2022 WUOF/SIU International Consultation on Urological Diseases: Kidney Cancer Screening and Epidemiology
Abstract
Epidemiology and Risk Factors for RCC
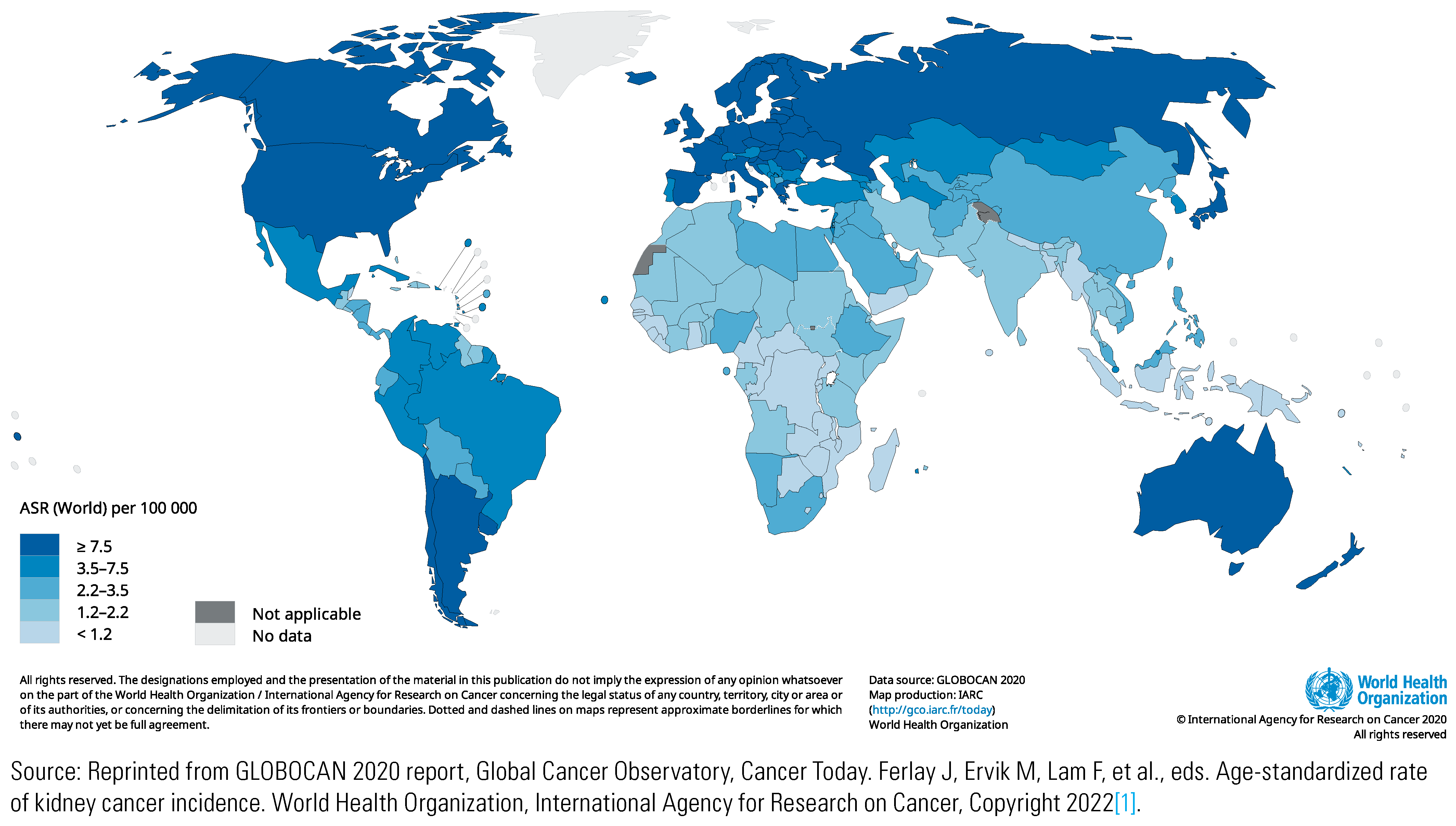
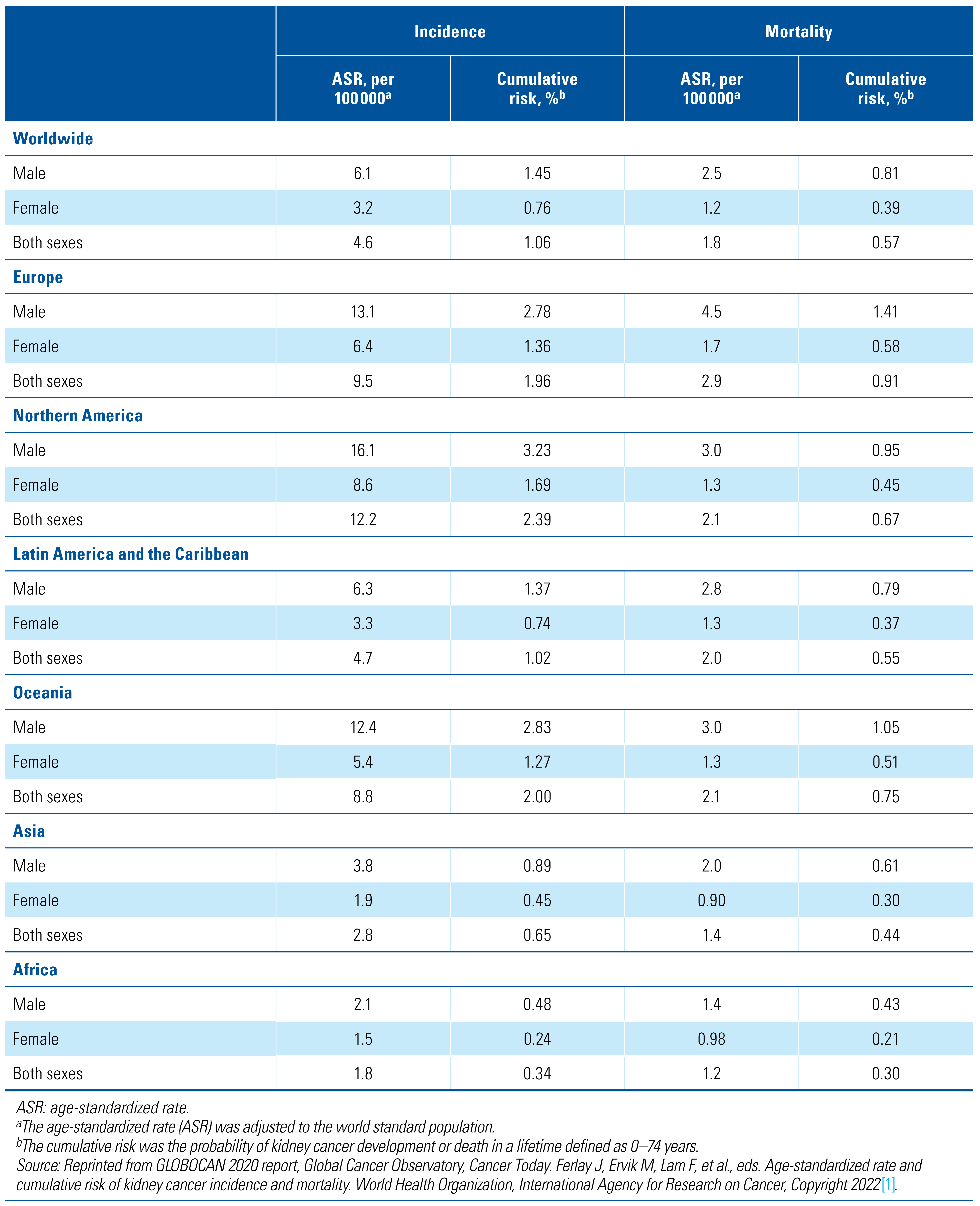
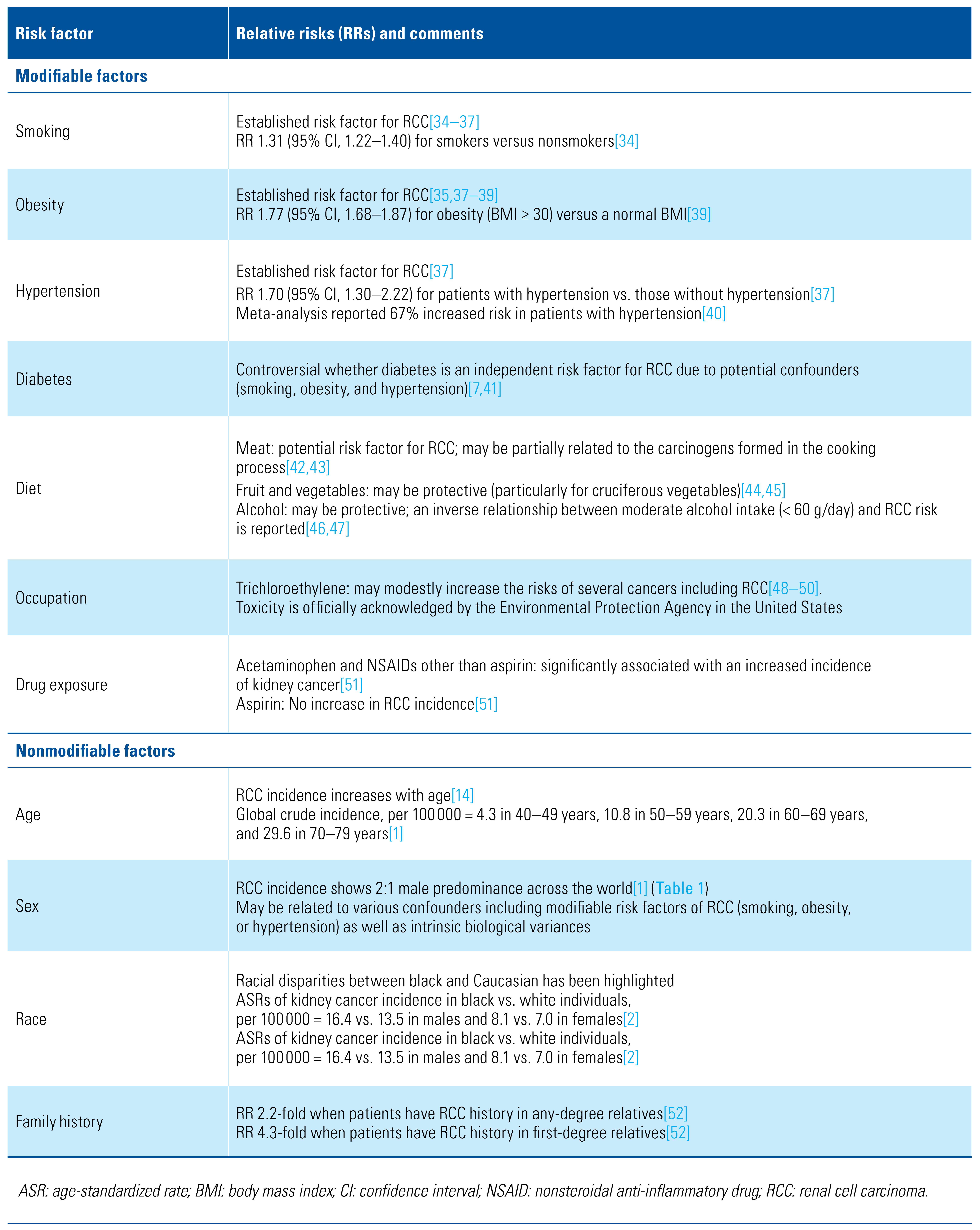
Incidence and Risk Factors
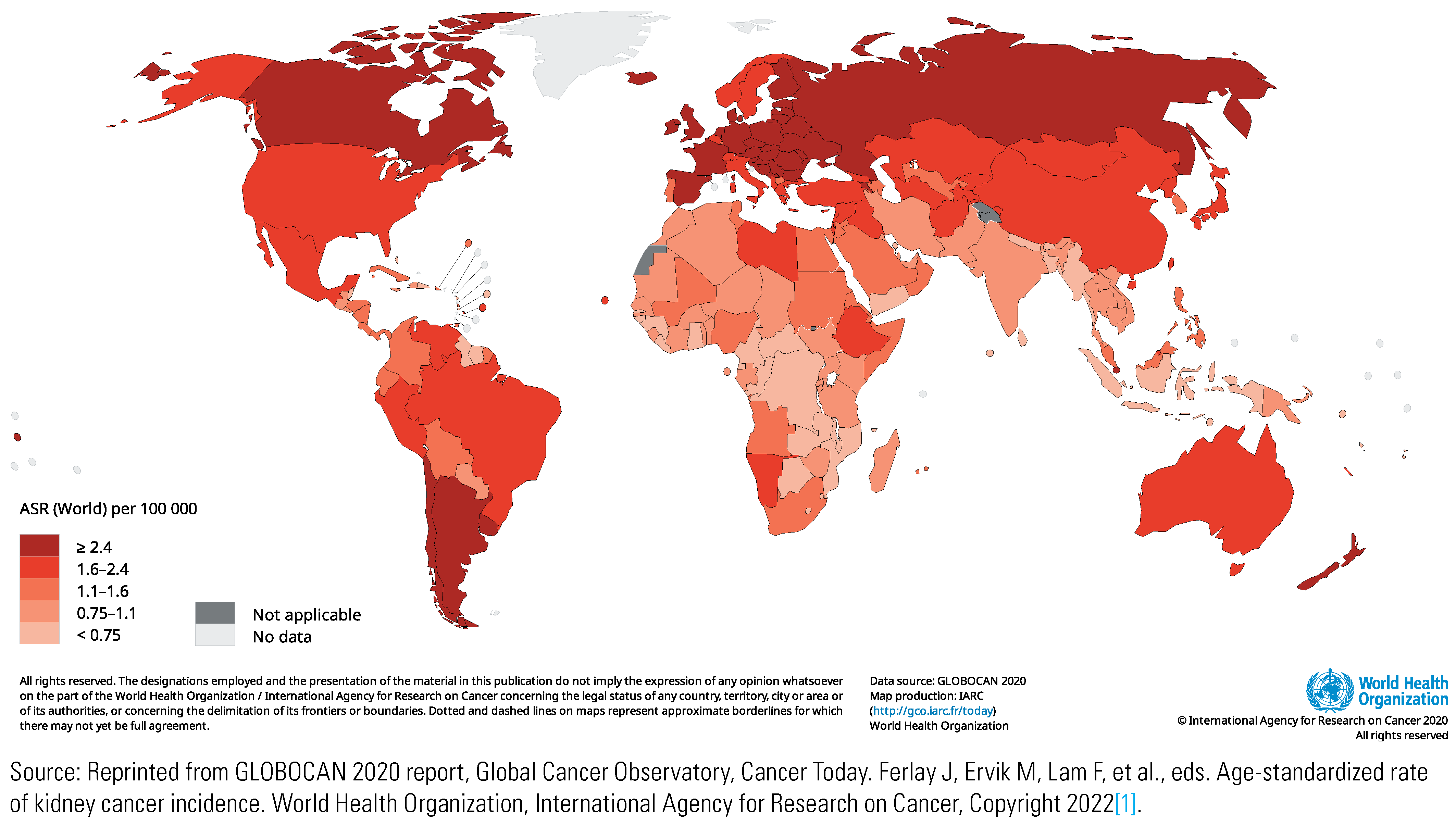
Mortality
Population Screening
Rationale for Screening
Screening Modality
Urinary Tests
Blood Tests
Ultrasound
Computed Tomography
Screening Population
Screening Implementation and Public Acceptability
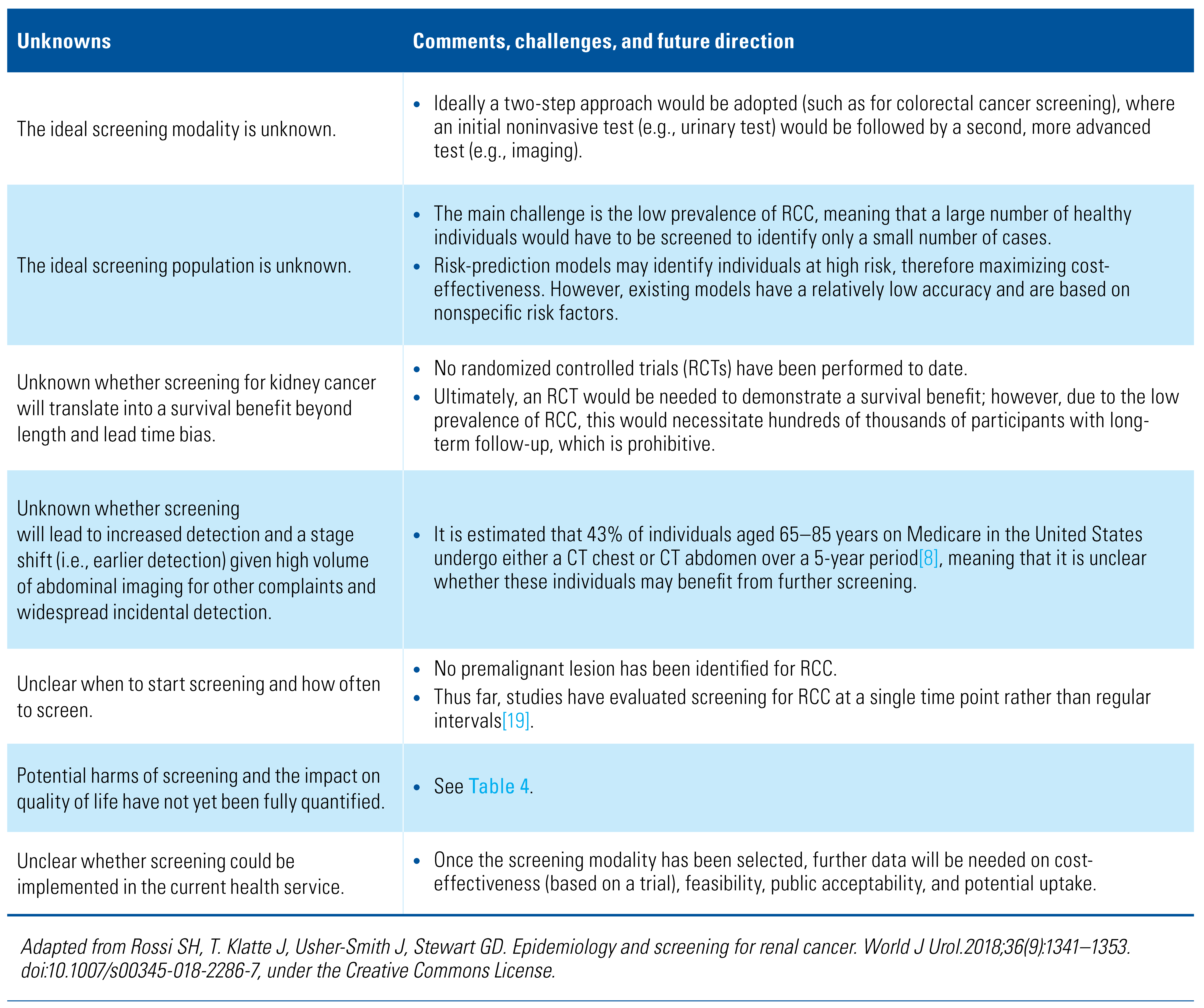
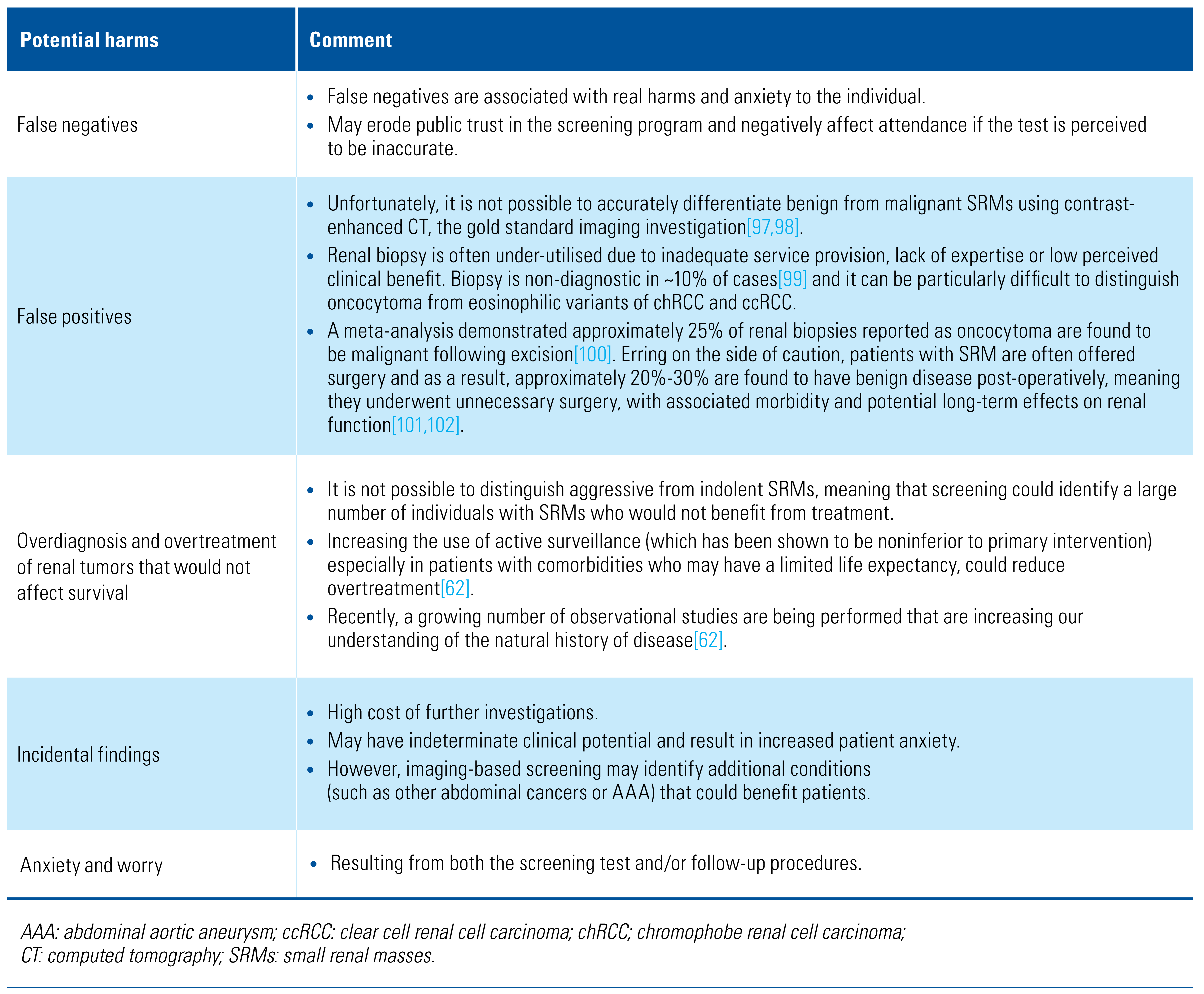
Unknown Benefits and Harms
Future Directions
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm ASR age-standardized rate |
| AUC | area under the curve BMI body mass index |
| ccRCC | clear cell renal cell carcinoma |
| chRCC | chromophobe renal cell carcinoma |
| CT | computed tomography |
| ctDNA | circulating tumor DNA |
| KIM-1 | kidney injury molecule-1 |
| NV | nonvisible |
| RCC | renal cell carcinoma |
| RCT | randomized controlled trial |
| SRMs | small renal masses |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; et al. Global Cancer Observatory, Cancer Today. International Agency for Research on Cancer.2020. Available at: https://gco.iarc.fr/today. Accessed September 22, 2022.
- Ervik, M.; Lam, F.; Laversanne, M.; Ferlay, J.; Bray, F. Global Cancer Observatory, Cancer Over Time. International Agency for Research on Cancer.2020. Available at: https://gco.iarc.fr/overtime. Accessed September 22, 2022.
- Smittenaar, C.R.; Petersen, K.A.; Stewart, K.; Moitt, N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016, 115, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; et al. (Eds.) Cancer Incidence in Five Continents, Vol. XI. IARC Scientific Publication No. 166. Lyon: International Agency for Research on Cancer. Available from: https://publications.iarc.fr/597. Licence: CC BY-NC-ND 3.0 IGO.
- Nguyen, M.M.; Gill, I.S.; Ellison, L.M. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006, 176 (6 Pt 1), 2397–2400; discussion 400. [Google Scholar] [CrossRef]
- Hollingsworth, J.M.; Miller, D.C.; Daignault, S.; Hollenbeck, B.K. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006, 98, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.; Dong, L.M.; Devesa, S.S. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Skinner, J.S.; Schroeck, F.R.; Zhou, W.; Black, W.C. Regional variation of computed tomographic imaging in the United States and the risk of nephrectomy. JAMA Intern Med. 2018, 178, 221–227. [Google Scholar] [CrossRef]
- Michel, K.F.; Spaulding, A.; Jemal, A.; Yabroff, K.R.; Lee, D.J.; Han, X. Associations of Medicaid expansion with insurance coverage, stage at diagnosis, and treatment among patients with genitourinary malignant neoplasms. JAMA Netw Open. 2021, 4, e217051. [Google Scholar] [CrossRef] [PubMed]
- Javier-DesLoges, J.F.; Yuan, J.; Soliman, S.; Hakimi, K.; Meagher, M.F.; Ghali, F.; et al. Evaluation of insurance coverage and cancer stage at diagnosis among low-income adults with renal cell carcinoma after passage of the Patient Protection and Affordable Care Act. JAMA Netw Open. 2021, 4, e2116267. [Google Scholar] [CrossRef]
- Kane, C.J.; Mallin, K.; Ritchey, J.; Cooperberg, M.R.; Carroll, P.R. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008, 113, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Gupta, M.; Joice, G.A.; Srivastava, A.; Alam, R.; Allaf, M.E.; et al. Clinical stage migration and survival for renal cell carcinoma in the United States. Eur Urol Oncol. 2019, 2, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Kondo, T.; Tanabe, K. Stage migration of renal cell carcinoma at a single Japanese university hospital: 24-year study. Int J Urol. 2014, 21, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Cancer Research UK Kidney Cancer Statistics. Available at: http:// www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer. Accessed September 22, 2022.
- Lalani, A.A.; McGregor, B.A.; Albiges, L.; Choueiri, T.K.; Motzer, R.; Powles, T.; et al. Systemic treatment of metastatic clear cell renal cell carcinoma in 2018: current paradigms, use of immunotherapy, and future directions. Eur Urol. 2019, 75, 100–110. [Google Scholar] [CrossRef]
- Hughes-Hallett, A.; Browne, D.; Mensah, E.; Vale, J.; Mayer, E. Assessing the impact of mass media public health campaigns. Be Clear on Cancer ‘blood in pee’: a case in point. BJU Int. 2016, 117, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.H.; Klatte, T.; Usher-Smith, J.; Stewart, G.D. Epidemiology and screening for renal cancer. World J Urol. 2018, 36, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Usher-Smith, J.; Simmons, R.K.; Rossi, S.H.; Stewart, G.D. Current evidence on screening for renal cancer. Nat Rev Urol. 2020, 17, 637–642. [Google Scholar] [CrossRef]
- Guo, J.; Ma, J.; Sun, Y.; Qin, S.; Ye, D.; Zhou, F.; et al. Chinese guidelines on the management of renal cell carcinoma (2015 edition). Chin Clin Oncol. 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Clark, P.E.; Chang, S.S.; Karam, J.A.; Souter, L.; Uzzo, R.G. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA Guideline: Part I. J Urol. 2021, 206, 199–208. [Google Scholar] [CrossRef]
- Richard, P.O.; Violette, P.D.; Bhindi, B.; Breau, R.H.; Kassouf, W.; Lavalle, L.T.; et al. Canadian Urological Association guideline: Management of small renal masses - Summary of recommendations. Can Urol Assoc J. 2022, 16, E61–E75. [Google Scholar] [CrossRef]
- Kanesvaran, R.; Porta, C.; Wong, A.; Powles, T.; Ng, Q.S.; Schmidinger, M.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with renal cell carcinoma. ESMO Open. 2021, 6, 100304. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Bhatt, J.; Avery, J.; Laupacis, A.; Coan, K.; Basappa, N.; et al. The kidney cancer research priority-setting partnership: Identifying the top 10 research priorities as defined by patients, caregivers, and expert clinicians. Can Urol Assoc J. 2017, 11, 379–387. [Google Scholar] [CrossRef]
- Rossi, S.H.; Blick, C.; Handforth, C.; Brown, J.E.; Stewart, G.D.; Renal Cancer Gap Analysis Collaborative. Essential research priorities in renal cancer: a modified Delphi Consensus Statement. Eur Urol Focus. 2020, 6, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.H.; Fielding, A.; Blick, C.; Handforth, C.; et al. Setting research priorities in partnership with patients to provide patient-centred urological cancer care. Eur Urol. 2019, 75, 891–893. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.; Abel, E.J.; Albiges, L.; Bex, A.; Brugarolas, J.; Bukowski, R.M.; et al. Summary from the Kidney Cancer Association’s Inaugural Think Thank: Coalition for a Cure. Clin Genitourin Cancer. 2021, 19, 167–175. [Google Scholar] [CrossRef]
- The Kidney Cancer UK patient survey report 2018. Available at: https://www.kcuk.org.uk/2018/08/16/2018-kidney-cancer-patient-survey/. Accessed September 22, 2022.
- Fenton, J.J.; Weiss, N.S. Screening computed tomography: will it result in overdiagnosis of renal carcinoma? Cancer. 2004, 100, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.; Rota, M.; Catto, J.W.; La Vecchia, C. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016, 70, 458–466. [Google Scholar] [CrossRef]
- Lotan, Y.; Karam, J.A.; Shariat, S.F.; Gupta, A.; Roupret, M.; Bensalah, K.; et al. Renal-cell carcinoma risk estimates based on participants in the prostate, lung, colorectal, and ovarian cancer screening trial and national lung screening trial. Urol Oncol. 2016, 34, 167.e9–e16. [Google Scholar] [CrossRef]
- Hunt, J.D.; van der Hel, O.L.; McMillan, G.P.; Boffetta, P.; Brennan, P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005, 114, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Macleod, L.C.; Hotaling, J.M.; Wright, J.L.; Davenport, M.T.; Gore, J.L.; Harper, J.; et al. Risk factors for renal cell carcinoma in the VITAL study. J Urol. 2013, 190, 1657–1661. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; et al. Body fatness and cancer--viewpoint of the IARC Working Group. N Engl J Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2014, 135, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, X.; Zou, S.Y.; Shi, B.M. Blood pressure and kidney cancer risk: meta- analysis of prospective studies. J Hypertens. 2017, 35, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Iwasaki, M.; Otani, T.; Sasazuki, S.; Noda, M.; Tsugane, S. Diabetes mellitus and the risk of cancer: results from a large-scale population- based cohort study in Japan. Arch Intern Med. 2006, 166, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.R.; Schwartz, K.L.; Colt, J.S.; Dong, L.M.; Ruterbusch, J.J.; Purdue, M.P.; et al. Meat-cooking mutagens and risk of renal cell carcinoma. Br J Cancer. 2011, 105, 1096–104. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.R.; Cross, A.J.; Graubard, B.I.; Park, Y.; Ward, M.H.; Rothman, N.; et al. Large prospective investigation of meat intake, related mutagens, and risk of renal cell carcinoma. Am J Clin Nutr. 2012, 95, 155–162. [Google Scholar] [CrossRef]
- Liu, B.; Mao, Q.; Wang, X.; Zhou, F.; Luo, J.; Wang, C.; et al. Cruciferous vegetables consumption and risk of renal cell carcinoma: a meta-analysis. Nutr Cancer. 2013, 65, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, L. Cruciferous vegetables intake is associated with lower risk of renal cell carcinoma: evidence from a meta-analysis of observational studies. PLoS One. 2013, 8, e75732. [Google Scholar] [CrossRef]
- Lew, J.Q.; Chow, W.H.; Hollenbeck, A.R.; Schatzkin, A.; Park, Y. Alcohol consumption and risk of renal cell cancer: the NIH-AARP diet and health study. Br J Cancer. 2011, 104, 537–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jay, R.; Brennan, P.; Brenner Overvad, K.; Olsen, A.; Tjønneland, A.; et al. Alcohol consumption and the risk of renal cancers in the European Prospective Investigation into Cancer and Nutrition (EPIC). Wozniak, M.B.; Brennan, P.; Brenner, D.R.; Overvad, K.; Olsen, A.; Tjonneland, A.; et al. Int J Cancer. 2015, 137, 1953–1966, Urol Oncol. 2017, 35, 117. [Google Scholar] [CrossRef]
- Chiu, W.A.; Caldwell, J.C.; Keshava, N.; Scott, C.S. Key scientific issues in the health risk assessment of trichloroethylene. Environ Health Perspect. 2006, 114, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.S.; Chiu, W.A. Trichloroethylene cancer epidemiology: a consideration of select issues. Environ Health Perspect. 2006, 114, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Harth, V.; Bruning, T.; Bolt, H.M. Renal carcinogenicity of trichloroethylene: update, mode of action, and fundamentals for occupational standard setting. Rev Environ Health. 2005, 20, 103–118. [Google Scholar]
- Choueiri, T.K.; Je, Y.; Cho, E. Analgesic use and the risk of kidney cancer: a meta-analysis of epidemiologic studies. Int J Cancer. 2014, 134, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Clague, J.; Lin, J.; Cassidy, A.; Matin, S.; Tannir, N.M.; Tamboli, P.; et al. Family history and risk of renal cell carcinoma: results from a case-control study and systematic meta-analysis. Cancer Epidemiol Biomarkers Prev. 2009, 18, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Scelo, G.; Muller, D.C.; Riboli, E.; Johannson, M.; Cross, A.J.; Vineis, P.; et al. KIM-1 as a blood-based marker for early detection of kidney cancer: a prospective nested case-control study. Clin Cancer Res. 2018, 24, 5594–5601. [Google Scholar] [CrossRef]
- Wilson, J.M.; Jungner, Y.G. Principles and practice of mass screening for disease [article in Spanish]. Bol Oficina Sanit Panam. 1968, 65, 281–393. [Google Scholar] [PubMed]
- Harvey-Kelly, L.L.W.; Harrison, H.; Rossi, S.H.; Griffin, S.J.; Stewart, G.D.; Usher-Smith, J.A. Public attitudes towards screening for kidney cancer: an online survey. BMC Urol. 2020, 20, 170. [Google Scholar] [CrossRef]
- Rossi, S.H.; Prezzi, D.; Kelly-Morland, C.; Goh, V. Imaging for the diagnosis and response assessment of renal tumours. World J Urol. 2018, 36, 1927–1942. [Google Scholar] [CrossRef]
- Millet, I.; Doyon, F.C.; Hoa, D.; Thuret, R.; Merigeaud, S.; Serre, I.; et al. Characterization of small solid renal lesions: can benign and malignant tumors be differentiated with CT? AJR Am J Roentgenol. 2011, 197, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Marconi, L.; Dabestani, S.; Lam, T.B.; Hofmann, F.; Stewart, F.; Norrie, J.; et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol. 2016, 69, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Druskin, S.C.; Rowe, S.P.; Pierorazio, P.M.; Gorin, M.A.; Allaf, M.E. Surgical histopathology for suspected oncocytoma on renal mass biopsy: a systematic review and meta-analysis. BJU Int. 2017, 119, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Li, S.; Khandwala, Y.; Chung, K.J.; Park, H.K.; Chung, B.I. Association of prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States From 2007 to 2014. JAMA Surg. 2019, 154, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Vukina, J.; Smith, A.B.; Meyer, A.M.; Wheeler, S.B.; Kuo, t.-M.; et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J Urol. 2015, 193, 30–35. [Google Scholar] [CrossRef]
- Pierorazio, P.M.; Johnson, M.H.; Ball, M.W.; Gorin, M.A.; Trock, B.J.; Chang, P.; et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol. 2015, 68, 408–415. [Google Scholar] [CrossRef]
- Khadhouri, S.; Gallagher, K.M.; MacKenzie, K.R.; Shah, T.T.; Gao, C.; Moore, S.; et al. The IDENTIFY study: the investigation and detection of urological neoplasia in patients referred with suspected urinary tract cancer - a multicentre observational study. BJU Int. 2021, 128, 440–450. [Google Scholar] [CrossRef]
- Bangma, C.H.; Loeb, S.; Busstra, M.; Zhu, X.; El Bouazzaoui, S.; Refos, J.; et al. Outcomes of a bladder cancer screening program using home hematuria testing and molecular markers. Eur Urol. 2013, 64, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Messing, E.M.; Madeb, R.; Young, T.; Gilchrist, K.W.; Bram, L.; Greenberg, E.B.; et al. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006, 107, 2173–2179. [Google Scholar] [CrossRef]
- Sugimura, K.; Ikemoto, S.I.; Kawashima, H.; Nishisaka, N.; Kishimoto, T. Microscopic hematuria as a screening marker for urinary tract malignancies. Int J Urol. 2001, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sharp, V.J.; Barnes, K.T.; Erickson, B.A. Assessment of asymptomatic microscopic hematuria in adults. Am Fam Physician. 2013, 88, 747–754. [Google Scholar] [PubMed]
- Flitcroft, J.G.; Verheyen, J.; Vemulkar, T.; Welbourne, E.N.; Rossi, S.H.; Wlesh, S.J.; et al. Early detection of kidney cancer using urinary proteins: a truly non-invasive strategy. BJU Int. 2022, 129, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.J.; Mellnick, V.M.; Luo, J.; Siegel, M.J.; Figenshau, R.S.; Bhayani, S.; et al. Evaluation of urine aquaporin-1 and perilipin-2 concentrations as biomarkers to screen for renal cell carcinoma: a prospective cohort study. JAMA Oncol. 2015, 1, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Richards, D.; Cohn, A.; Tummala, M.; Lapham, R.; Cosgrove, D.; et al. Clinical validation of a targeted methylation-based multi- cancer early detection test using an independent validation set. Ann Oncol. 2021, 32, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Nadauld, L.D.; McDonnell, C.H., 3rd; Beer, T.M.; Liu, M.C.; Klein, E.A.; Hudnut, A.; et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021, 13, 3501. [Google Scholar] [CrossRef] [PubMed]
- The NHS Galleri Test. Available at: https://www.nhs-galleri.org/. Accessed September 22, 2022.
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat Commun. 2020, 11, 3475. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Rossi, S.H.; Stewart, G.D. Re: clinical validation of a targeted methylation- based multi-cancer early detection test using an independent validation set. Eur Urol. 2022, 82, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.G.; Moser, T.; Mouliere, F.; Field-Rayner, J.; Eldridge, M.; Riediger, A.L.; et al. Comprehensive characterization of cell-free tumor DNA in plasma and urine of patients with renal tumors. Genome Med. 2020, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, P.V.; Berchuck, J.E.; Korthauer, K.; Spisak, S.; Nassar, A.H.; Abou Alaiwi, S.; et al. Detection of renal cell carcinoma using plasma and urine cell- free DNA methylomes. Nat Med. 2020, 26, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.H.; Hsu, R.; Blick, C.; Goh, V.; Nathan, P.; Nicol, D.; et al. Meta-analysis of the prevalence of renal cancer detected by abdominal ultrasonography. Br J Surg. 2017, 104, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Spouge, A.R.; Wilson, S.R.; Wooley, B. Abdominal sonography in asymptomatic executives: prevalence of pathologic findings, potential benefits, and problems. J Ultrasound Med. 1996, 15, 763–767. [Google Scholar] [CrossRef]
- Fujii, Y.; Ajima, J.; Oka, K.; Tosaka, A.; Takehara, Y. Benign renal tumors detected among healthy adults by abdominal ultrasonography. Eur Urol. 1995, 27, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Mihara, S.; Kuroda, K.; Yoshioka, R.; Koyama, W. Early detection of renal cell carcinoma by ultrasonographic screening--based on the results of 13 years screening in Japan. Ultrasound Med Biol. 1999, 25, 1033–1039. [Google Scholar] [CrossRef]
- Tsuboi, N.; Horiuchi, K.; Kimura, G.; Kondoh, Y.; Yoshida, K.; Nishimura, T.; et al. Renal masses detected by general health checkup. Int J Urol. 2000, 7, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, Y.; Watanabe, Y.; Ozasa, K.; Hayashi, K.; Kawai, K. Validity of sonographic screening for the detection of abdominal cancers. J Clin Ultrasound. 2002, 30, 408–415. [Google Scholar] [CrossRef]
- Filipas, D.; Spix, C.; Schulz-Lampel, D.; Michaelis, J.; Hohenfellner, R.; Roth, S.; et al. Screening for renal cell carcinoma using ultrasonography: a feasibility study. BJU Int. 2003, 91, 595–599. [Google Scholar] [CrossRef]
- Malaeb, B.S.; Martin, D.J.; Littooy, F.N.; Lotan, Y.; Waters, W.B.; Flanigan, R.C.; et al. The utility of screening renal ultrasonography: identifying renal cell carcinoma in an elderly asymptomatic population. BJU Int. 2005, 95, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Warshauer, D.M.; McCarthy, S.M.; Street, L.; Bookbinder, M.J.; Glickman, M.G.; Richter, J.; et al. Detection of renal masses: sensitivities and specificities of excretory urography/linear tomography, US, and CT. Radiology. 1988, 169, 363–265. [Google Scholar] [CrossRef]
- Jamis-Dow, C.A.; Choyke, P.L.; Jennings, S.B.; Linehan, W.M.; Thakore, K.N.; Walther, M.M. Small (< or = 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology. 1996, 198, 785–788. [Google Scholar] [CrossRef] [PubMed]
- The US preventive services task force Final Recommendation Statement on Abdominal Aortic Aneurysm Screening. updated 2019. Available at: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/abdominal-aortic-aneurysm-screening. Accessed September 22, 2022.
- The National Health Service Abdominal Aortic Aneurysm Screening. Available at: https://www.nhs.uk/conditions/abdominal-aortic-aneurysm-screening/. Accessed September 22, 2022.
- Wanhainen, A.; Bjorck, M. The Swedish experience of screening for abdominal aortic aneurysm. J Vasc Surg. 2011, 53, 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Pelc, N.J. Recent and future directions in CT imaging. Ann Biomed Eng. 2014, 42, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Millor, M.; Bartolome, P.; Pons, M.J.; Bastarrika, G.; Beloqui, O.; Cano, D.; et al. Whole-body computed tomography: a new point of view in a hospital check-up unit? Our experience in 6516 patients. Radiol Med. 2019, 124, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Beinfeld, M.T.; Wittenberg, E.; Gazelle, G.S. Cost-effectiveness of whole- body CT screening. Radiology. 2005, 234, 415–422. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Graffy, P.M.; Perez, A.A.; Lubner, M.G.; Elton, D.C.; Summers, R.M. Opportunistic screening at abdominal CT: use of automated body composition biomarkers for added cardiometabolic value. Radiographics. 2021, 41, 524–542. [Google Scholar] [CrossRef]
- Crosbie, P.A.; Gabe, R.; Simmonds, I.; Kennedy, M.; Rogerson, S.; Ahmed, N.; et al. Yorkshire Lung Screening Trial (YLST): protocol for a randomised controlled trial to evaluate invitation to community-based low-dose CT screening for lung cancer versus usual care in a targeted population at risk. BMJ Open. 2020, 10, e037075. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.H.; Klatte, T.; Usher-Smith, J.A.; Fife, K.; Welsh, S.J.; Dabestani, S.; et al. A decision analysis evaluating screening for kidney cancer using focused renal ultrasound. Eur Urol Focus. 2021, 7, 407–419. [Google Scholar] [CrossRef]
- Harrison, H.; Thompson, R.E.; Lin, Z.; Rossi, S.H.; Stewart, G.D.; Griffin, S.J.; et al. Risk prediction models for kidney cancer: a systematic review. Eur Urol Focus. 2021, 7, 1380–1390. [Google Scholar] [CrossRef]
- Harrison, H.; Pennells, L.; Wood, A.; Rossi, S.H.; Stewart, G.D.; Griffin, S.J.; et al. Validation and public health modelling of risk prediction models for kidney cancer using the UK Biobank. BJU Int. 2022, 129, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Harrison, H.; Li, N.; Saunders, C.L.; Rossi, S.H.; Dennis, J.; Griffin, S.J.; et al. The current state of genetic risk models for the development of kidney cancer: a review and validation. BJU Int. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Usher-Smith, J.A.; Harvey-Kelly, L.L.W.; Rossi, S.H.; Harrison, H.; Griffin, S.J.; Stewart, G.D. Acceptability and potential impact on uptake of using different risk stratification approaches to determine eligibility for screening: A population-based survey. Health Expect. 2021, 24, 341–351. [Google Scholar] [CrossRef]
- Heleno, B.; Thomsen, M.F.; Rodrigues, D.S.; Jorgensen, K.J.; Brodersen, J. Quantification of harms in cancer screening trials: literature review. BMJ. 2013, 347, f5334. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.P.; Sheridan, S.L.; Lewis, C.L.; Barclay, C.; Vu, M.B.; Kistler, C.E.; et al. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA Intern Med. 2014, 174, 281–5. [Google Scholar] [CrossRef] [PubMed]
- Korenstein, D.; Chimonas, S.; Barrow, B.; Keyhani, S.; Troy, A.; Lipitz- Snyderman, A. Development of a conceptual map of negative consequences for patients of overuse of medical tests and treatments. JAMA Intern Med. 2018, 178, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
 |
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Rossi, S.H.; Tanaka, H.; Usher-Smith, J.A.; Bernhard, J.-C.; Fujii, Y.; Stewart, G.D. 2022 WUOF/SIU International Consultation on Urological Diseases: Kidney Cancer Screening and Epidemiology. Soc. Int. Urol. J. 2022, 3, 371-385. https://doi.org/10.48083/XBCX3386
Rossi SH, Tanaka H, Usher-Smith JA, Bernhard J-C, Fujii Y, Stewart GD. 2022 WUOF/SIU International Consultation on Urological Diseases: Kidney Cancer Screening and Epidemiology. Société Internationale d’Urologie Journal. 2022; 3(6):371-385. https://doi.org/10.48083/XBCX3386
Chicago/Turabian StyleRossi, Sabrina H., Hajime Tanaka, Juliet A. Usher-Smith, Jean-Christophe Bernhard, Yasuhisa Fujii, and Grant D. Stewart. 2022. "2022 WUOF/SIU International Consultation on Urological Diseases: Kidney Cancer Screening and Epidemiology" Société Internationale d’Urologie Journal 3, no. 6: 371-385. https://doi.org/10.48083/XBCX3386
APA StyleRossi, S. H., Tanaka, H., Usher-Smith, J. A., Bernhard, J.-C., Fujii, Y., & Stewart, G. D. (2022). 2022 WUOF/SIU International Consultation on Urological Diseases: Kidney Cancer Screening and Epidemiology. Société Internationale d’Urologie Journal, 3(6), 371-385. https://doi.org/10.48083/XBCX3386





