References
- Ali, A.S.; Vasdev, N.; Shanmuganathan, S.; Paez, E.; Dark, J.H.; Manas, D.; et al. The surgical management and prognosis of renal cell cancer with IVC tumour thrombus: 15-years of experience using a multi-specialty approach at a single UK referral center. Urol. Oncol. 2013, 31, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.; Edge, S.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Marshall, F.F. Surgery of renal cell carcinoma with inferior vena caval involvement. Semin. Urol. 1989, 7, 186–190. [Google Scholar]
- Slaton, J.W.; Balbay, M.D.; Levy, D.A.; Pisters, L.L.; Nesbitt, J.C.; Swanson, D.A.; et al. Nephrectomy and vena caval thrombectomy in patients with metastatic renal cell carcinoma. Urology. 1997, 50, 673–677. [Google Scholar] [CrossRef]
- Kim, H.L.; Zisman, A.; Han, K.R.; Figlin, R.A.; Belldegrun, A.S. Prognostic significance of venous thrombus in renal cell carcinoma. Are renal vein and inferior vena cava involvement different? J. Urol. 2024, 171 Pt 1, 588–591. [Google Scholar] [CrossRef]
- Moinzadeh, A.; Libertino, J.A. Prognostic significance of tumour thrombus level in patients with renal cell carcinoma and VTTombus extension. Is all T3b the same? J. Urol. 2004, 171 Pt 1, 598–601. [Google Scholar] [CrossRef]
- Sosa, R.E.; Muecke, E.C.; Vaughan, E.D., Jr.; McCarron, J.P., Jr. Renal cell carcinoma extending into the inferior vena cava: The prognostic significance of the level of vena caval involvement. J. Urol. 1984, 132, 1097–1100. [Google Scholar] [CrossRef]
- Wagner, B.; Patard, J.J.; Méjean, A.; Bensalah, K.; Verhoest, G.; Zigeuner, V.; et al. Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur. Urol. 2009, 55, 452–459. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Blute, M.L. Surgery for vena caval tumour extension in renal cancer. Curr. Opin. Urol. 2009, 19, 473–477. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Sengupta, S.; Blute, M.L. Renal cell carcinoma: Vena caval involvement. BJU Int. 2007, 99 Pt B, 1239–1244. [Google Scholar] [CrossRef]
- Granberg, C.F.; Boorjian, S.A.; Schaff, H.V.; Orszulak, T.A.; Leibovich, B.C.; Lohse, C.M.; et al. Surgical management, complications, and outcome of radical nephrectomy with inferior vena cava tumour thrombectomy facilitated by vascular bypass. Urology 2008, 72, 148–152. [Google Scholar] [CrossRef]
- Blute, M.L.; Boorjian, S.A.; Leibovich, B.C.; Lohse, C.M.; Frank, I.; Karnes, R.J. Results of inferior vena caval interruption by greenfield filter, ligation or resection during radical nephrectomy and tumour thrombectomy. J. Urol. 2007, 178, 440–445; discussion 444. [Google Scholar] [CrossRef]
- Chiappini, B.; Savini, C.; Marinelli, G.; Martin Suarez, S.; Di Eusanio, M.; Fiorani, V.; et al. Cavoatrial tumour thrombus: Single-stage surgical approach with profound hypothermia and circulatory arrest, including a review of the literature. J. Thorac. Cardiovasc. Surg. 2002, 124, 684–688. [Google Scholar] [CrossRef]
- Neves, R.J.; Zincke, H. Surgical treatment of renal cancer with vena cava extension. BJU Int. 1987, 59, 390–395. [Google Scholar] [CrossRef]
- Gohji, K.; Yamashita, C.; Ueno, K.; Shimogaki, H.; Kamidono, S. Preoperative computerized tomography detection of extensive invasion of the inferior vena cava by renal cell carcinoma: Possible indication for resection with partial cardiopulmonary bypass and patch grafting. J. Urol. 1994, 152 Pt 1, 1993–1996; discussion 1997. [Google Scholar] [CrossRef]
- Psutka, S.P.; Boorjian, S.A.; Thompson, R.H.; Schmit, G.D.; Schmitz, J.J.; Bowere, T.C.; et al. Clinical and radiographic predictors of the need for inferior vena cava resection during nephrectomy for patients with renal cell carcinoma and caval tumour thrombus. BJU Int. 2015, 116, 388–396. [Google Scholar] [CrossRef]
- Hutchinson, R.; Rew, C.; Chen, G.; Woldu, S.; Krabbe, L.-M.; Mieissner, M.; et al. The adverse survival implications of bland thrombus in renal cell carcinoma with venous tumor thrombus. Urology 2018, 115, 119–124. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Huang, Q.; Panic, A.; Shen, D.; Jia, W.; et al. Prognostic role of bland thrombus in patients treated with resection of renal cell carcinoma with inferior vena cava tumour thrombus. Urol. Oncol. 2021, 39, 302–e301. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Hong, P.; Zhu, G.; Tang, S.; Zhao, X.; et al. A predictive model for tumour invasion of the inferior vena cava wall using multimodal imaging in patients with renal cell carcinoma and inferior vena cava tumour thrombus. Biomed. Res. Int. 2020, 2020, 9530618. [Google Scholar]
- Weiss, V.L.; Braun, M.; Perner, S.; Harz, A.; Vorreuther, R.; Kristiansen, G.; et al. Prognostic significance of VTT consistency in patients with renal cell carcinoma (RCC). BJU Int. 2014, 113, 209–217. [Google Scholar] [CrossRef]
- Bertini, R.; Roscigno, M.; Freschi, M.; Strada, E.; Angiolilli, D.; Petralia, G.; et al. Impact of VTT consistency (solid vs friable) on cancer-specific survival in patients with renal cell carcinoma. Eur. Urol. 2011, 60, 358–365. [Google Scholar] [CrossRef]
- Psutka, S.P.; Leibovich, B.C. Management of inferior vena cava tumour thrombus in locally advanced renal cell carcinoma. Ther. Adv. Urol. 2015, 7, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, G.; Soloway, M. Renal cell carcinoma invading the hepatic veins. Cancer 2001, 92, 1836–1842. [Google Scholar] [CrossRef]
- Ciancio, G.; Vaidya, A.; Savoie, M.; Soloway, M. Management of renal cell carcinoma with level III thrombus in the inferior vena cava. J. Urol. 2002, 168 Pt 1, 1374–1377. [Google Scholar] [CrossRef]
- Kume, H.; Kameyama, S.; Kasuya, Y.; Tajima, A.; Kawabe, K. Surgical treatment of renal cell carcinoma associated with Budd-Chiari syndrome: Report of four cases and review of the literature. Eur. J. Surg. Oncol. 1999, 25, 71–75. [Google Scholar] [CrossRef]
- Lessard, L.; Bach, D.; Wall, W.; Luke, P.P. Intrahepatic extension of renal cell carcinoma tumour thrombus causing Budd-Chiari syndrome. Can. Urol. Assoc. J. 2011, 5, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.J.; Thompson, R.H.; Margulis, V.; Heckman, J.E.; Merril, M.M.; Darwish, O.M.; et al. Perioperative outcomes following surgical resection of renal cell carcinoma with inferior vena cava thrombus extending above the hepatic veins: A contemporary multicenter experience. Eur. Urol. 2014, 66, 584–592. [Google Scholar] [CrossRef]
- Blute, M.L.; Leibovich, B.C.; Lohse, C.M.; Cheville, J.C.; Zincke, H. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and VTT. BJU Int. 2004, 94, 33–41. [Google Scholar] [CrossRef]
- Parekh, D.J.; Cookson, M.S.; Chapman, W.; Harrell FJr Wells, N.; Chang, S.S.; et al. Renal cell carcinoma with renal vein and inferior vena caval involvement: Clinicopathological features, surgical techniques and outcomes. J. Urol. 2005, 173, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Paik, E.; Rezaei, A.; Vu, D.N. Where there is blood, there is a way: Unusual collateral vessels in superior and inferior vena cava obstruction. Radiographics 2010, 30, 67–78. [Google Scholar] [CrossRef]
- Wotkowicz, C.; Wszolek, M.F.; Libertino, J.A. Resection of renal tumours invading the vena cava. Urol. Clin. N. Am. 2008, 35, 657–671. [Google Scholar] [CrossRef]
- Lawindy, S.M.; Kurian, T.; Kim, T.; Mangar, D.; Armstrong, P.A.; Alsina, A.E.; et al. Important surgical considerations in the management of renal cell carcinoma (RCC) with inferior vena cava (IVC) tumour thrombus. BJU Int. 2012, 110, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.Y.; Van Veldhuizen, P.; Muehlebach, G.; Johnson, P.; Williamson, T.; Holzbeierlein, J.M. The perioperative management of an inferior vena caval tumour thrombus in patients with renal cell carcinoma. Urol. Oncol. 2013, 31, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.F.; Song, Y.; Na, Y.Q. Value of abdominal ultrasound scan, CT and MRI for diagnosing inferior vena cava tumour thrombus in renal cell carcinoma. Chin. Med. J. 2009, 122, 2299–2302. [Google Scholar] [PubMed]
- Calderone, C.E.; Tuck, B.C.; Gray, S.H.; Porter, K.K.; Rais-Bahrami, S. The role of transesophageal echocardiography in the management of renal cell carcinoma with VTTombus. Echocardiography 2018, 35, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Cost, N.G.; Delacroix SEJr Sleeper, J.P.; Smith, P.J.; Youssef, R.F.; Chapin, B.F.; et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumour thrombus. Eur. Urol. 2011, 59, 912–918. [Google Scholar] [CrossRef]
- Bigot, P.; Fardoun, T.; Bernhard, J.C.; Xylinas, E.; Berger, J.; Rouprêt, M.; et al. Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: Is it useful? World J. Urol. 2014, 32, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Gu, L.; Wang, L.; Huang, Q.; Wang, B.; Guo, G.; et al. Role of presurgical targeted molecular therapy in renal cell carcinoma with an inferior vena cava tumour thrombus. OncoTargets Ther. 2018, 11, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Kondo, T.; Takagi, T.; Iizuka, J.; Nagashima, Y.; Tanabe, K. Limited benefit of targeted molecular therapy for inferior vena cava thrombus associated with renal cell carcinoma. Int. J. Clin. Oncol. 2017, 22, 767–773. [Google Scholar] [CrossRef]
- Ujike, T.; Uemura, M.; Kawashima, A.; Nagahara, A.; Fujita, K.; Miyagawa, Y.; et al. Clinical and histopathological effects of presurgical treatment with sunitinib for renal cell carcinoma with inferior vena cava tumour thrombus at a single institution. Anticancer. Drugs 2016, 27, 1038–1043. [Google Scholar] [CrossRef]
- Horn, T.; Thalgott, M.K.; Maurer, T.; Hauner, K.; Schulz, S.; Fingerle, A.; et al. Presurgical treatment with sunitinib for renal cell carcinoma with a level III/IV vena cava tumour thrombus. Anticancer. Res. 2012, 32, 1729–1735. [Google Scholar]
- Tanaka, Y.; Hatakeyama, S.; Hosogoe, S.; Tanaka, T.; Hamano, I.; Kusaka, A.; et al. Presurgical axitinib therapy increases fibrotic reactions within tumour thrombus in renal cell carcinoma with thrombus extending to the inferior vena cava. Int. J. Clin. Oncol. 2018, 23, 134–141. [Google Scholar] [CrossRef]
- Terakawa, T.; Hussein, A.A.; Bando, Y.; Guru, K.A.; Furukawa, J.; Shigemura, K.; et al. Presurgical pazopanib for renal cell carcinoma with inferior vena caval thrombus: A single-institution study. Anticancer. Drugs 2018, 29, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.D.; Welsh, S.J.; Ursprung, S.; Gallagher, F.A.; Mendichovszky, I.; Riddick, A.; Eisen, T.; et al. NAXIVA: A phase II neoadjuvant study of axitinib for reducing extent of VTTombus in clear cell renal cell cancer (RCC) with venous invasion. J. Clin. Oncol. 2021, 39 (Suppl. 6), 275. [Google Scholar] [CrossRef]
- Skinner, D.G.; Pritchett, T.R.; Lieskovsky, G.; Boyd, S.D.; Stiles, Q.R. Vena caval involvement by renal cell carcinoma. Surgical resection provides meaningful long-term survival. Ann. Surg. 1989, 210, 387–392; discussion 392–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciancio, G.; Livingstone, A.S.; Soloway, M. Surgical management of renal cell carcinoma with tumour thrombus in the renal and inferior vena cava: The University of Miami experience in using liver transplantation techniques. Eur. Urol. 2007, 51, 988–994; discussion 994–995. [Google Scholar] [CrossRef] [PubMed]
- Davydov, M.I.; Matveev, V.B. Surgical management of renal cell carcinoma with tumour thrombosis of renal vein and inferior vena cava. Cancer Urol. 2005, 1, 8–15. [Google Scholar]
- Davydov, M.I.; Matveev, V.B.; Volkova, M.I.; et al. Predictors of the immediate results of thrombectomy in kidney cancer patients with venous tumour thrombosis. Cancer Urol. 2014, 10, 31–39. [Google Scholar]
- Daylami, R.; Amiri, A.; Goldsmith, B.; Troppmann, C.; Schneider, P.D.; Khatri, V.P. Inferior vena cava leiomyosarcoma: Is reconstruction necessary after resection? J. Am. Coll. Surg. 2010, 210, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ayyathurai, R.; Garcia-Roig, M.; Gorin, M.A.; González, J.; Manoharan, M.; Kava, B.R.; et al. Bland thrombus association with tumour thrombus in renal cell carcinoma: Analysis of surgical significance and role of inferior vena caval interruption. BJU Int. 2012, 110 Pt B, E449–E455. [Google Scholar] [CrossRef] [PubMed]
- Bratslavsky, G.; Cheng, J.S. Robotic-assisted radical nephrectomy with retrohepatic vena caval tumour thrombectomy (Level III) combined with extended retroperitoneal lymph node dissection. Urology 2015, 86, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; de Castro Abreu, A.L.; Gill, I.S. Robotic inferior vena cava thrombus surgery: Novel strategies. Curr. Opin. Urol. 2014, 24, 140–147. [Google Scholar] [CrossRef]
- Varkarakis, I.M.; Bhayani, S.B.; Allaf, M.E.; Inagaki, T.; Gonzalgo, M.L.; Jarrett, T.W. Laparoscopic-assisted nephrectomy with inferior vena cava tumor thrombectomy: Preliminary results. Urology 2004, 64, 925–929. [Google Scholar] [CrossRef]
- Kovac, J.R.; Luke, P.P. Hand-assisted laparoscopic radical nephrectomy in the treatment of a renal cell carcinoma with a level II vena cava thrombus. Int. Braz. J. Urol. 2010, 36, 327–331. [Google Scholar] [CrossRef]
- Shao, P.; Li, J.; Qin, C.; Lv, Q.; Ju, X.; Li, P.; et al. Laparoscopic radical nephrectomy and inferior vena cava thrombectomy in the treatment of renal cell carcinoma. Eur. Urol. 2015, 68, 115–122. [Google Scholar] [CrossRef]
- Ramirez, D.; Maurice, M.J.; Cohen, B.; Krishnamurthi, V.; Haber, G.-P. Robotic level III IVC tumor thrombectomy: Duplicating the open approach. Urology 2016, 90, 204–247. [Google Scholar] [CrossRef]
- Gill, I.S.; Metcalfe, C.; Abreu, A.; Duddalwar, V.; Chopra, S.; Cunningham, M.; et al. Robotic level III inferior vena cava tumor thrombectomy: Initial series. J. Urol. 2015, 194, 929–938. [Google Scholar] [CrossRef]
- Savage, S.J.; Gill, I.S. Laparoscopic radical nephrectomy for renal cell carcinoma in a patient with level I renal vein tumor thrombus. J. Urol. 2000, 163, 1243–1244. [Google Scholar] [CrossRef]
- Fergany, A.F.; Gill, I.S.; Schweizer, D.K.; Kaouk, J.H.; ElFettouh, H.A.; Cherullo, E.E.; et al. Laparoscopic radical nephrectomy with level II vena caval thrombectomy: Survival porcine study. J. Urol. 2002, 168, 2629–2631. [Google Scholar] [CrossRef]
- Abaza, R.; Shabsigh, A.; Castle, E.; Allaf, M.; Hu, J.C.; Rogers, C.; Memon, M.; et al. Multi-institutional experience with robotic nephrectomy with inferior vena cava tumor thrombectomy. J. Urol. 2016, 195 Pt 1, 865–871. [Google Scholar] [CrossRef]
- Abaza, R. Initial series of robotic radical nephrectomy with vena caval tumor thrombectomy. Eur. Urol. 2011, 59, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, H.; Ma, X.; Zhang, X.; Gu, L.; Li, X.; et al. Robot-assisted laparoscopic inferior vena cava thrombectomy: Different sides require different techniques. Eur. Urol. 2016, 69, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.R.; Muntener, M.; Bagga, H.S.; Brito, F.A.R.; Sulman, A.; Jarrett, T.W. Pure laparoscopic radical nephrectomy with level II vena caval thrombectomy. Urology 2006, 68, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.W.; Gorin, M.A.; Jayram, G.; Pierorazio, P.M.; Allaf, M.E. Robot-assisted radical nephrectomy with inferior vena cava tumor thrombectomy: Technique and initial outcomes. Can. J. Urol. 2015, 22, 7666–7670. [Google Scholar]
- Gupta, N.P.; Ansari, M.S.; Khaitan, A.; Sivaramakrishna, M.S.; Hemal, A.K.; Dogra, P.N.; et al. Impact of imaging and thrombus level in management of renal cell carcinoma extending to veins. Urol. Int. 2004, 72, 129–134. [Google Scholar] [CrossRef]
- Scott, K.; Manwaring, J.; Amankwah, K.; Bratslavsky, G. Robotic assisted caval replacement for recurrent renal cell carcinoma invading the wall of the inferior vena cava. Urology 2022, 161, 131–134. [Google Scholar] [CrossRef]
- Desai, M.M.; Gill, I.S.; Ramani, A.P.; Matin, S.F.; Kaouk, J.H.; Campero, J.M.; et al. Laparoscopic radical nephrectomy for cancer with level I renal vein involvement. J. Urol. 2003, 169, 487–491. [Google Scholar] [CrossRef]
- Ball, M.W.; Gorin, M.A.; Jayram, G.; Pieroazio, P.M.; Allaf, M. Robot-assisted radical nephrectomy with inferior vena cava tumor thrombectomy: Technique and initial outcomes. Can. J. Urol. 2015, 22, 7666–7670. [Google Scholar] [PubMed]
- Lee, J.Y.; Mucksavage, P. Robotic radical nephrectomy with vena caval tumor thrombectomy: Experience of novice robotic surgeons. Korean J. Urol. 2012, 53, 879–882. [Google Scholar] [CrossRef]
- Riches, E.W.; Griffiths, I.H.; Thackray, A.C. New growths of the kidney and ureter. Br. J. Urol. 1951, 23, 297–356. [Google Scholar] [CrossRef]
- Skinner, D.G.; Pfister, R.F.; Colvin, R. Extension of renal cell carcinoma into the vena cava: The rationale for aggressive surgical management. J. Urol. 1972, 107, 711–716. [Google Scholar] [CrossRef]
- Kirkali, Z.; Van Poppel, H. A critical analysis of surgery for kidney cancer with vena cava invasion. Eur. Urol. 2007, 52, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.G.; Tilki, D.; Dall’Era, M.A.; Durbin-Johnson, B.; Carballido, J.A.; Chandrasekar, T.; et al. Cardiopulmonary bypass has no significant impact on survival in patients undergoing nephrectomy and level III-IV inferior vena cava thrombectomy: Multi-institutional analysis. J. Urol. 2015, 194, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Shuch, B.; Crispen, P.L.; Leibovich, B.C.; LaRochelle, J.C.; Pouliot, F.; Pantuck, A.J.; et al. Cardiopulmonary bypass and renal cell carcinoma with level IV tumour thrombus: Can deep hypothermic circulatory arrest limit perioperative mortality? BJU Int. 2011, 107, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, G.; Gonzalez, J.; Shirodkar, S.P.; Angulo, J.C.; Soloway, M.S. Liver transplantation techniques for the surgical management of renal cell carcinoma with tumor thrombus in the inferior vena cava: Stepby-step description. Eur. Urol. 2011, 59, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.G.; Raheem, O.A.; Elmusharaf, E.; Madhavan, P.; Tolan, M.; Lynch, T.H. Renal cell carcinoma with IVC and atrial thrombus: A single centre’s 10 year surgical experience. Surgeon 2013, 11, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.C.J.; Salika, T.; Maw, J.; Lyratzopoulos, G.; Gnanapragasam, V.J.; Armitage, J.N. Influence of hospital volume on nephrectomy mortality and complications: A systematic review and meta-analysis stratified by surgical type. BMJ Open 2017, 7, e016833. [Google Scholar] [CrossRef]
- Yap, S.A.; Horovitz, D.; Alibhai, S.M.; Abouassaly, R.; Timilshina, N.; Finelli, A. Predictors of early mortality after radical nephrectomy with renal vein or inferior vena cava thrombectomy—A population-based study. BJU Int. 2012, 110, 1283–1288. [Google Scholar] [CrossRef]
- Toren, P.; Abouassaly, R.; Timilshina, N.; Kulkarni, G.; Alibhai, S.; Finelli, A. Results of a national population-based study of outcomes of surgery for renal tumors associated with inferior vena cava thrombus. Urology 2013, 82, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.J.; Spiess, P.E.; Margulis, V.; Master, V.A.; Mann, M.; Zargar-Shostari, K.; et al. Cytoreductive nephrectomy for renal cell carcinoma with venous tumor thrombus. J. Urol. 2017, 198, 281–288. [Google Scholar] [CrossRef]
- Martinez-Salamanca, J.I.; Linares, E.; González, J.; Bertini, R.; Carballido, J.A.; Chromecki, T.; et al. Lessons learned from the International Renal Cell Carcinoma-Venous Thrombus Consortium (IRCC-VTC). Curr. Urol. Rep. 2014, 15, 404. [Google Scholar] [CrossRef]
- Shuch, B.; Larochelle, J.C.; Onyia, T.; Vallera, C.; Margulis, D.; Pantuck, A.J.; et al. Intraoperative thrombus embolization during nephrectomy and tumor thrombectomy: Critical analysis of the University of CaliforniaLos Angeles experience. J. Urol. 2009, 181, 492–498; discussion 498–499. [Google Scholar] [CrossRef]
- Abel, E.J.; Thompson, R.H.; Margulis, V.; et al. Perioperative outcomes following surgical resection of renal cell carcinoma with inferior vena cava thrombus extending above the hepatic veins: A contemporary multicenter experience. Eur. Urol 2014, 66, 584–592. [Google Scholar] [CrossRef]
- Reese, A.C.; Whitson, J.M.; Meng, M.V. Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urol. Oncol. 2013, 31, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Whitson, J.M.; Reese, A.C.; Meng, M.V. Population based analysis of survival in patients with renal cell carcinoma and venous tumor thrombus. Urol. Oncol. 2013, 31, 259–263. [Google Scholar] [CrossRef]
- Haddad, A.Q.; Wood, C.G.; Abel, E.J.; Krabbe, L.-M.; Darwish, O.M.; Thompson, R.H.; et al. Oncologic outcomes following surgical resection of renal cell carcinoma with inferior vena caval thrombus extending above the hepatic veins: A contemporary multicenter cohort. J. Urol. 2014, 192, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Hirono, M.; Kobayashi, M.; Tsushima, T.; Obara, W.; Shinohara, N.; Ito, K.; et al. Members of the Japanese Society of Renal Cancer. Impacts of clinicopathologic and operative factors on short-term and longterm survival in renal cell carcinoma with venous tumor thrombus extension: A multi-institutional retrospective study in Japan. BMC Cancer 2013, 13, 447. [Google Scholar] [CrossRef]
- Pouliot, F.; Shuch, B.; Larochelle, J.C.; Panticuk, A.; Belledegrun, A.S. Contemporary management of renal tumors with venous tumor thrombus. J. Urol. 2010, 184, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Salamanca, J.I.; Huang, W.C.; Millan, I.; Bertini, R.; Bianco, F.J.; Carballido, J.A.; et al. International Renal Cell Carcinoma-Venous Thrombus Consortium. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol. 2011, 59, 120–127. [Google Scholar] [CrossRef]
- Kaushik, D.; Linder, B.J.; Thompson, R.H.; Eisenberg, M.S.; Lohse, C.M.; Cheville, J.C.; et al. The impact of histology on clinicopathologic outcomes for patients with renal cell carcinoma and venous tumor thrombus: A matched cohort analysis. Urology 2013, 82, 136–141. [Google Scholar] [CrossRef]
- Tilki, D.; Hu, B.; Nguyen, H.G.; Dall’Era, M.A.; Bertini, R.; Carballido, J.A.; et al. Impact of synchronous metastasis distribution on cancer specific survival in renal cell carcinoma after radical nephrectomy with tumor thrombectomy. J. Urol. 2015, 193, 436–442. [Google Scholar] [CrossRef]



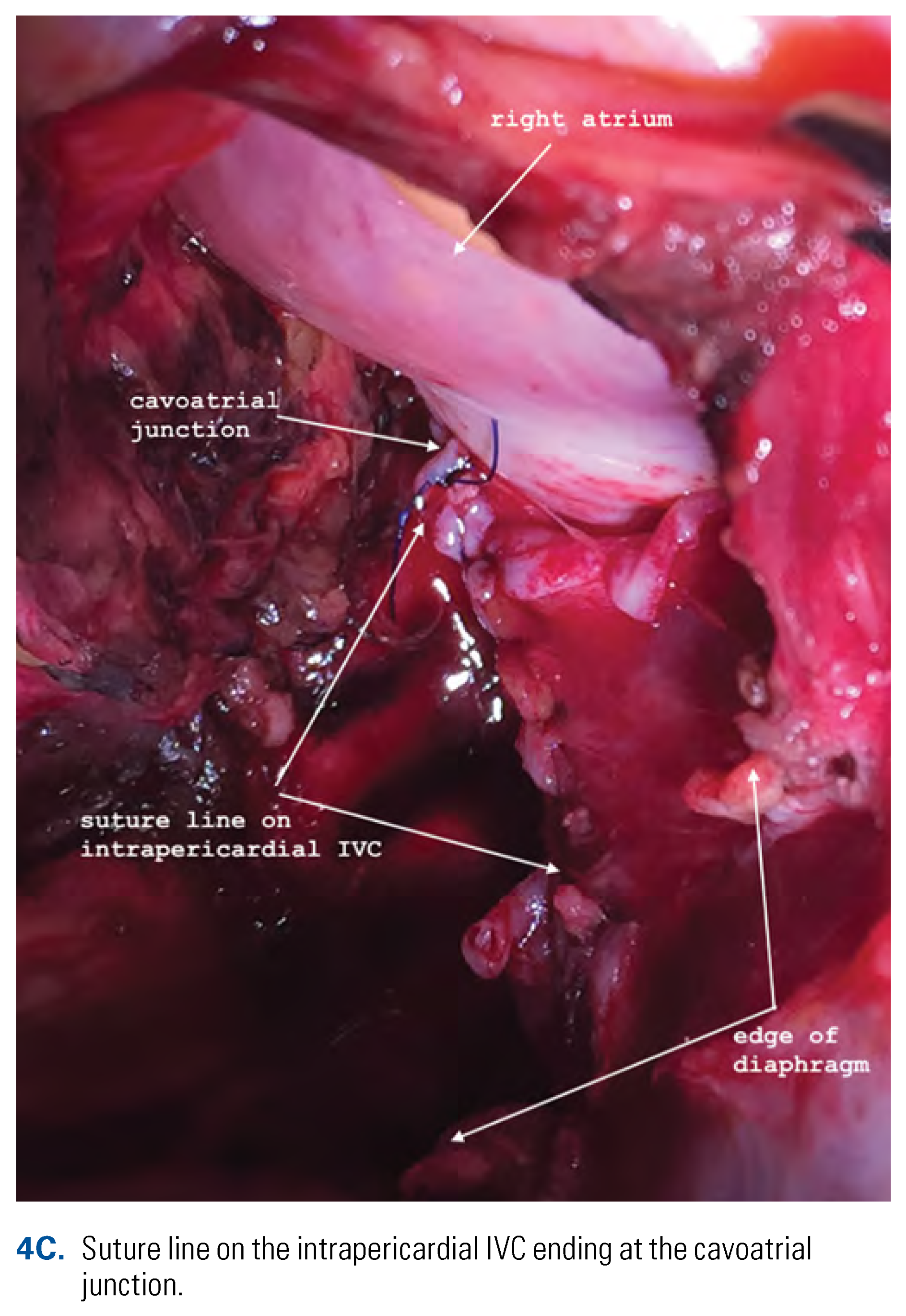
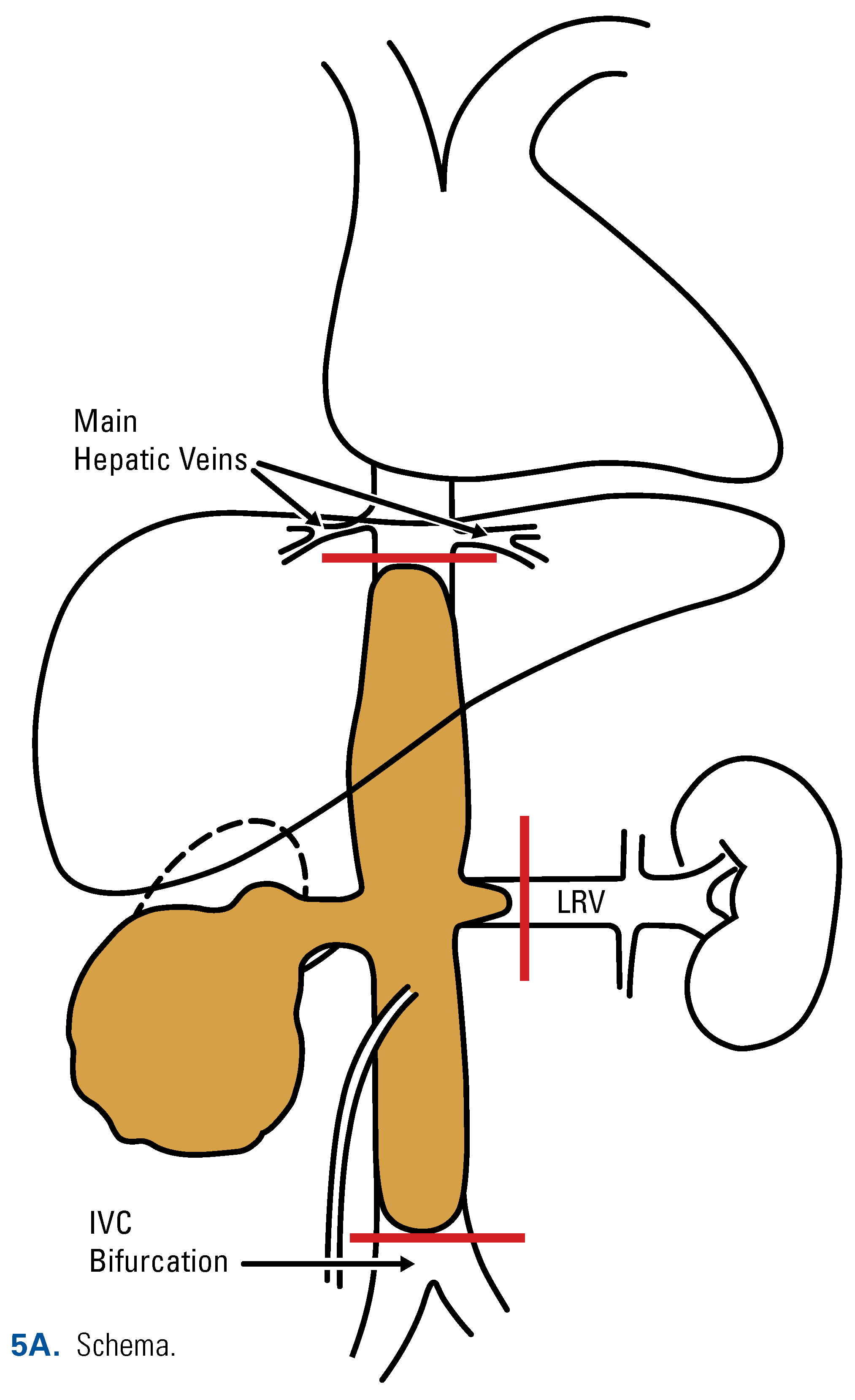
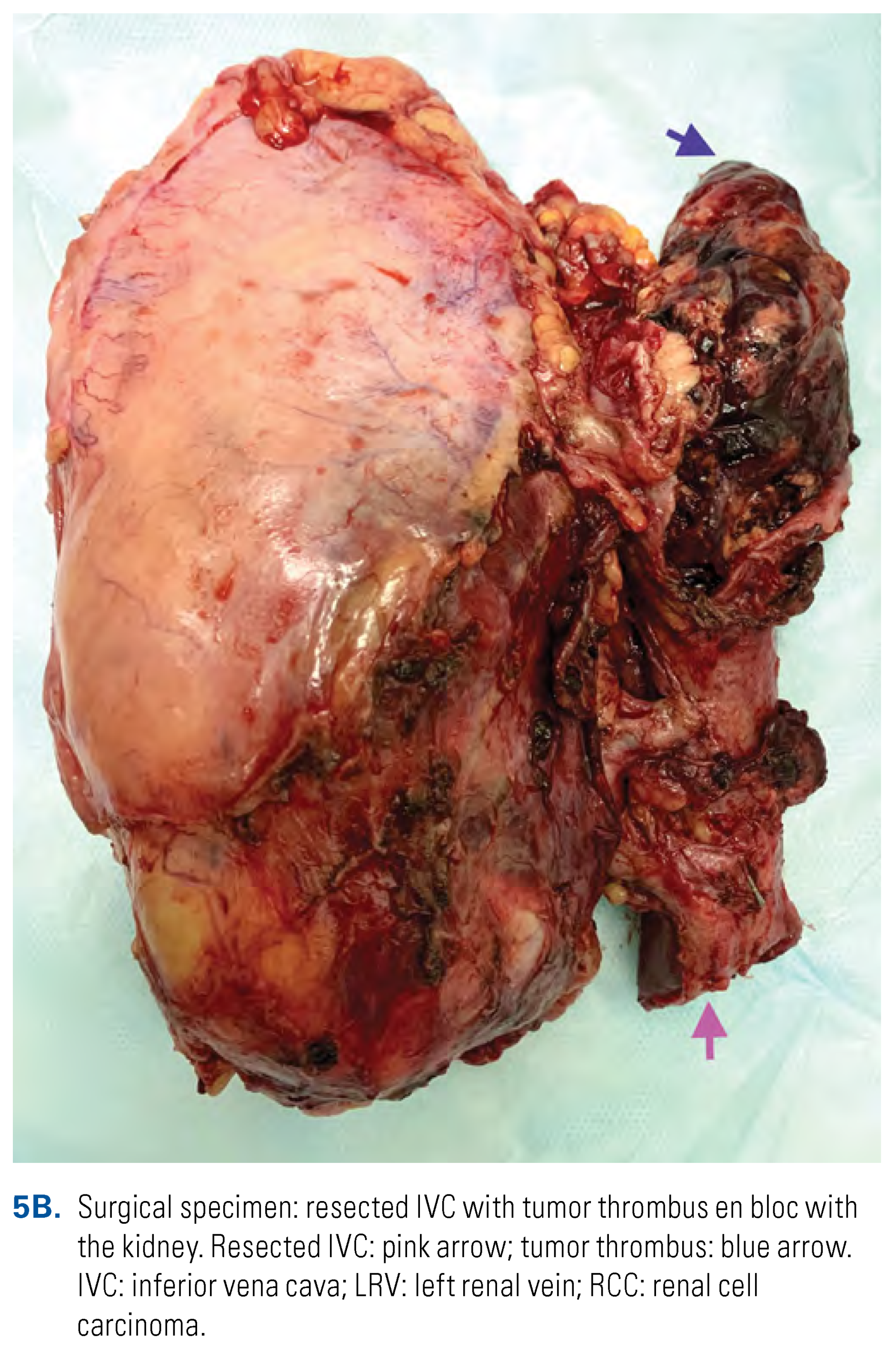
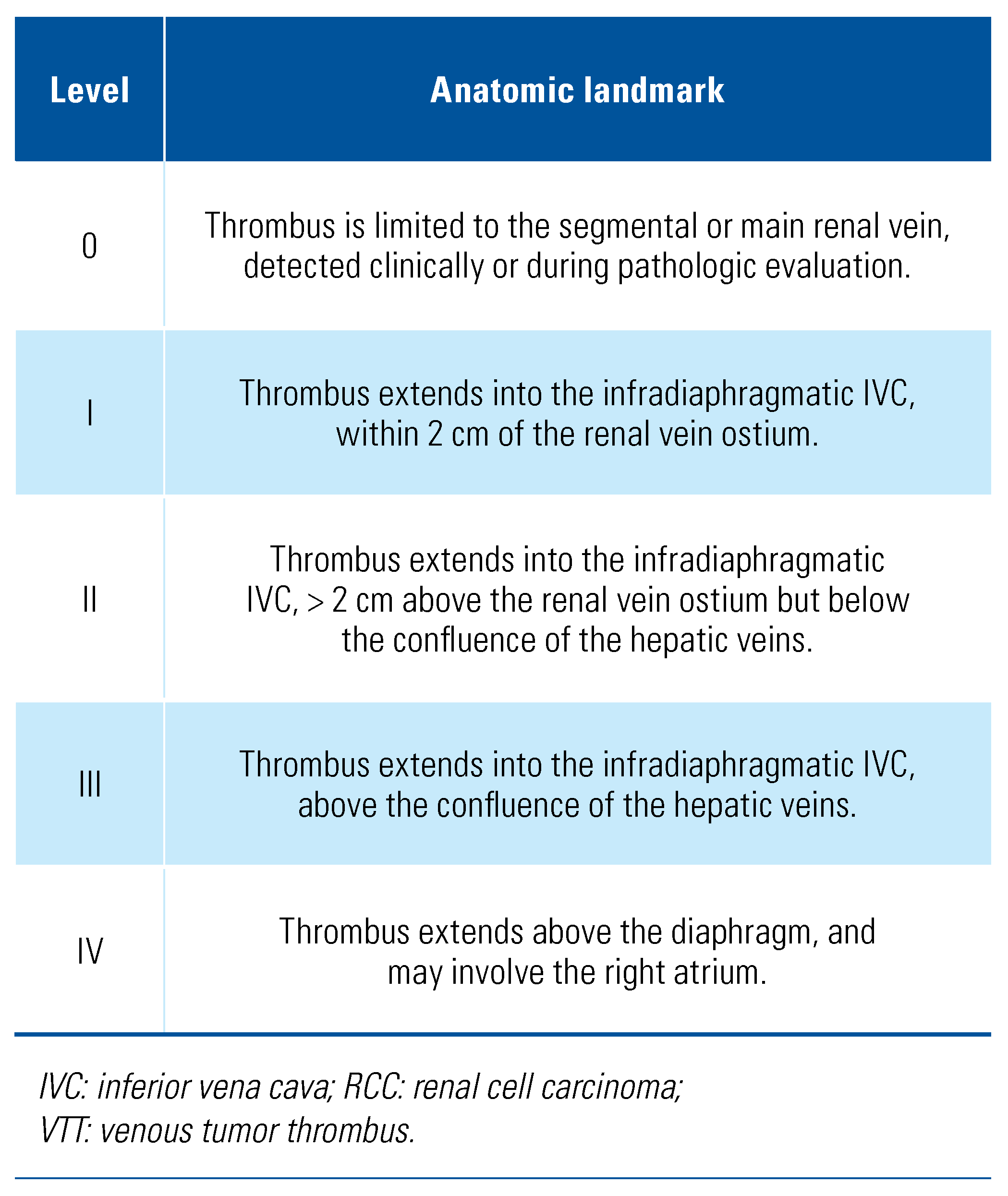
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Matveev, V.B.; Psutka, S.P.; Stewart, G.D.; Bratslavsky, G.; Abel, E.J. 2022 WUOF/SIU International Consultation on Urological Diseases: Management of Locally Advanced Renal Cell Carcinoma. Soc. Int. Urol. J. 2022, 3, 451-463. https://doi.org/10.48083/EGWH6536
Matveev VB, Psutka SP, Stewart GD, Bratslavsky G, Abel EJ. 2022 WUOF/SIU International Consultation on Urological Diseases: Management of Locally Advanced Renal Cell Carcinoma. Société Internationale d’Urologie Journal. 2022; 3(6):451-463. https://doi.org/10.48083/EGWH6536
Chicago/Turabian StyleMatveev, Vsevolod B., Sarah P. Psutka, Grant D. Stewart, Gennady Bratslavsky, and E. Jason Abel. 2022. "2022 WUOF/SIU International Consultation on Urological Diseases: Management of Locally Advanced Renal Cell Carcinoma" Société Internationale d’Urologie Journal 3, no. 6: 451-463. https://doi.org/10.48083/EGWH6536
APA StyleMatveev, V. B., Psutka, S. P., Stewart, G. D., Bratslavsky, G., & Abel, E. J. (2022). 2022 WUOF/SIU International Consultation on Urological Diseases: Management of Locally Advanced Renal Cell Carcinoma. Société Internationale d’Urologie Journal, 3(6), 451-463. https://doi.org/10.48083/EGWH6536





