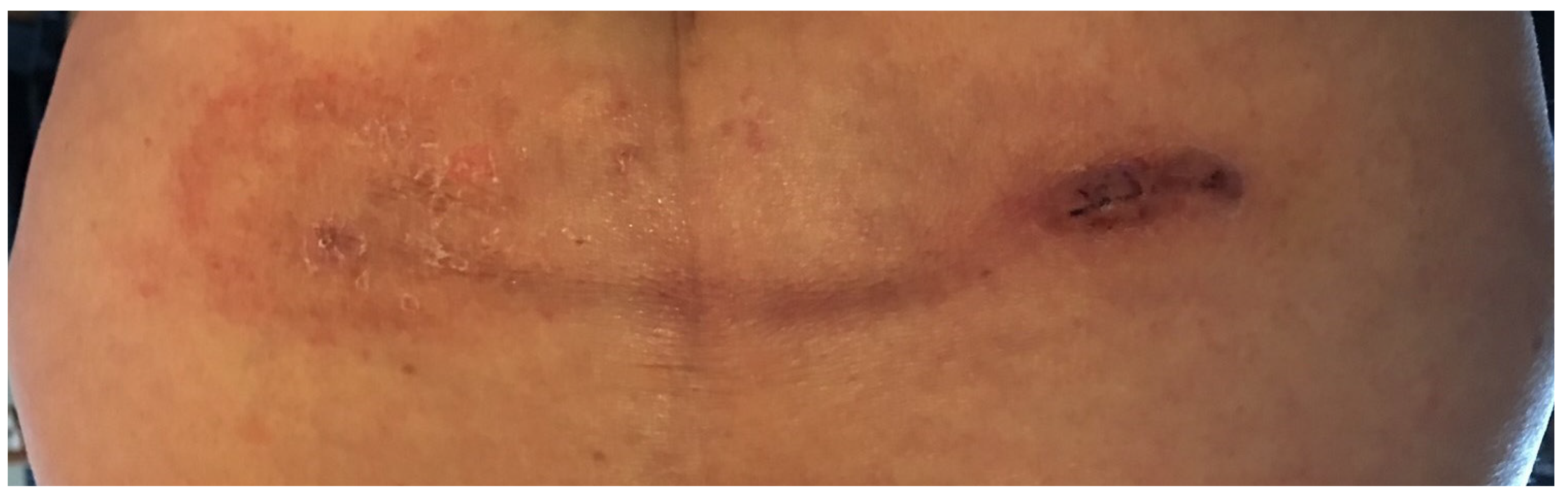
A 77-year-old female with history of lymphoma status post radiation therapy presented with approximately 10 years of intermittent urinary retention, managed with clean intermittent catheterization. Urodynamic studies revealed minimal detrusor contraction (3 cm H2O) and a low amplitude, interrupted flow curve with maximum flow 9.5 mL/sec, average flow 2 mL/second, and appropriate EMG silencing. Cystoscopy was within normal limits. We proceeded with staged placement of Medtronic InterStim device. After stage 1 placement of the sacral neuromodulation device, the patient reported voiding larger volumes with less straining (an approximate 80% subjective improvement), denied further episodes of urinary retention, and her PVR improved from between 100 mL and 300 mL to 50 mL. However, she rapidly developed an erythematous skin reaction overlying the device lead (Figure 1). Physical examination revealed a discrete area of erythema overlying the neuromodulation lead from percutaneous entrance to exit site. The initial differential diagnosis included infection versus inflammatory response. Due to concern for infection, the device was promptly removed, at which time no purulence was noted along the course of the device components. Subsequent patch allergy testing revealed a strong reaction to vanadium, which is often used in titanium alloy, an external component of the InterStim implant. Both newer generation Medtronic and Axonics devices contain this titanium alloy; therefore, because of allergic intolerance to all available sacral neuromodulation devices, the patient’s urinary symptoms were subsequently managed with percutaneous tibial nerve stimulation. While there are documented cases of adverse events after sacral neuromodulation including device migration [1,2], device infection [3], and gluteal hematoma [4], to our knowledge there are no cases in the literature describing a hypersensitivity to sacral neuromodulation devices. The American Contact Dermatitis Society does not recommend preoperative hypersensitivity evaluation for patients undergoing hardware implantation but does recommend a risk-benefit analysis when considering device re-implantation in patients who demonstrate hypersensitivity to a metal implant [5].
Figure 1.
Conflicts of Interest
None declared. Patient Consent: Obtained.
References
- Karapanos, L.; Chon, S.H.; Kokx, R.; Schmautz, M.; Heidenreich, A. A rare case of tined lead migration of InterStim device into the rectum with subsequent novel combined surgical-endoscopic removal technique. Turk. J. Urol. 2020, 46, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Negron, J.M.; Derisavifard, S.; Goldman, H.B. Sacral neuromodulation lead twisting causes migration and loss of efficacy. Female Pelvic Med. Reconstr. Surg. 2020, 26, e13–e15. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.H.; Grewal, S. Bacterial colonization rate of InterStim and infection outcome with staged testing. Urology 2013, 82, 1255–1260. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Mahdy, A. Extensive gluteal hematoma following InterStim implant: A case report. Int. Urogynecol. J. 2012, 23, 1805–1807. [Google Scholar] [CrossRef] [PubMed]
- Schalock, P.C.; Crawford, G.; Nedorost, S.; Scheinman, P.L.; Atwater, A.R.; Mowad, C.; et al. Patching testing for evaluation of hypersensitivity to implanted metal devices: A perspective from the American Contact Dermatitis Society. Dermatitis 2016, 27, 241–247. [Google Scholar] [CrossRef]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.