Abstract
Objectives To compare the performance of micro-ultrasound (mUS) with multi-parametric magnetic resonance imaging (mpMRI) in detecting clinically significant prostate cancer. Materials and Methods Retrospective data from consecutive patients with any indication for prostate biopsy in 2 academic institutions were included. The operator, blinded to mpMRI, would first scan the prostate and annotate any mUS lesions. All mUS lesions were biopsied. Any mpMRI lesions that did not correspond to mUS lesion upon unblinding were additionally biopsied. Grade group (GG) ≥ 2 was considered clinically significant cancer. The Jeffreys interval method was used to compare performance of mUS with mpMRI with the non-inferiority limit set at −5%. Results Imaging and biopsy were performed in 82 patients with 153 lesions. mUS had similar sensitivity to mpMRI (per-lesion analysis: 78.4% versus 72.5%), but lower specificity, positive predictive value, negative predictive value, and area under the curve. Micro-ultrasound found GG ≥ 2 in 13% of cases missed by mpMRI, while mpMRI found GG ≥ 2 in 11% of cases missed by mUS. The difference 0.020 (95% CI −0.070 to 0.110) was not statistically significant (P = 0.33). Conclusion The sensitivity of mUS in detecting GG ≥ 2 disease was similar to that of mpMRI, but the specificity was lower. Further evaluation with a larger sample size and experienced operators is warranted.
Introduction
Prostate biopsies are usually guided by transrectal ultrasound (TRUS) platforms that operate at 6 to 9 MHz [1]. Systematic biopsies of the prostate, the mainstay of prostate cancer diagnosis for the last 30 years, have many limitations, including both under-diagnosis of significant cancer and the identification of many men with clinically insignificant cancer. Multi-parametric magnetic resonance imaging (mpMRI) detects clinically significant cancer on the basis of alterations in cell density and ductal anatomy [2,3,4]. The Prostate Imaging Reporting and Data System (PI-RADS 2.0) was developed to standardize interpretation and reporting of mpMRI findings [5].
Many national guidelines now recommend mpMRI before TRUS biopsy [6,7]. Multi-parametric MRI with targeted biopsy appears to reduce the number of men requiring a biopsy and the over-detection of clinically insignificant cancer without compromising significant cancer detection [2,8]. However, in many regions of the world access to mpMRI is limited, and performing an mpMRI in all men at risk is not feasible. Multi-parametric MRI before biopsy also means 2 procedures, requiring that the patient to return for fusion targeted biopsy if suspicious lesions are detected.
To address these limitations, a novel micro-ultrasound (mUS) platform that operates at 29MHz was developed. This system generates high-resolution (70µ) images of the prostate. This resolution, compared with 200µ in conventional ultrasound, is the diameter of prostatic ducts, and therefore detects alterations in ductal anatomy. This offers the opportunity for improved detection of high-grade cancers characterized by loss of normal acinar lumens and tighter cellular packing. As with PI-RADS for mpMRI, a prostate risk identification using mUS (PRI-MUS) protocol has been developed to standardize sonographic lesions [9]. In addition to superior resolution, this technology offers the convenience of conventional ultrasound such as real-time imaging and targeted biopsy during the same procedure, office-based set-up, relatively easy access compared with MRI, and considerably less expensive equipment. The mUS platform is novel, and evidence regarding its accuracy in prostate cancer diagnosis is lacking. This study compares the performance of mUS in detecting clinically significant prostate cancer with that of mpMRI.
Materials and Methods
Inclusion and Exclusion Criteria
Data for this study were retrospectively gathered from electronic health records. We included consecutive patients between April 2019 and April 2020 who had any indication for prostate biopsy: (1) elevated PSA, (2) abnormal digital rectal examination (DRE), and/or (3) any suspicious mpMRI lesions. All patients must have had an mpMRI of the prostate before biopsy within the last year. mpMRIs were 3T with an abdominal coil. Data were obtained from Sunnybrook Health Sciences Centre and Johns Hopkins University School of Medicine. These procedures were performed as a standard of care, and research ethics board approval was not required or sought for the study.
Procedure
A single urologist who had been trained on the device performed the TRUS biopsies or provided direct supervision of the fellow. All patients were prepared with prophylactic antibiotics and enema and placed in the left lateral position as for standard TRUS biopsy. TRUS was performed using the mUS platform that operated at 29 MHz with a side-firing mUS probe. Critically, for the patients in this study, high-resolution ultrasound was performed and formally annotated with the operator scrupulously blinded to the patient’s prostate cancer history and mpMRI findings.
The prostate gland was measured and scanned for any visible target lesions. TRUS abnormalities were formally annotated and given a PRI-MUS score. The TRUS annotation was “locked” before the mpMRI findings were reviewed. One percent lignocaine was then injected into the prostate-seminal vesicle angle as local anaesthesia. The operator then reviewed the mpMRI. The locations of visible mpMRI lesions were identified from the radiologist’s report. Targeted samples of every mUS and mpMRI lesions were performed. In most cases, 3 cores were taken of each region of interest. Cognitive targeting was performed on mpMRI-only visible lesions. We did not perform systematic biopsy.
Analysis
Clinically significant prostate cancer was defined as GG ≥ 2. Per target lesion and per-patient analyses were performed. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver operating characteristic (ROC) curve were used as performance measures for mUS and mpMRI. The added value of mUS was the number of cases with PRI-MUS ≥ 3 and GG ≥ 2 in which mpMRI was PI-RADS < 3, or PI-RADS ≥ 3 and GG < 2. The added value of mpMRI was the number of cases with PI-RADS ≥ 3 and GG ≥ 2 in which mUS was PRI-MUS < 3, or PRI-MUS ≥ 3 and GG < 2. The Jeffreys interval method was used to compare the performance of mUS with that of mpMRI, with the non-inferiority limit set at −5%. P ≤ 0.05 was considered significant. All statistical calculations were performed using IBM SPSS Statistics Version 26.
Results
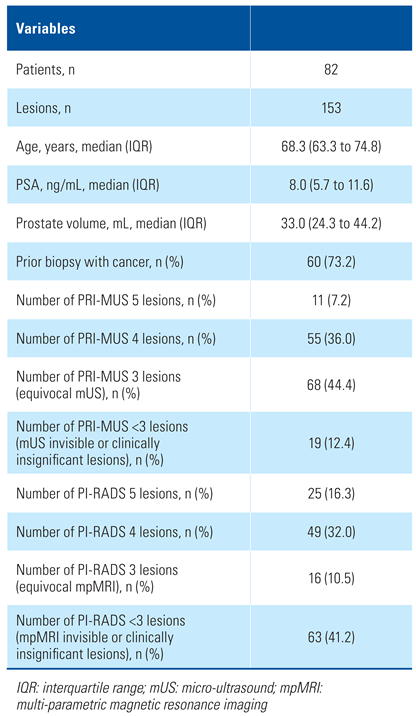
Between April 2019 and April 2020, imaging and biopsy were performed in 82 patients with 153 lesions. GG ≥ 2 prostate cancer was diagnosed in 33 patients (40.2%) and 51 (33.3%) of the biopsied lesions. The median age was 68.3 (IQR = 63.3 to 74.8) years, and median PSA was 8.0 (IQR = 5.7 to 11.6) ng/mL. Sixty patients (73.2%) had been diagnosed with prostate cancer in the past. Among them, 39 patients (65%) were on active surveillance, and 21 patients (35%) were being followed up after prior focal therapy. Table 1 shows the breakdown of normal, equivocal, and suspicious mpMRI and mUS studies.

Table 1.
Patient demographics.
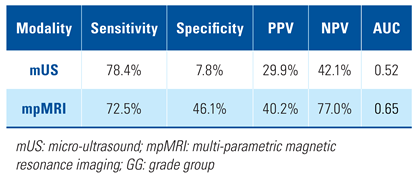
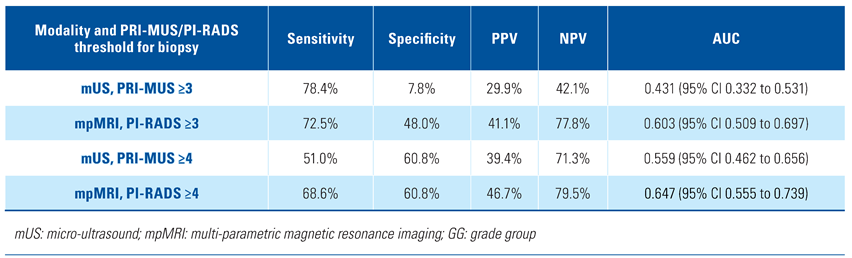
The per-lesion analysis of mUS and mpMRI performance in detection of GG ≥ 2 prostate cancer showed similar sensitivity with mUS (78.4%) and mpMRI (72.5%). There was concordance in 73 lesions (47.7%) between mUS and mpMRI, ie, visible on both imaging modalities. The specificity, PPV, NPV, and AUC of mUS were lower than mpMRI (Table 2). When the threshold for biopsy was set at PRI-MUS/PI-RADS ≥ 3, mUS maintained similar sensitivity but lower specificity, PPV, NPV, and AUC than mpMRI. However, when the threshold for biopsy was set at PRI-MUS/PI-RADS ≥ 4, mpMRI showed better sensitivity, PPV, NPV, and AUC than mUS. The specificity of the 2 imaging modalities was equal in this case at 60.8% (Table 3).

Table 2.
Per-lesion analysis of performance metrics comparing mUS and mpMRI for detection of GG ≥ 2 PCa.

Table 3.
Per-lesion analysis of performance metrics comparing mUS and mpMRI for detection of GG ≥ 2 PCa at various PRI-MUS/PI-RADS thresholds for biopsy.
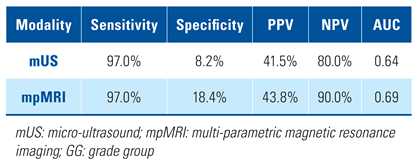
In per-patient analysis, the sensitivity of mUS was again similar to that of mpMRI (97.0% versus 97.0%). The specificity, PPV, NPV, and AUC of mUS in per-patient analysis were lower than mpMRI (Table 4). Micro-ultrasound found GG ≥ 2 in 13% of cases missed by mpMRI while mpMRI found GG ≥ 2 in 11% of cases missed by mUS. The difference 0.020 (95% CI −0.070 to 0.110) was not statistically significant (P = 0.33).

Table 4.
Per-patient analysis of performance metrics comparing mUS and mpMRI for detection of GG ≥ 2 PCa.
Twenty-one lesions (13.7%) detected by mUS in this study were located anteriorly. Seven of these (33.3%) were clinically significant cancers. One (14.3%) was not detected by mpMRI. Twenty-five mpMRI lesions (16.3%) were labelled as anterior. Eleven (44.0%) were clinically significant cancer, and 1 (9.1%) was missed by mUS.
Discussion
Micro-ultrasound technology that offers high-resolution real-time images of the prostate has the potential to enhance prostate cancer diagnosis. Little has been published on the performance of mUS compared with mpMRI. Preliminary studies demonstrate comparable sensitivity and specificity to mpMRI [10,11,12,13,14]. A limitation of many of these studies is the lack of rigorous blinding of the mpMRI results when interpreting the mUS. The results of this current study represent an early comparative experience with mUS blinded to mpMRI at 2 academic institutions.
Our awareness of the learning curve mandated an aggressive approach to identifying and biopsying subtle abnormalities to minimize the risk of missing significant cancer. This resulted in a small number of biopsied locations, n = 19 (12.4%) assigned as invisible (PRI-MUS < 3). In contrast, 40% of biopsied lesions were assigned PI-RADS < 3 on mpMRI. In a series reported by a highly experienced mpMRI centre, the proportion of non-suspicious mpMRI cases was as high as 49%, and equivocal cases were only 6% [15]. We biopsied considerably more equivocal mUS lesions (PRI-MUS 3) n = 68 (44.4%) compared with only 16 (10.5%) PI-RADS 3 mpMRI lesions. This is likely because this is an early experience with the mUS technology, and the operators were anxious not to miss cancers. It is likely that the low specificity will improve with experience, as clinicians become more familiar with non-significant mUS patterns and acquire the confidence to exclude these from biopsy. Refraining from biopsy of smaller PRI-MUS 3 lesions will facilitate this.
This inclusive strategy resulted in a comparable sensitivity to mpMRI in the detection of GG ≥ 2 disease (78.4% versus 72.5%) in the per-lesion analysis. This was also apparent in the per-patient analysis in which the sensitivity of mUS was 97.0% compared with 97.0% for mpMRI, using a biopsy threshold of PRI-MUS/PI-RADS ≥ 3. This approach with mUS resulted in low specificity (7.8%) with an AUC of 0.52 in per-lesion analysis. Per-patient analysis also showed low specificity (8.2%) and AUC 0.64 at a biopsy threshold of PRI-MUS ≥ 3. Despite the difference in specificity, the AUCs of mUS and mpMRI were similar. This trend of high sensitivity but low specificity with mUS was also apparent in another contemporary study [16]. Importantly, we demonstrated that mUS was able to detect GG ≥ 2 in 13% of cases not diagnosed by mpMRI. Wiemer et al. analyzed 159 patients and found 20 (12.6%) patients who were negative on targeted mpMRI-guided biopsy had in fact harboured clinically significant prostate cancer when biopsied with mUS guidance [17]. Their finding in this aspect was very similar to our result.
About 44% of the mUS scans were categorized as PRI-MUS 3. Among the 68 PRI-MUS 3 lesions, 14 (20.6%) were found to be significant cancers. In contrast, only 2 (12.5%) of the PI-RADS 3 lesions showed clinically significant cancer. Much as the PI-RADS grading system has been repeatedly modified over the last decade, the low specificity of PRI-MUS 3 in this series suggests that further refinement of the PRI-MUS 3 pattern is warranted.
For example, MRI studies published in the years 1985 to 1993 showed the AUC for seminal vesicle invasion (SVI) was 0.57 ± 0.25. Subsequent articles published between 1993 and 2001 described an AUC for SVI of 0.64 ± 0.21 [18]. In a later study, the AUC for tumour localization ranged from 0.72 to 0.83 with mpMRI [19]. The AUC for GG ≥ 2 prostate cancer in the present study was 0.647 (95% CI 0.555 to 0.739) if PI-RADS ≥ 4 lesions were biopsied. Improved accuracy of mpMRI can be attributed to better technology and image quality, as well as greater individual and collective experience in image interpretation and refinement of the criteria for each score.
There are concerns that mUS may not detect anterior tumours effectively because of the reduced depth of tissue visualization at higher ultrasound frequencies. This is a major limiting factor only in men with marked prostatomegaly. We did not encounter this problem in the present study. Among the 7 anterior mUS lesions with GG ≥ 2 prostate cancer, 1 was missed by mpMRI. Micro-ultrasound did not detect 1 of 11 GG ≥ 2 prostate cancer that were anteriorly located on the basis of mpMRI findings. Therefore, it appears that mUS and mpMRI were similar in their ability to diagnose anterior tumours. A recent publication demonstrated that mUS was also compatible with the transperineal biopsy approach [20]. This method could be considered if there are anterior lesions that are harder to reach via the transrectal route.
The key strength of this study, compared with most other studies, was that the operators were blinded to mpMRI findings prior to mUS. This approach gave us an unbiased reflection of the mUS performance.
The study had limitations. All patients had at least 1 target lesion seen on mUS and/or mpMRI. Patients with both negative mpMRI and mUS were excluded from the analysis, since in most cases they did not have a biopsy. Therefore, the true NPV for significant cancer cannot be accurately estimated.
Sixty patients (73.2%) had prior diagnosis of prostate cancer. Therefore, the results of this study might not reflect the performance of mUS in men who have only clinical suspicion of cancer. Although we included 22 patients who did not have prior prostate cancer, this number was too small to yield a reliable conclusion from this sub-group.
Patients in this cohort had targeted biopsies only, without systematic biopsies. The role of systematic biopsies in men having targeted biopsies is evolving, and recent data emphasize the importance of systematic biopsies in higher risk patients. Had systematic biopsies been performed, prostate cancer would undoubtedly have been found in some of the patients in this trial who had negative targeted biopsies.
Blinding to the mpMRI results before annotating the ultrasound findings was self-imposed by the clinicians performing the biopsy. Having one clinician document the mUS findings and a second clinician performing the biopsy after reviewing the mpMRI findings would have enhanced this process, but it was not feasible. In addition, the mpMRI lesions were not targeted with image fusion technology. It should be taken into consideration when interpretating the results of this study.
Conclusion
Micro-ultrasound is an appealing alternative to mpMRI by virtue of reduced cost, complexity, and absence of contrast requirement. In this study, the sensitivity of mUS in detecting clinically significant prostate cancer was found to be similar to that of mpMRI. The specificity of mUS was found to be lower than MRI. Further evaluation with a larger sample size and operators who have surmounted the learning curve is warranted.
Conflicts of Interest
None declared.
Abbreviations
| AUC | area under the curve GG grade group |
| mpMRI | multi-parametric magnetic resonance imaging mUS micro-ultrasound |
| NPV | negative predictive value |
| PI-RADS | Prostate Imaging Reporting and Data System PPV positive predictive value |
| PRI-MUS | prostate risk identification using micro-ultrasound TRUS transrectal ultrasound |
References
- Rohrbach, D.; Wodlinger, B.; Wen, J.; Mamou, J.; Feleppa, E. High-frequency quantitative ultrasound for imaging prostate cancer using a novel micro-ultrasound scanner. Ultrasound Med. Biol. 2018, 44, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; et al. Diagnostic accuracy of multiparametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet. 2017, 389, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Brown, A.M.; Sankineni, S.; Wood, B.J.; Pinto, P.A.; Choyke, P.L. Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. C A Cancer J. Clin 2016, 66, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Mowatt, G.; Scotland, G.; Boachie, C.; Cruickshank, M.; Ford, J.A.; Fraser, C.; et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: A systematic review and economic evaluation. Health Technol. Assess. 2013, 17, 1–281. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Choyke, P.L. Pirads 2.0: What is new? Diagnostic Interv. Radiol. 2015, 21, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; De Santis, M.; Gillessen, S.; Grummet, J.; et al. EAU – EANM – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. Available online: https://uroweb.org/wp-content/ uploads/EAU-EANM-ESUR-ESTRO-SIOG-Guidelines-on-Prostate Cancer-2019.pdf (accessed on 11 June 2020).
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; et al. NCCN clinical practice guidelines in oncology. prostate cancer V2.2020. Available online: https://www.nccn.org/professionals/ physician_gls/pdf/prostate_detection.pdf (accessed on 11 June 2020). [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; et al.; for the PRECISION Study Group Collaborators MRI-targeted or standard biopsy for prostate-cancer diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Eure, G.; Fradet, V.; Hyndman, M.E.; McGrath, T.; Wodlinger, B.; et al. Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: Creation of the micro-ultrasound protocol for prostate risk identification. J. Urol. 2016, 196, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Eure, G.; Fanney, D.; Lin, J.; Wodlinger, B.; Ghai, S. Comparison of conventional transrectal ultrasound, magnetic resonance imaging, and micro-ultrasound for visualizing prostate cancer in an active surveillance population: A feasibility study. Can. Urol. Assoc. J. 2019, 13, E70–E77. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Saita, A.; Lazzeri, M.; Paciotti, M.; Maffei, D.; Lista, G.; et al. Comparison of the diagnostic accuracy of micro-ultrasound and magnetic resonance imaging/ultrasound fusion targeted biopsies for the diagnosis of clinically significant prostate cancer. Eur. Urol. Oncol. 2019, 2, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Claros, O.R.; Tourinho-Barbosa, R.R.; Fregeville, A.; Gallardo, A.C.; Muttin, F.; Carneiro, A.; et al. comparison of initial experience with transrectal magnetic resonance imaging cognitive guided micro-ultrasound biopsies versus established transperineal robotic ultrasound magnetic resonance imaging fusion biopsies for prostate cancer. J. Urol. 2020, 203, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Socarrás, M.E.R.; Rivas, J.G.; Rivera, V.C.; Elbers, J.R.; González, L.L.; Mercado, I.M.; et al. Prostate mapping for cancer diagnosis: The Madrid protocol. Transperineal prostate biopsies using mpMRI fusion and micro ultrasound guided biopsies. J. Urol. 2020, 204, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Cornud, F.; Lefevre, A.; Flam, T.; Dumonceau, O.; Galiano, M.; Soyer, P.; et al. MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: Initial results of a single-center study. Eur. Radiol. 2020, 30, 4838–4846. [Google Scholar] [CrossRef] [PubMed]
- van der Leest, M.; Erik Cornel, E.; Bas Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: A large prospective multicenter clinical study. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Cornud, F.; Lefevre, A.; Flam, T.; Dumonceau, O.; Galiano, M.; Soyer, P.; et al. MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: Initial results of a single-center study. Eur. Radiol. 2020, 30, 4838–4846. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, L.; Hollenbach, M.; Heckmann, R.; Kittner, B.; Plage, H.; Reimann, M.; et al. Evolution of Targeted Prostate Biopsy by Adding Micro Ultrasound to the Magnetic Resonance Imaging Pathway. Eur. Urol. Focus. 2020, 7, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, M.R.; Jager, G.J.; Laheij, R.J.; Verbeek, A.L.M.; van Lier, H.J.; Barentsz, J.O.; et al. Local staging of prostate cancer using magnetic resonance imaging: A meta-analysis. Eur. Radiol. 2002, 12, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Mullerad, M.; Hricak, H.; Kuroiwa, K.; Pucar, D.; Chen, H.-N.; Kattan, M.W.; et al. Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J. Urol. 2005, 174, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Socarrás, M.E.; Gomez Rivas, J.; Cuadros Rivera, V.; Reinoso Elbers, J.; Llanes González, L.; Michel Mercado, I.; et al. Prostate mapping for cancer diagnosis: The Madrid protocol. transperineal prostate biopsies using multiparametric magnetic resonance imaging fusion and micro-ultrasound guided biopsies. J. Urol. 2020, 204, 726–733. [Google Scholar] [CrossRef] [PubMed]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.