Abstract
Background: Follicle-stimulating hormone (FSH) dysregulation plays a potential role in prostate cancer progression. The objective of this study was to evaluate whether higher FSH levels during androgen deprivation therapy (ADT) for recurrent prostate cancer could predict the development of castration-resistant prostate cancer (CRPC), prostate cancer-specific survival (CSS), and overall survival (OS). Methods: Serum FSH levels were measured in cryopreserved samples of the continuous ADT arm of the PR.7 trial, supplemented with analogous samples from a large contemporaneous biobank. Univariate and multivariate analyses assessed the relationship between FSH tertiles and time to CRPC, as well as CSS, and OS. Results: A total of 172 patients were included in our analysis. Of these, 54 patients (31%) developed CRPC during the 9-year follow-up. Median FSH for the tertiles was 4.35, 6.13, and 11.32 mIU/mL. FSH tertiles were not significantly associated with the time to CRPC, or with CSS or OS. FSH levels were not a significant prognostic factor for these oncologic outcomes. Conclusion: As previously reported, the use of gonadotropin-releasing hormone (GnRH) antagonists for ADT has significantly more suppression of FSH levels than GnRH agonists. Our results do not suggest that differences in circulating FSH 1 year following ADT initiation influence long-term oncologic outcomes or development of CRPC.
1. Introduction
There is mounting evidence that follicle-stimulating hormone (FSH) dysregulation may play a role in prostate cancer development and progression [1]. Selective expression of FSH receptor by endothelial cells has been demonstrated in a wide range of tumors as well as in the majority of tumor metastases, including prostate cancer [2,3]. Higher preoperative levels of serum FSH have been linked with lower levels of testosterone, higher grade cancer, and extraprostatic extension [4,5]. In patients undergoing androgen deprivation therapy (ADT) for advanced prostate cancer, an association between serum FSH levels and the time to the development of castration-resistant prostate cancer (CRPC) has been reported in a retrospective, single-center study [6].
ADT has also been associated with a higher risk of cardiovascular events [7,8]. Moreover, in the context of ADT, data suggest that FSH can promote cardiovascular, metabolic, and skeletal comorbidities through atherosclerotic plaque formation, metabolic syndrome, insulin resistance, and bone resorption [9,10]. Of particular interest, recent reports have highlighted the differences in cardiovascular events between gonadotropin-releasing hormone (GnRH) agonists and antagonists. For instance, a randomized phase II study by Margel et al. suggested that GnRH antagonist therapy, which lowers circulating FSH levels, is associated with an 18% absolute risk reduction in major adverse cardiovascular and cerebrovascular events [11]. More recently, the randomized phase III HERO trial comparing leuprolide with the oral GnRH antagonist relugolix has shown a 54% relative risk reduction of major adverse cardiovascular events (MACE) [12].
An important difference between GnRH agonists and antagonists during ADT is superior suppression of serum FSH with antagonists, which has been a leading hypothesis in explaining the differences in cardiovascular and other comorbidities. However, the role of FSH biology and its impact on clinical outcomes remains to be fully defined. The role of FSH suppression on clinical outcomes and safety has so far been researched mainly in studies comparing GnRH agonists with antagonists. In the CS21 trial, rapid decrease in PSA and more profound suppression of FSH was noted with degarelix compared with leuprolide, including upon crossover from leuprolide to degarelix [13]. Few studies have reported on the association of FSH levels before or during ADT, as an independent prognostic factor, on clinical outcomes. In a recent study, Kourbanhoussen et al. showed no significant association between FSH levels before prostatectomy with biochemical recurrence, time to CRPC, time to metastasis, or occurrence of MACE [14]. In the context of ADT, it has been reported that FSH levels could predict time to the development of CRPC [6].
In this study, using cryopreserved serum samples from the PR.7 trial with long annotated clinical follow-up [15], we sought to evaluate whether higher FSH levels correlate with lower overall survival (OS) and cancer-specific survival (CSS), as well as a reduced time to development of CRPC.
2. Methods
Institutional research ethics approval for this retrospective study was obtained from the CHU de Québec-Université Laval (2016-2835) and from the Duke University Institutional Review Board (Pro00044627).
For this analysis, we identified 163 patients with cryopreserved serum samples taken 12 months post randomization from the continuous androgen deprivation (CAD) arm of the PR.7 trial for whom demographic information and long-term clinical follow-up were available in the Canadian Cancer Trials Group (CCTG) database. Informed consent was obtained for the biobank for all participants in the PR.7 trial.
For 100 of those patients, serum samples taken 24 and 36 months post randomization were also available and were included in our analysis. Details of the PR.7 trial are provided in the original publication [15]. Notably, all patients received GnRH agonists in this cohort.
To increase our statistical power, we included 9 additional contemporaneous cryopreserved serum samples from patients identified in a large biobank at Duke University with long clinical follow-up.
These patients also received continuous ADT following PSA recurrence following radiotherapy and met the inclusion criteria of the PR.7 trial.
FSH levels were measured using 20 µL of plasma with immunoassays according to the manufacturer’s instructions (Cayman Chemicals, Ann Arbor, MI, US). Statistical analyses assessed the relationship between FSH levels and clinical outcomes such as, OS, CSS, and time to CRPC. CRPC was defined as at least 2 rising PSA values or as new or progressing metastases despite castrate levels of testosterone (<1.7 mmol/L). Patients were divided into tertiles based on FSH levels during CAD and Kaplan-Meier survival curves estimated, OS, CSS, and time to CRPC for these tertiles. Cox multivariable proportional hazards analyses were performed for the same outcomes, with adjustment for baseline clinical factors: age, Gleason score, baseline PSA, time since radiotherapy, and Eastern Cooperative Oncology Group (ECOG) status.
3. Results
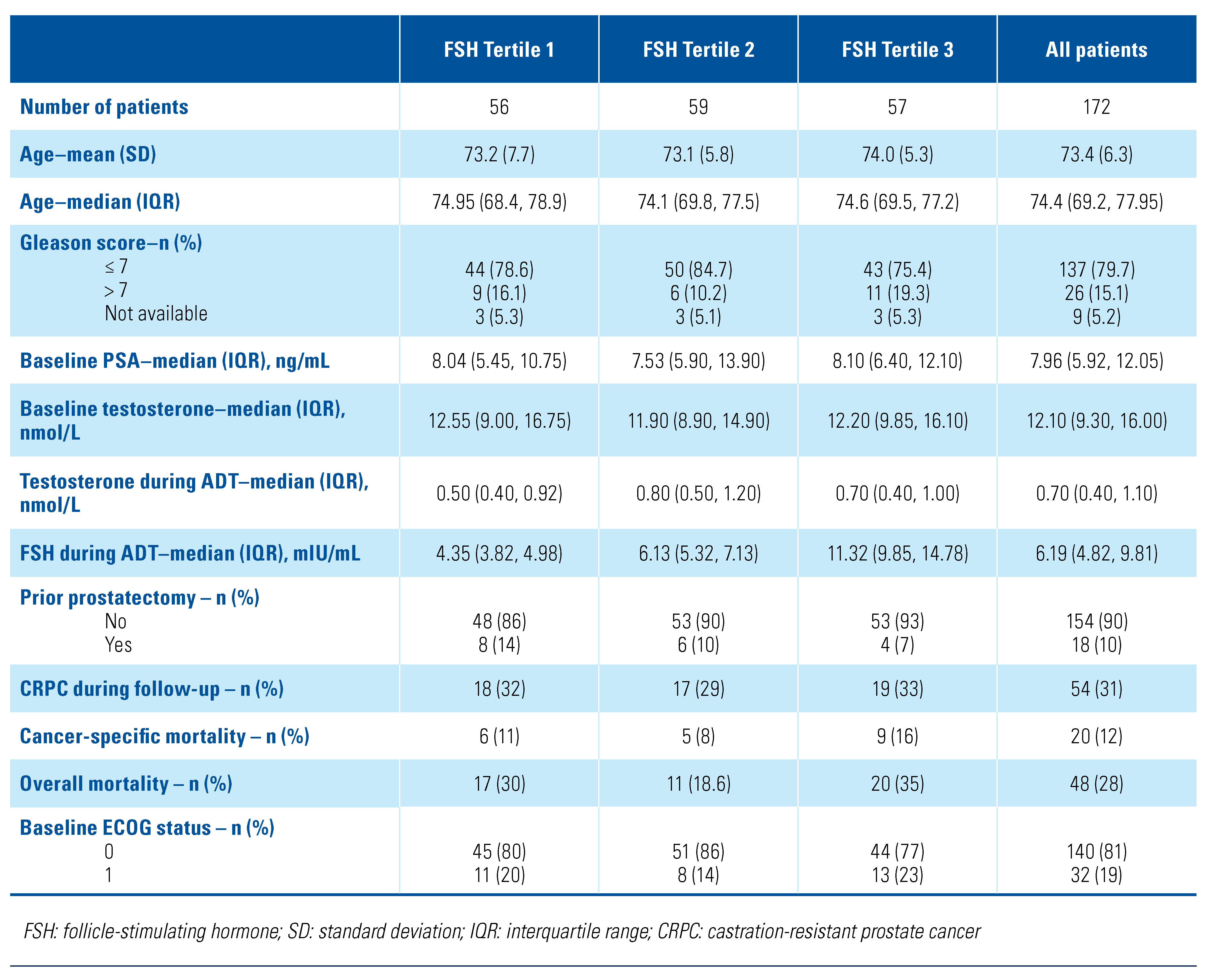
A total of 172 patients were included in this study. The mean age was 73.4 years, and almost 80% of patients had a Gleason score ≤ 7 at diagnosis. The median FSH during ADT was 6.19 IU/mL (interquartile range: 4.82 to 9.81). Median FSH for the tertiles was 4.35, 6.13, and 11.32 mIU/mL. Only 10% of patients had prostatectomy before ADT. Over the 9 years of follow-up, 54 patients (31%) developed CRPC, and 48 patients (28%) died, of whom 20 (12%) died of prostate cancer. Demographic and clinical characteristics are outlined by FSH tertile and for the whole cohort in Table 1. Baseline patient characteristics were similar across all tertiles, except that Gleason scores were similar for tertiles 1 and 3, while tertile 2 had fewer patients with a score > 7.
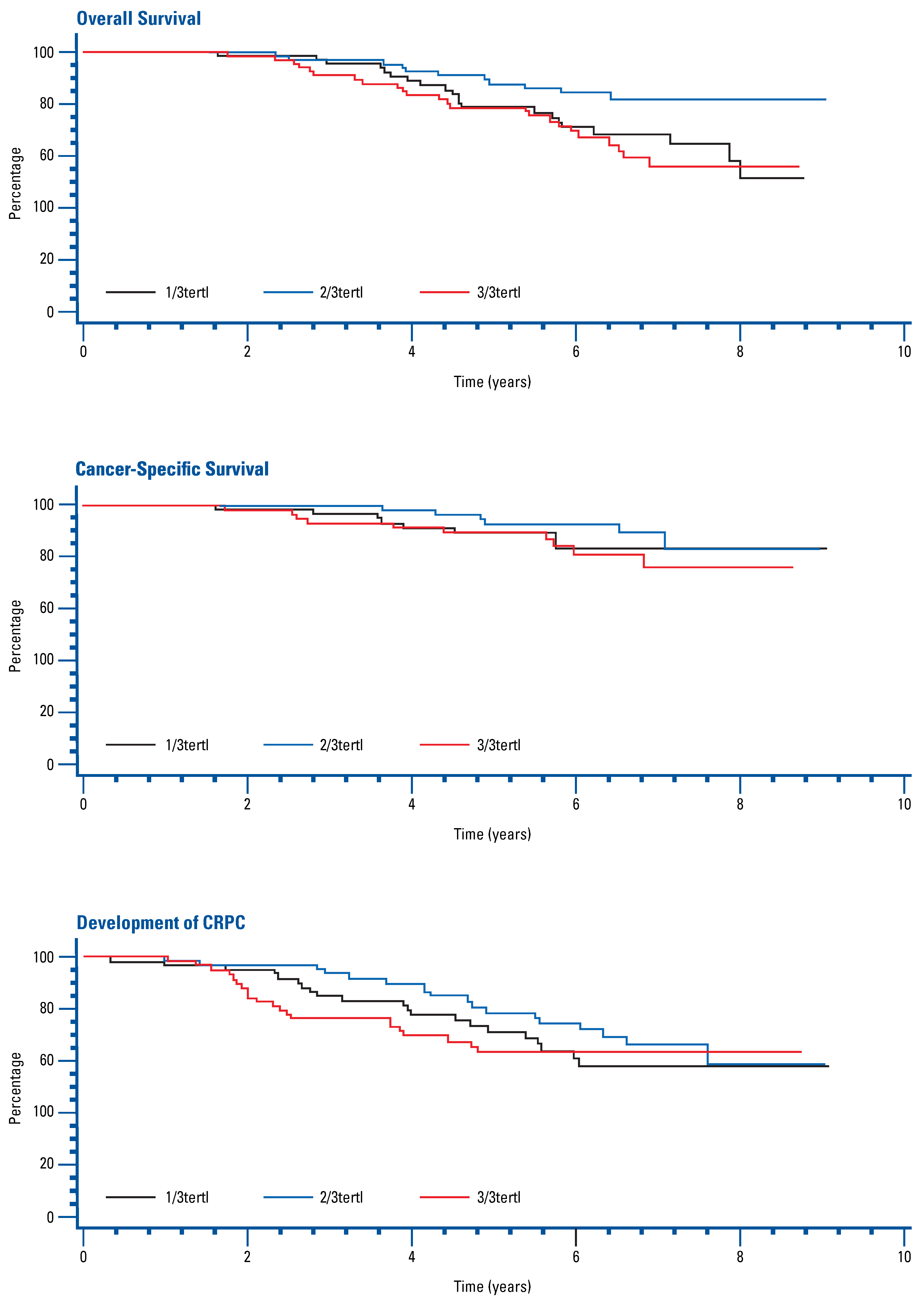
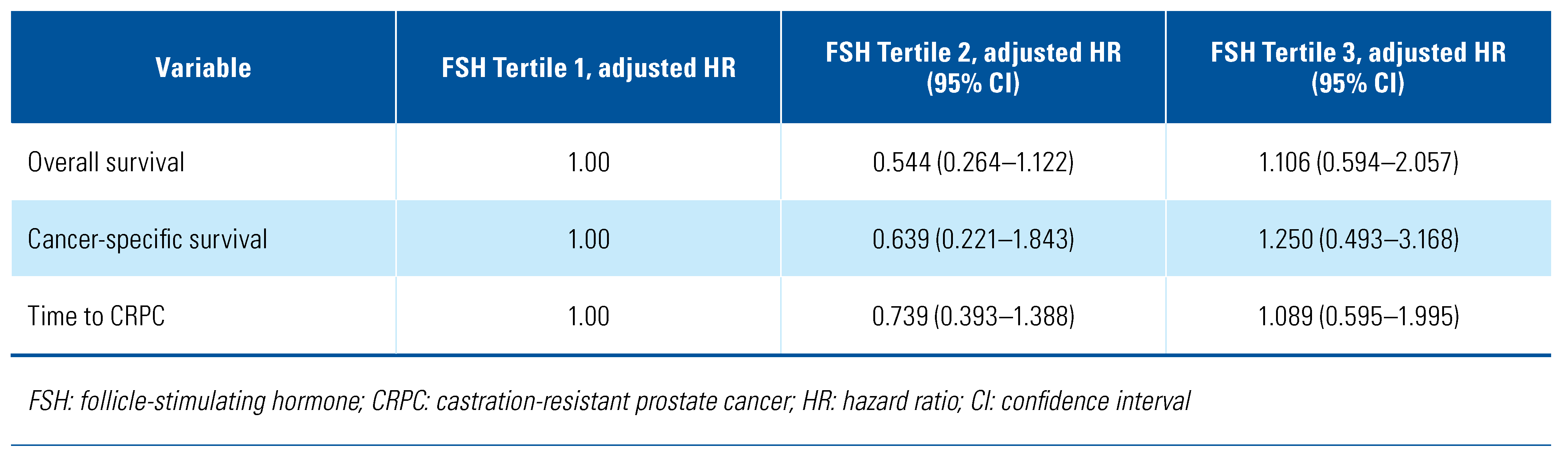
Univariate and multivariate Cox regression analysis adjusted for age, Gleason score, baseline PSA, time since radiotherapy, and ECOG status found no significant association between FSH tertile and OS, CSS, or time to development of CRPC (Figure 1, Table 2).
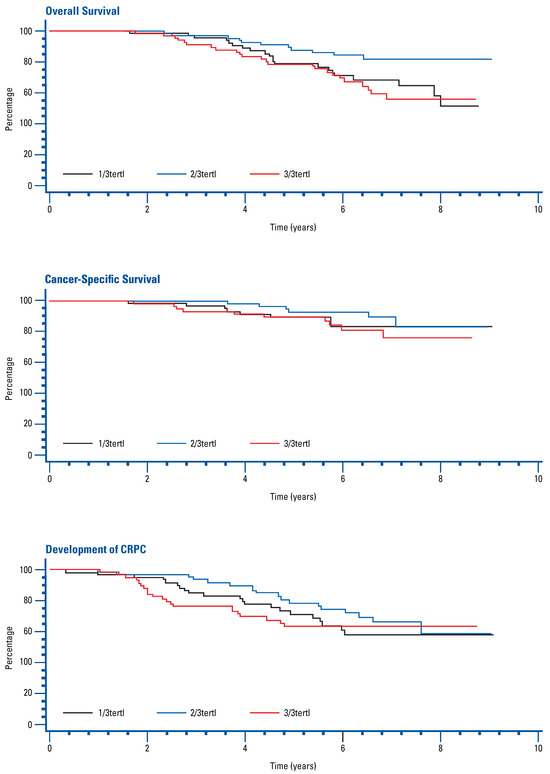
Figure 1.
Kaplan-Meier curves for overall survival, cancer-specific survival, and development of CRPC by FSH tertile.

Table 2.
Association between FSH tertile and long-term clinical outcomes.
For the subset of 100 patients with 3 annual FSH measurements after randomization, median and maximum FSH levels were similarly analyzed, but no statistically significant association was found. Lastly, within the latter subset of patients, a comparison of patients with increasing FSH levels (≥30% from baseline) with those who had no increase (<30%) yielded no significant association with the clinical outcomes.
4. Discussion
The role of FSH in prostate cancer clinical outcomes and comorbidities during ADT is not yet fully understood. In this analysis, we were not able to demonstrate a significant association between circulating FSH levels during ADT and OS, CSS, or time to development of CRPC.
Given that low testosterone levels before radical prostatectomy have been associated with poor prognosis [16,17,18,19], it would be reasonable to hypothesize that physiologic feedback increase in FSH might also be a marker for adverse disease course. However, a recent retrospective study of 492 patients showed no association between FSH levels and long-term oncologic and cardiovascular outcomes [14]. Although several studies found some evidence for preoperative FSH levels as a prognostic marker for more aggressive disease in patients undergoing radical prostatectomy [4,5,20], we found no differences in long-term clinical outcomes for advanced prostate cancer under ADT. Few studies have directly investigated the prognostic value of FSH levels during ADT. For example, a prior study of patients treated with ADT reported that higher FSH levels may predict shorter time to development of CRPC [6]. Nonetheless, FSH measurement in this retrospective cohort occurred in less than a third of patients, raising the potential for selection bias. Our contradictory results feature a greater number of patients, a lower proportion of patients who later developed CRPC, and longer follow-up.
Our study has a reasonably sized, multi-centric cohort of patients with long-term clinical follow-up from a robust clinical trial. Limitations intrinsic to the cohort of the clinical trial are discussed elsewhere [21]. However, the limitations of this study include the fact that only one FSH measurement was available for the main analysis 1 year into ADT and 3 measurements over 3 years for a subset of patients. There was also no standardization of the time of sampling during the day, which may have small effects on circulating FSH levels due to circadian rhythms. No details were available on whether patients had medical comorbidities that might have affected FSH production. Finally, the number of events limits the power to detect differences between the FSH tertiles.
Accumulating research comparing GnRH agonists with antagonists suggests GnRH antagonists are associated with lower overall mortality and cardiovascular events [22]. Superior FSH suppression has been hypothesized to play an important role in this comparison. Nonetheless, our results suggest FSH values following ADT with GnRH agonists do not predict survival. Thus, our results cast doubt on whether FSH levels are causative in any relationship between GnRH antagonists and cardiovascular outcomes. This is concordant with the recent report by Kourbanhoussen et al. [14].
In summary, this retrospective analysis does not suggest that circulating FSH levels during ADT for recurrent prostate cancer predict clinical outcomes. Further clinical research is warranted to better understand the role of FSH level in prostate cancer patients.
Funding
This project was funded by a Fonds de Recherche du Québec – Santé Clinician-Scientist Award (#32774) and a Prostate Cancer Canada Movember Discovery Grant (D2016-1393).
Conflicts of Interest
Paul Toren reports research funding from Bristol-Myers-Squibb and Janssen, as well as personal fees as a consultant from Sanofi, Ferring, Astellas, Bayer, and Abbvie.
Abbreviations
| ADT | androgen deprivation therapy |
| CRPC | castration-resistant prostate cancer CSS cancer-specific survival |
| CSS | cancer-specific survival |
| FSH | follicle-stimulating hormone |
| GnRH | gonadotropin-releasing hormone OS overall survival |
| OS | overall survival |
References
- Crawford, E.D.; Rove, K.O.; Schally, A.V. The role of the FSH system in the development and progression of prostate cancer. Am. J. Hematol. Oncol. 2014, 10, 5–13. [Google Scholar]
- Radu, A.; Pichon, C.; Camparo, P.; Antoine, M.; Allory, Y.; Couvelard, A.; et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N. Engl. J. Med. 2010, 363, 1621–1630. [Google Scholar] [CrossRef]
- Siraj, A.; Desestret, V.; Antoine, M.; Fromont, G.; Huerre, M.; Sanson, M.; et al. Expression of follicle stimulating hormone receptor by the vascular endothelium in tumor metastases. BMC Cancer 2013, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, A.B.; Siracusano, S.; de Luyk, N.; Corsi, P.; Sebben, M.; Tafuri, A.; et al. Simultaneous measurements of follicle stimulating hormone and total testosterone and associations in clinically localized prostate cancer. Curr. Urol. 2017, 10, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Terado, Y.; Sakamaki, K.; Inoue, M.; Nakajima, A.; Lu, Y.; et al. Serum level of follicle-stimulating hormone is associated with extraprostatic extension of prostate cancer. Prostate Int. 2013, 1, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Hoare, D.; Skinner, T.A.A.; Black, A.; Siemens, R.D. Serum follicle-stimulating hormone levels predict time to development of castration-resistant prostate cancer. Can. Urol. Assoc. J. 2015, 9, 122–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carneiro, A.; Sasse, A.D.; Wagner, A.A.; Peixoto, G.; Kataguiri, A.; Neto, A.S.; et al. Cardiovascular events associated with androgen deprivation therapy in patients with prostate cancer: A systematic review and meta-analysis. World J. Urol. 2015, 33, 1281–1289. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Mahar, A.L.; Satkunasivam, R.; Herschorn, S.; Kodama, R.T.; Lee, Y.; et al. Cardiovascular and skeletal-related events following localized prostate cancer treatment: Role of surgery, radiotherapy, and androgen deprivation. Urology 2016, 97, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Schally, A.V.; Pinthus, J.H.; Block, N.L.; Rick, F.G.; Garnick, M.B.; et al. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol. Oncol. 2017, 35, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Schally, A.V. The role of FSH and LH in prostate cancer and cardiometabolic comorbidities. Can. J. Urol. 2020, 27, 10167–10173. [Google Scholar] [PubMed]
- Margel, D.; Peer, A.; Ber, Y.; Shavit-Grievink, L.; Tabachnik, T.; Sela, S.; et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J. Urol. 2019, 202, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D.; Saad, F.; Cookson, M.S.; George, D.J.; Saltzstein, D.R.; Tutrone, R.; et al. , for the HERO Study Investigators. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N. Engl. J. Med. 2020, 382, 2187–2196. [Google Scholar] [CrossRef]
- Crawford, E.D.; Tombal, B.; Keane, T.; Boccardo, F.; Miller, K.; Shore, N.; et al. FSH suppression and tumour control in patients with prostate cancer during androgen deprivation with a GnRH agonist or antagonist. Scand. J. Urol. 2018, 52, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kourbanhoussen, K.; Joncas, F.-H.; Wallis, C.J.D.; Hovington, H.; Dagenais, F.; Fradet, Y.; et al. Follicle-stimulating hormone (FSH) levels prior to prostatectomy are not related to long-term oncologic or cardiovascular outcomes for men with prostate cancer. Asian J. Androl. 2021. [Google Scholar] [CrossRef]
- Crook, J.M.; O’Callaghan, C.J.; Duncan, -G.; Dearnaley, D.P.; Higano, C.S.; Horwitz, E.M.; et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N. Engl. J. Med. 2012, 367, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Xylinas, E.; Ploussard, G.; Durand, X.; Fabre, A.; Salomon, L.; Allory, Y.; et al. Low pretreatment total testosterone (<3 ng/mL) predicts extraprostatic disease in prostatectomy specimens from patients with preoperative localized prostate cancer. BJU Int. 2011, 107, 1400–1403. [Google Scholar] [CrossRef]
- Dai, B.; Qu, Y.; Kong, Y.; Ye, D.; Yao, X.; Zhang, S.; et al. Low pretreatment serum total testosterone is associated with a high incidence of Gleason score 8-10 disease in prostatectomy specimens: Data from ethnic Chinese patients with localized prostate cancer. BJU Int. 2012, 110, E667–E672. [Google Scholar] [CrossRef]
- García-Cruz, E.; Piqueras, M.; Huguet, J.; Peri, L.; Izquierdo, L.; Musquera, M.; et al. Low testosterone levels are related to poor prognosis factors in men with prostate cancer prior to treatment. BJU Int. 2012, 110, E541–546. [Google Scholar] [CrossRef]
- Llukani, E.; Katz, B.F.; Agalliu, I.; Lightfoot, A.; Yu, S.-J.S.; Kathrins, M.; Lee, Z.; et al. Low levels of serum testosterone in middle-aged men impact pathological features of prostate cancer. Prostate Int. 2017, 5, 17–23. [Google Scholar] [CrossRef]
- Heracek, J.; Urban, M.; Sachova, J.; Kuncova, J.; Eis, V.; Mandys, V.; et al. The endocrine profiles in men with localized and locally advanced prostate cancer treated with radical prostatectomy. Neuro Endocrinol. Lett. 2007, 28, 45–51. [Google Scholar]
- Klotz, L.; O’Callaghan, C.; Ding, K.; Toren, P.; Dearnaley, D.; Higano, C.S.; Horwitz, E.; et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: A secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J. Clin. Oncol. 2015, 33, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Abufaraj, M.; Iwata, T.; Kimura, S.; Haddad, A.; Al-Ani, H.; Abusubaih, L.; et al. Differential Impact of Gonadotropin-releasing Hormone Antagonist Versus Agonist on Clinical Safety and Oncologic Outcomes on Patients with Metastatic Prostate Cancer: A Meta-analysis of Randomized Controlled Trials. Eur. Urol. 2020. [Google Scholar] [CrossRef] [PubMed]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.