Abstract
Objectives To report 1-year results of the ROBUST II study investigating the safety and efficacy of a paclitaxel-coated balloon for the treatment of recurrent urethral strictures. Methods Subjects were adult men with a single anterior urethral stricture ≤ 3 cm in length and at least 2 prior stricture treatments. After treatment with the Optilume urethral drug-coated balloon (DCB), subjects were followed through 1 year. The primary safety endpoint was the rate of treatment-related serious complications at 90 days post-procedure. Efficacy outcomes included symptomatic assessments, erectile function measured using the International Index of Erectile Function (IIEF), Qmax, and anatomic success. Results Sixteen men with an average of 4.1 prior dilations were treated with the DCB. Anatomic success was achieved at 6 months in 73%. Average IPSS improved from 18.4 to 6.0 at 1 year (P < 0.001). Qmax improved from 6.9 mL/sec to 20.8 mL/sec (P < 0.001). There was no change in IIEF. Four subjects received additional treatment within 1 year. There were no treatment-related serious complications. Conclusions Short-term follow-up of men with urethral stricture treated with the Optilume DCB showed durable anatomic results at 6 months and sustained symptomatic improvement through 1 year. Treatment with the device was safe.
Introduction
Urethral stricture disease occurs in approximately 0.6% of men [1]. Formation of scar tissue leads to narrowing of the urethral lumen resulting in obstructive lower urinary tract symptoms (LUTS) and associated morbidities that negatively impact patient quality of life [1]. Several treatment options are available for stricture, including rigid rod or balloon dilation, direct visual urethrotomy (DVIU), and urethroplasty [2]. Although dilation and DVIU are widely used for stricture treatment, durability is poor, with long-term stricture-free rates estimated between 8% and 30% after a single treatment [3,4,5]. Furthermore, multiple treatments of the same stricture lead to progressively worse outcomes, with success rates approaching 0% at 2 years after a third treatment. Urethroplasty is recommended for patients with recurrent strictures or strictures > 2 cm long and has reported success rates > 80% depending on approach and stricture characteristics [6].
Safe and durable minimally invasive solutions for stricture treatment are needed given the lack of long-term benefit from endoscopic procedures. The Optilume urethral drug-coated balloon (Urotronic, Inc., Plymouth, MN, US) is the first drug-coated balloon (DCB) developed for the management of urethral stricture disease. The device combines mechanical balloon dilation for immediate symptomatic relief with the localized delivery of paclitaxel to maintain long-term urethral patency. Paclitaxel acts to inhibit cell division thereby preventing new tissue growth and scar tissue formation that can lead to stricture recurrence. The Optilume DCB was initially evaluated in 53 patients in Latin America in the ROBUST I study, which showed symptomatic improvement through 2 years after treatment in men with urethral strictures ≤ 2 cm long and an average of 1.7 prior dilations [7,8]. At 2 years, 70% of subjects had an improvement in International Prostate Symptom Score (IPSS) of at least 50% without retreatment [7]. The ROBUST II trial was conducted to gain initial experience with the device in the United States and in patients with longer strictures (≤ 3 cm).
Materials and Methods
Study Design
ROBUST II is an industry sponsored prospective, multicenter, non-randomized, open label study designed to assess the safety and efficacy of the Optilume DCB for the treatment of anterior urethral stricture at 5 investigational sites in the United States (ClinicalTrials. gov: NCT03270384). Institutional review board approval was obtained for all study sites.
A baseline retrograde urethrogram was performed to obtain urethral and stricture measurements used to inform balloon size selection and define stricture characteristics. Balloons with diameters of 18F, 24F, and 30F and lengths of 30 and 50 mm were available for use. Per physician discretion, strictures were dilated directly with the Optilume DCB or pre-dilated with an uncoated balloon, rigid rods, or DVIU. The selected DCB was inflated to the rated burst pressure and held for at least 5 minutes (Figure 1). A 12F or 14F Foley catheter was inserted after treatment. Subject follow-up occurred at Foley removal (2 to 5 days), 30 days, 90 days, 6 months, and 1 year.
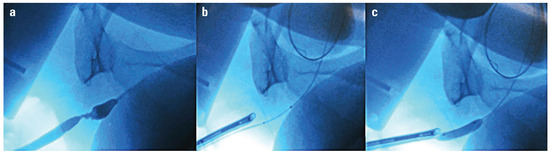
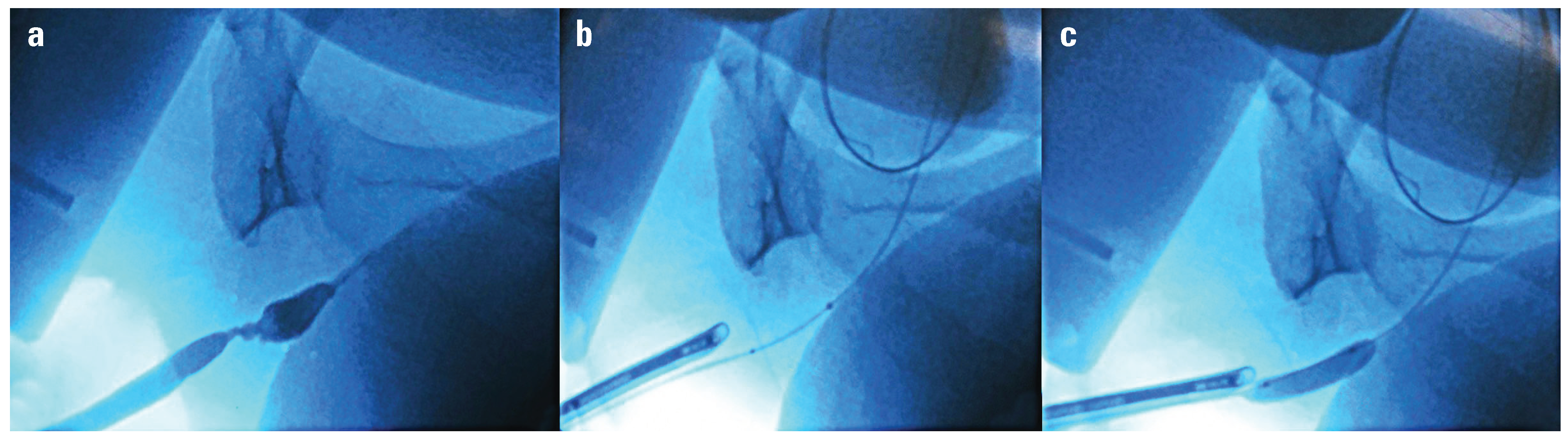
Figure 1.
DCB alignment utilizing fluoroscopy. a. Retrograde urethrogram showing bulbar stricture b. Markers visible across the stricture c. Balloon inflated across stricture.
Study Population
Subjects included adult men with a single anterior urethral stricture ≤ 3 cm in length with lumen diameter < 12F, at least 2 prior endoscopic treatments of the stricture, bothersome LUTS, IPSS ≥ 13, and peak urinary flow (Qmax) < 15 mL/sec. Self-catheterization was not considered a prior dilation. Patients were excluded if they had prior urethroplasty, radical prostatectomy, pelvic radiation, artificial urinary sphincter or urethral stent, or stricture dilation or incision within 6 weeks. Additional exclusions were diagnosis of lichen sclerosus, urinary stone passage within 6 weeks, chronic renal failure, neurogenic bladder, and history of carcinoma of the bladder or prostate within the last 5 years. Eight of the 16 subjects had exhibited stricture recurrence within 6 months prior to enrollment. Written informed consent was obtained from all subjects prior to study specific assessments.
Study Endpoints
The primary safety endpoint was the rate of treatment-related serious complications at 90 days defined as a composite of formation of fistula, new strictures requiring intervention, unresolved de novo stress urinary incontinence requiring > 1 pad/day, and urethral rupture. Any change in sexual function was evaluated using the “overall satisfaction” domain of the International Index of Erectile Function (IIEF).
Efficacy endpoints included IPSS, anatomic success at 6 months, a urethral stricture-specific patient-reported outcome measure (PROM) [9], Qmax, and freedom from repeat intervention. The IPSS responder rate was defined as the percent of subjects with ≥ 50% improvement in IPSS without repeat treatment. Anatomic success was assessed by the ability to pass a 16F flexible cystoscope through the treatment site. Subjects re-treated with the study device or who received other treatment for their stricture prior to the 6-month visit were considered failures for the anatomic success and repeat intervention endpoints.
Pain was assessed using the visual analog scale (VAS) before and after treatment. Adverse events were adjudicated by the study medical monitor.
Statistical Methods
An intent-to-treat analysis was performed for all endpoints using a complete case approach with no imputation for missing data. Descriptive statistics were used for data summaries including mean and standard deviation for continuous variables and percentages or proportions for categorical measures. The significance of improvements from baseline were assessed using a 2-sided Student t test, in which P < 0.05 indicated significance.
Results
The study enrolled 16 subjects from December 2017 to April 2019, and all were treated with the Optilume DCB. Nine subjects completed the 1-year follow-up visit. Four subjects exited prior to or at the 6-month visit (3 treatment failures, 1 withdrawn consent), and 3 did not complete the 1-year visit (2 due to COVID-19 pandemic and 1 was re-treated with the Optilume DCB just prior to the 1-year visit).
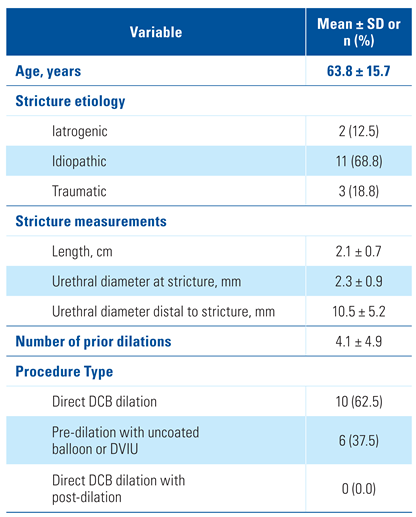
Baseline Characteristics and Treatment Procedure
The study cohort included men with an average age of 63.8 years and 4.1 prior dilations of the treated stricture (Table 1). Stricture etiology was idiopathic (68.8%), traumatic (18.8%), or iatrogenic (12.5%). All subjects had bulbar strictures with an average length of 2.1 cm and urethral diameter of 2.3 mm. Ten subjects underwent direct dilation with the Optilume DCB, and 6 were pre- dilated with an uncoated balloon or DVIU. The DCB diameter used was 30F in 14/16 subjects (87.5%) and 24F in 2/16 subjects (12.5%), per surgeon discretion. Subjects experienced minimal pain after the procedure, with mean VAS scores of 1.7 ± 2.3 at baseline, 2.0 ± 2.0 at treatment, 1.1 ± 1.2 at Foley removal, and 0.3 ± 0.6 at 30 days.

Table 1.
Baseline characteristics and procedure type.
Safety
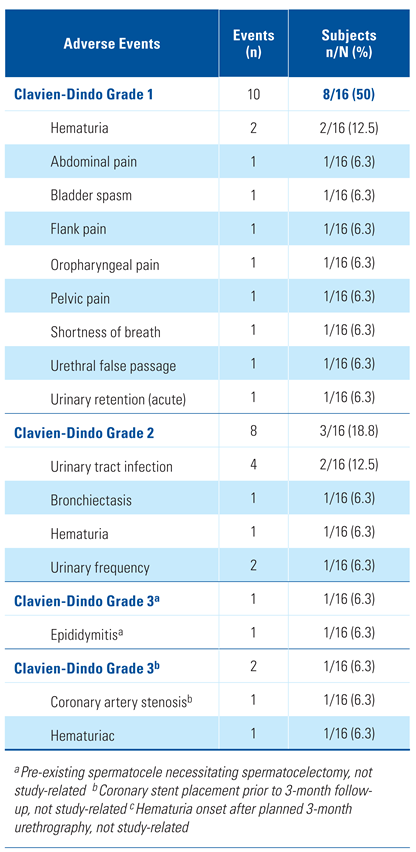
A total of 21 adverse events in 10 subjects were reported in the study, most frequently urinary tract infection in 12.5% (2/16) and hematuria in 18.8% (3/16). The majority of the events (85.7%, 18/21) were Clavien-Dindo grade I-II (Table 2). None of the subjects experienced a serious treatment-related complication through 90 days post-procedure (0/16, 0.0%). Grade III events included 1 event each of bronchiectasis, coronary artery stenosis, and hematuria (post 3-month urethrogram). All Grade III events resolved within 2 weeks of onset, and none were related to the study device. There were 4 device-related events: 2 mild events of hematuria, 1 case of mild bladder spasms, and 1 case of acute urinary retention within 24 hours of Foley catheter removal. All 4 events resolved without sequelae within a month of onset.

Table 2.
Adverse events.
There was no negative impact on sexual function through 1 year following treatment with the Optilume DCB (Table 2). The average IIEF score improved from 6.7 at baseline to 7.3 at 1 year (P = 0.596).
Efficacy
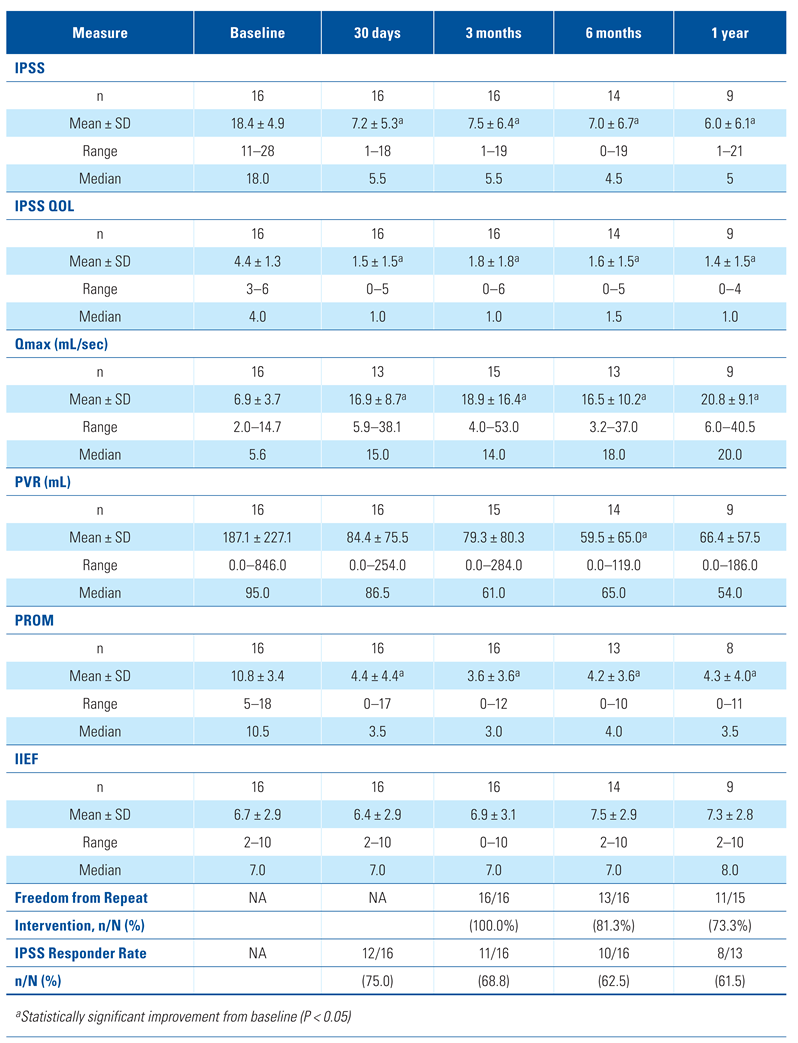
Efficacy outcome measures showed sustained improvement through 1 year post-procedure (Table 3). The average IPSS decreased from 18.4 at baseline to 7.5 at 90 days, 7.0 at 6 months, and 6.0 at 1 year (P < 0.001). The IPSS responder rate was 75.0% (12/16) at 30 days and 61.5% (8/13) at 1 year. The average PROM score also improved after the procedure, decreasing from 10.8 at baseline to 3.6 at 90 days, 4.2 at 6 months, and 4.3 at 1 year (P < 0.001). Quality of life as measured by IPSS QOL improved from 4.4 at baseline to 1.4 at 1 year (P < 0.001).

Table 3.
Results summary.
Voiding function measured by Qmax and post-void residual (PVR) urine volume also improved. Average peak urinary flow increased from 6.9 mL/sec at baseline to 18.9 mL/sec at 3 months, 16.5 mL/sec at 6 months, and 20.8 mL/sec at 1 year (P < 0.001). The PVR volume improved from 187.1 mL at baseline to 79.3 mL, 59.5 mL, and 66.4 mL at 3 months, 6 months, and 1 year respectively, although the decrease was not statistically significant (P = 0.134).
The anatomic success rate at 6 months was 73.3% (11/15). One subject did not have a cystoscopy performed at their 6-month visit and is considered missing for this analysis. Of the 13 subjects who completed the 6-month cystoscopy, 2 were considered failures. Two additional subjects were considered failures due to recurrence of their stricture requiring repeat treatment prior to the 6-month visit (1 re-treated with Optilume DCB, 1 urethroplasty). The rate of anatomic success was not significantly impacted by the decision to directly dilate with the DCB or pre-dilate prior to DCB. The subgroup of subjects treated with direct DCB exhibited an anatomic success rate of 77.8% (7/9), while the anatomic success rate in those subjects receiving pre-dilation with uncoated balloon or DVIU was 66.7% (4/6).
A total of 4 subjects received repeat treatment through 1 year, resulting in a rate of freedom from repeat intervention of 73.3% (11/15). Two subjects were re-treated with the DCB, and 2 subjects underwent urethroplasty.
Discussion
Results of the ROBUST II study showed that treatment of recurrent anterior urethral stricture with the minimally invasive Optilume DCB was safe and achieved durable anatomic results at 6 months, with sustained reduction in severity of LUTS through 1 year.
The study cohort represents a refractory population that is associated with poor endoscopic treatment outcomes. Subjects had moderate-to-severe LUTS at baseline with an IPSS of 18.4. The majority of subjects (81.3%) had strictures that were ≥ 2 cm long; for these patients, current guidelines recommend urethroplasty as initial treatment given low success rates of dilation or DVIU [6]. All subjects had at least 2 prior dilations of the treated stricture and an average of 4.1 prior interventions, meaning treatment with the Optilume DCB represented the fifth dilation of the stricture on average.
Symptom severity as measured by IPSS decreased to 6.0 at 1 year, indicating that subjects were experiencing mild symptoms post-treatment. This represents an average improvement in IPSS of 12.4 points (67.4%) and is clinically meaningful based on a minimal clinically important difference of 6 points for patients with severe LUTS [10]. Changes in the PROM score for anterior urethral stricture mirrored the IPSS, with an average of 10.8 at baseline improving to 4.3 at 1 year, representing a change of 60.2%. Improvement in subjective symptom measures were accompanied by improvement in objective measures of voiding function. The average Qmax improved 201.4%, from 6.9 mL/sec at baseline to 20.8 mL/sec at 1 year (P < 0.001). Peak flow at 1 year was higher than the 15 mL/sec typically used to define patients free from clinically significant stricture recurrence. Further, the observed reductions in PVR urine volume showed that more complete voiding could be achieved after treatment with the Optilume DCB.
Flexible cystoscopy demonstrated an anatomic success rate of 73% at 6 months. Despite these subjects having longer stricture lengths and a higher number of prior failed treatments, this was similar to the anatomic success rate previously reported in ROBUST I of 76% at the same time point [8]. In contrast, published success rates for dilation or urethrotomy after 3 repeat dilations is around 20% to 30% at 6 months, which decreases to 0% at 24 months [3,5]. Median time to stricture recurrence with endoscopic treatment is 6 to 12 months; for patients with 3 treatments, the median time to recurrence has been reported to be about 4.5 months [3]. Results for the initial 6-month period following treatment are critical because a hazard function analysis showed that the risk of stricture recurrence after endoscopic treatment is greatest at 6 months [11]. The 6-month timepoint was therefore chosen in this study to assess anatomic success.
Data collected to date support the safety of the device and procedure. Adverse events were generally mild and resolved shortly after onset. Most events were associated with urethral stricture disease or were common post urinary intervention. Erectile function was not affected by treatment.
The advantages of dilation or DVIU are hampered by low long-term success rates. Multiple groups have investigated the use of antifibrotic agents to augment traditional urethrotomy in an attempt to achieve the durability of open urethroplasty [12]. Two small randomized controlled trials have investigated urethrotomy with or without triamcinolone intralesional injection with mixed results; one study showed a lower rate of stricture recurrence in the triamcinolone treated group and the other study showed no difference between groups in the rate of stricture recurrence [13,14]. Mitomycin C as well as hyaluronic acid and carboxymethyl- cellulose have also been evaluated in small randomized controlled trials showing encouraging results, although long-term follow-up is lacking and the safety of mitomycin C has recently been questioned [15,16,17,18]. Indeed, even the combination of all 3 drugs has been proposed as a potential treatment [19]. To date, there is a lack of robust evidence to support adjunctive treatment with steroids, mitomycin C, or hyaluronidase. One potential advantage of a drug-coated balloon is the ability to deliver the drug more evenly around the circumference and along the length of the stricture, as compared with multiple manual injections of antifibrotic agents.
The Optilume DCB procedure is minimally invasive, has low complication rates, and leverages current methods of treating stricture. The potential to treat longer strictures with minimally invasive procedures would allow more patients access to interventions with intent- to-cure and with lower associated morbidity. In contrast to ROBUST I, subjects in ROBUST II could undergo direct DCB treatment without prior stricture dilation with an uncoated balloon or DVIU. The rate of anatomic success at 6 months for subjects treated directly with the DCB was similar to the rate for those treated with pre-dilation. Data reported previously in an animal model show urethral concentration of paclitaxel dropping by 73% at 7 days post-procedure, and low serum levels at all time points [7].
There were several important limitations in this early phase study. The open label study lacked a control arm, and the sample size was small. Patients with higher risk of stricture recurrence, including those with bladder neck contractures, prior radiotherapy, or lichen sclerosus were excluded from the study; it is unknown how the DCB treatment would have performed in these subgroups. This small study was intended as an initial evaluation of the safety and efficacy of the therapy in the United States before initiation of the ROBUST III randomized controlled pivotal trial which is ongoing. Finally, while the 1-year outcomes of this study are encouraging, longer follow-up is required to assess treatment durability with the Optilume DCB. Follow-up is expected up to 5 years and results from ROBUST I have already shown a functional treatment success rate of 70% at 2 years [7].
Conclusions
Early results of the ROBUST II study showed that treatment of recurrent male anterior urethral stricture with the Optilume DCB was safe and showed durable anatomic results at 6 months and sustained symptomatic improvement through 1 year. Data are preliminary, and follow-up to 5 years is planned.
Conflicts of Interest
The study was sponsored and funded by Urotronic Inc.
Abbreviations
| DCB | drug-coated balloon |
| DVIU | direct vision internal urethrotomy |
| IIEF | International Index of Erectile Dysfunction |
| IPSS | International Prostate Symptom Score |
| LUTS | lower urinary tract symptoms |
| PROM | patient-reported outcome measure PVR post-void residual |
| Qmax | peak urinary flow |
References
- Santucci, R.A.; Joyce, G.F.; Wise, M. Male urethral stricture disease. J. Urol. 2007, 177, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Verla, W.; Oosterlinck, W.; Spinoit, A.F.; Waterloos, M. A comprehensive review emphasizing anatomy, etiology, diagnosis, and treatment of male urethral stricture disease. Biomed. Res. Int. 2019, 2019, 9046430. [Google Scholar] [CrossRef] [PubMed]
- Heyns, C.F.; Steenkamp, J.W.; De Kock, M.L.; Whitaker, P. Treatment of male urethral strictures: Is repeated dilation or internal urethrotomy useful? J. Urol. 1998, 160, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Bullock, T.L.; Brandes, S.B. Adult anterior urethral strictures: A national practice patterns survey of board certified urologists in the United States. J. Urol. 2007, 177, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Santucci, R.; Eisenberg, L. Urethrotomy has a much lower success rate than previously reported. J. Urol. 2010, 183, 1859–1862. [Google Scholar] [CrossRef] [PubMed]
- Wessells, H.; Angermeier, K.W.; Elliott, S.; Gonzalez, C.M.; Kodama, R.; Peterson, A.C.; et al. Male urethral stricture: American Urological Association guideline. J. Urol. 2017, 197, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.A.; Virasoro, R.; DeLong, J.M.; Estrella, R.E.; Pichardo, M.; Lay, R.R.; et al. A drug-coated balloon treatment for urethral stricture disease: Two-year results from the ROBUST I study. Can. Urol. Assoc. J. 2021, 5, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Virasoro, R.; DeLong, J.M.; Mann, R.A.; Estrella, R.E.; Pichardo, M.; Lay, R.R.; et al. A drug-coated balloon treatment for urethral stricture disease: Interim results from the ROBUST I study. Can. Urol. Assoc. J. 2020, 14, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Sciberras, J.; Mangera, A.; Brett, A.; Watkin, N.; N’Dow, J.M.; et al. Defining a patient-reported outcome measure for urethral stricture surgery. Eur. Urol. 2011, 60, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Williford, W.O.; Chang, Y.; Machi, M.; Jones, K.M.; Walker-Corkery, E.; et al. Benign prostatic hyperplasia specific health status measures in clinical research: How much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J. Urol. 1995, 154, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, J.W.; Heyns, C.F.; de Kock, M.L. Internal urethrotomy versus dilation as treatment for male urethral strictures: A prospective, randomized comparison. J. Urol. 1997, 157, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.M.; Venkatesan, K. Endoscopic management of urethral stricture: Review and practice algorithm for management of male urethral stricture disease. Curr. Urol. Rep. 2018, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli Tabassi, K.; Yarmohamadi, A.; Mohammadi, S. Triamcinolone injection following internal urethrotomy for treatment of urethral stricture. Urol. J. 2011, 8, 132–136. [Google Scholar] [PubMed]
- Mazdak, H.; Izadpanahi, M.H.; Ghalamkari, A.; Kabiri, M.; Khorrami, M.H.; Nouri-Mahdavi, K.; et al. Internal urethrotomy and intraurethral submucosal injection of triamcinolone in short bulbar urethral strictures. Int. Urol. Nephrol. 2010, 42, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Mazdak, H.; Meshki, I.; Ghassami, F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur. Urol. 2007, 51, 1089–1092; discussion 92. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Kang, D.H.; Choi, H.Y.; Jeong, T.Y.; Ha, U.S.; Han, J.H.; et al. The effects of hyaluronic acid and carboxymethylcellulose in preventing recurrence of urethral stricture after endoscopic internal urethrotomy: A multicenter, randomized controlled, single-blinded study. J. Endourol. 2013, 27, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Shahzad, M.; Orakzai, N.; Khan, I.; Ahmad, M. Efficacy of mitomycin C in reducing recurrence of anterior urethral stricture after internal optical urethrotomy. Korean J. Urol. 2015, 56, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, J.D.; Broghammer, J.A.; Smith, T.G.; 3rd Voelzke, B.B.; Erickson, B.A.; McClung, C.D.; et al. Intralesional injection of mitomycin C at transurethral incision of bladder neck contracture may offer limited benefit: TURNS Study Group. J. Urol. 2015, 193, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Garg, N.; Singh, S.K.; Mandal, A.K. Efficacy of optical internal urethrotomy and intralesional injection of Vatsala-Santosh PGI tri-Inject (triamcinolone, mitomycin C, and hyaluronidase) in the treatment of anterior urethral stricture. Adv. Urol. 2014, 2014, 192710. [Google Scholar] [CrossRef] [PubMed]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.