Abstract
Objectives Since the introduction of the first master–slave robotic platform for surgical procedures, there have been ongoing modifications and development of new platforms, but there is still a paucity of commercially available systems. Our study aims to identify all master–slave robotic surgical platforms currently commercially available or in development around the world with applications in urologic surgery. Methods A scoping literature search was performed using PRISMA methodology to identify all relevant publications in English in PubMed, PubMed Central, and Embase, with additional information being obtained from official company websites. Results Ten robotic platforms with either proven or potential application in urologic surgery were identified: the da Vinci surgical system (Intuitive), Senhance surgical system (Transentrix), Versius Surgical (CMR Ltd.), Enos surgical system (Titan Medical), Revo –I (Meere Company), MiroSurge (DLR), Avatera System (Avatera Medical), Hugo Surgical Robot (Medtronic), Ottava (J&J, Ethicon, Areus), and Hinotori (Medicaroid Corporation). Conclusions This review highlights the distinct features of emerging master–slave robotic platforms with applications in urologic surgery. Research and development are now focused on finding wider applications, improving outcomes, increasing availability, and reducing cost. Additional research is required comparing newly developed master–slave robotic platforms with those already well established.
Introduction
The field of urology has played a major role in the advancement of surgical robotics, dating back to the MONA robot, the prototype of the modern day da Vinci surgical robot [1]. The most advanced surgical robotic platforms currently are the ‘‘master–slave systems’’ in which the surgeon controls robotic arms remotely from a console. In a dry laboratory setting, Choussein and colleagues were able to demonstrate that robot-assisted laparoscopy nearly eliminated operative handedness, which persists in conventional laparoscopy [2]. Robotic platforms can be categorized by some of their key features, and there are many similarities amongst them. One major distinction is the open versus the closed robotic platform console. In an open console, there is no ability for the operator to fix his or her head in position; instead, the head can be moved freely during the procedure. These alterations in the field of view from the display during an operation can result in errors and decreased efficiency [3]. A development in newer generations of robotic platforms is the addition of haptic feedback from the robot for the operating physician. Abiri and colleagues tested a multi-modal system for haptic force feedback in robotic surgery that results in nearly a 50% force reduction in comparison to a system with no haptic feedback [4].
Laparoendoscopic single-site surgery (LESS) is another feature of surgical robotic platforms that is an advancement from original laparoscopy. One disadvantage of LESS is the challenge of instruments interfering with one another, although early success has been reported [5,6,7]. No head-to-head comparison of any robotic platforms from different companies exists in the urologic literature. This paper provides a scoping review on established and emerging master–slave robotic platforms in urologic surgery.
Methods
In the absence of a clear population, intervention, comparison, and outcome research question, we elected to perform a systematic scoping review of the currently available literature on this topic [8]. The goal of this article is not to compare surgical or oncologic outcomes of procedures performed with the different robotic platforms, but to highlight the emerging technologies in robotic platforms. Three databases were searched for articles published in English. PubMed and PubMed Central (n = 3644) were searched using the following terms: “robotic surgical procedures,” “robotics,” “urology,” “urology department, hospital,” “urologic surgical procedures,” “urologic surgical procedures, male.” Embase (n = 3252) was searched using the following terms: “robot,” “robotics,” “robotic surgical procedures,” “robot assisted surgery,” “urology,” “child urology,” “urologic surgery.” Additional records were identified through screening the citations of selected texts (n = 20; Figure 1). Additional information was obtained by searching through google.com (Google Inc, Mountain View, United States) and official company websites (n = 9). All case reports, case series, cohort studies, and randomized controlled trials written in English were included. Articles were excluded if they were abstracts, editorials, expert opinions, or review articles, if they did not report the surgical platform used, or if the platform has been discontinued. Preferred reporting items for systematic reviews and meta- analyses (PRISMA) guidelines were used to ensure reproducibility of our scoping review [9].
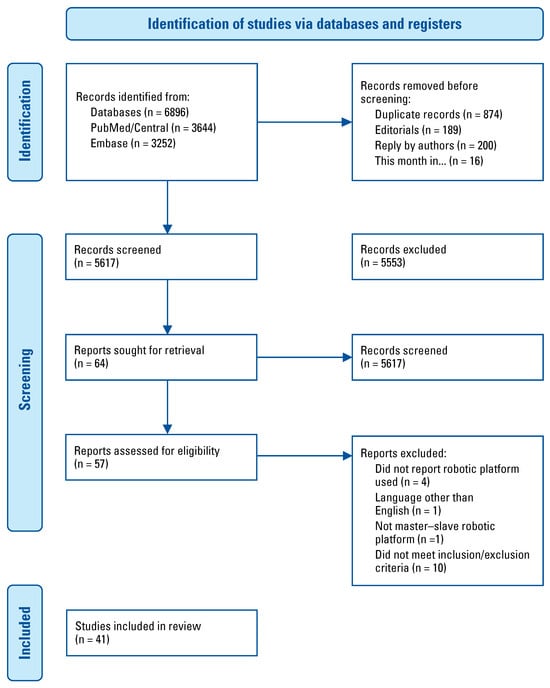
Figure 1.
PRISMA flow diagram [10].
Results
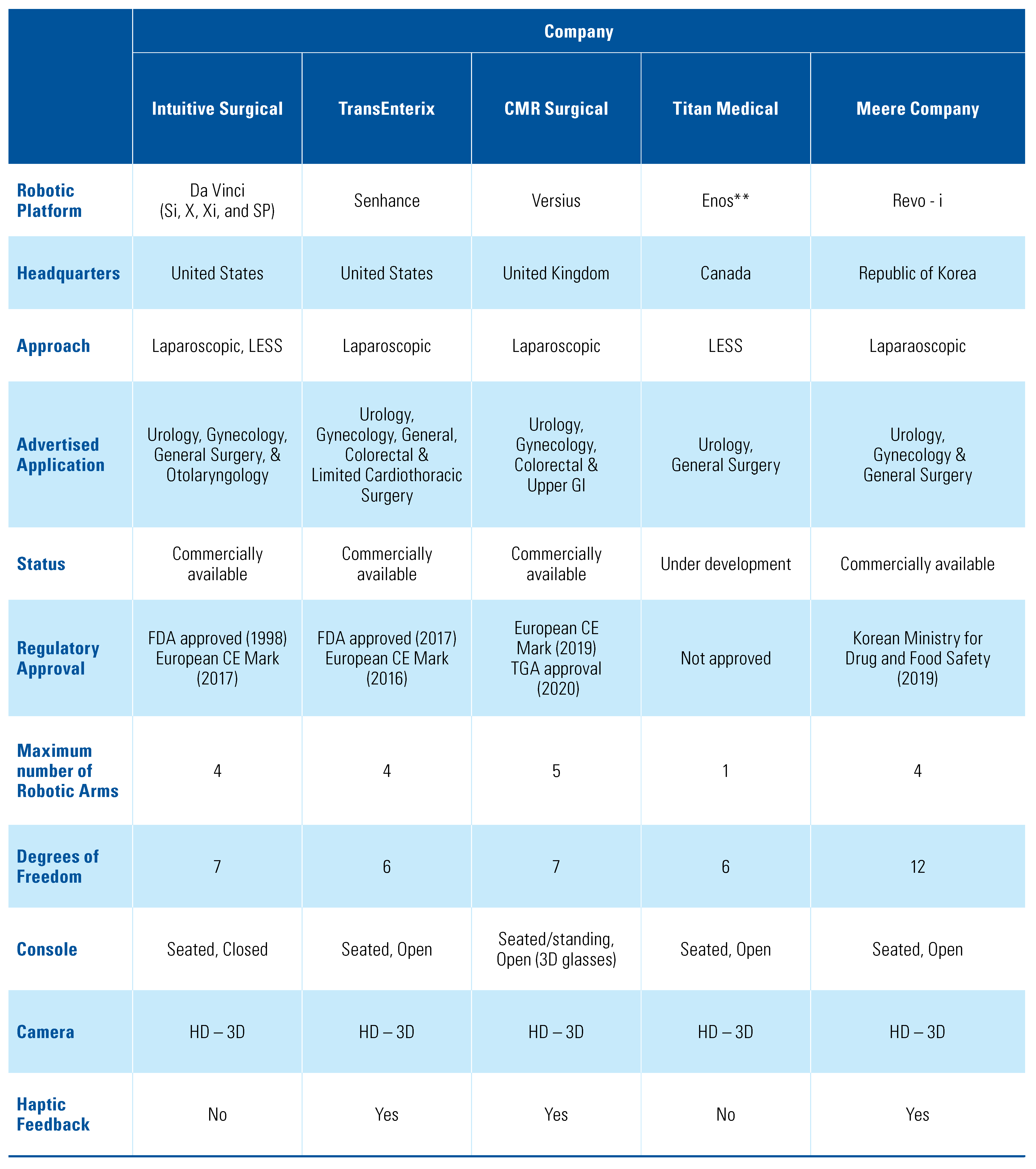
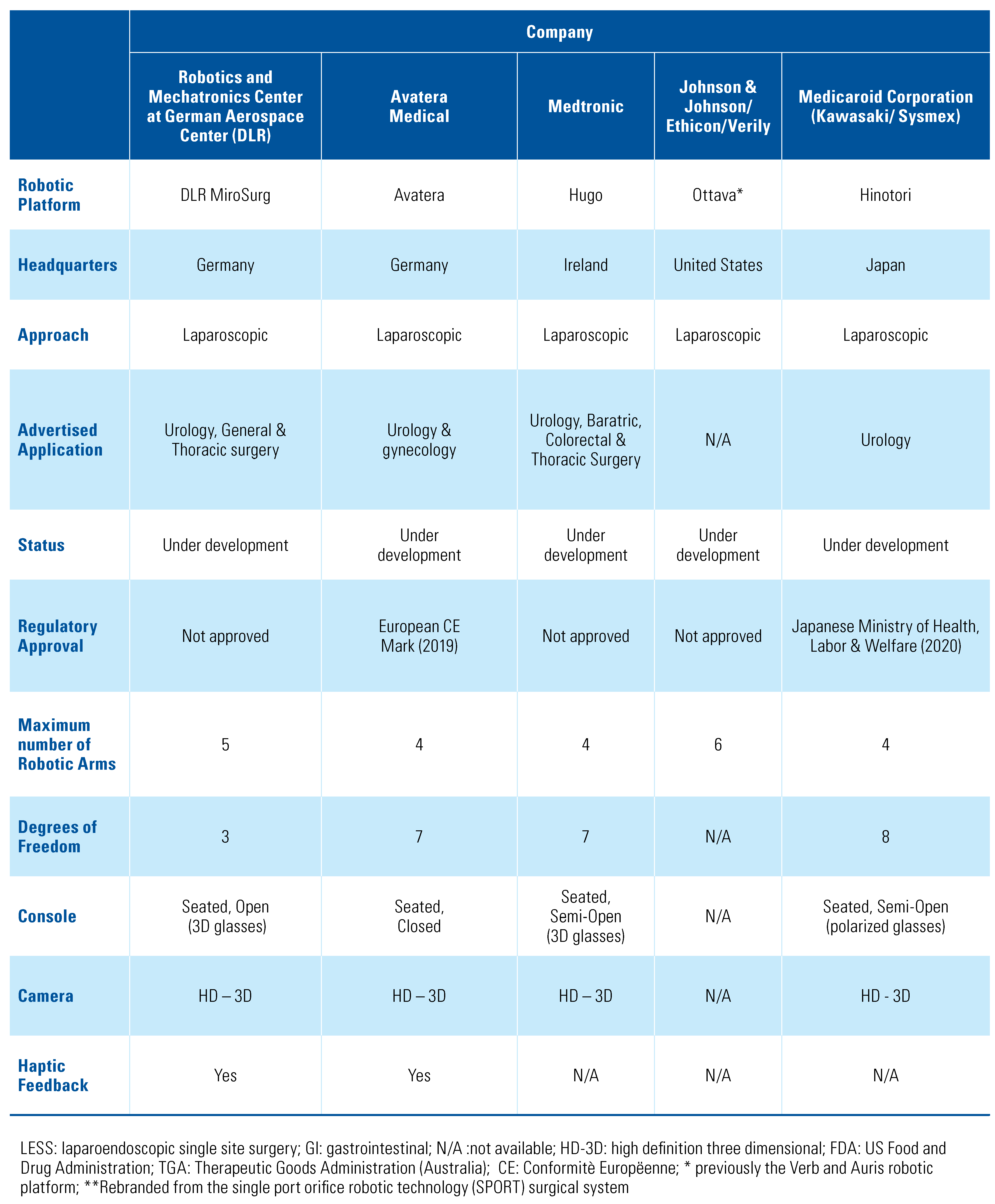
After initial screening of titles, 57 articles underwent final review, and 41 articles were included in the final paper (Figure 1) [10]. A total of 10 master–slave surgical robots were identified through our scoping systematic review. For each surgical platform we sought to provide information regarding the current company with proprietary rights to the device, whether or not it had regulatory approval, the main features of the robotic platform, and a review of any pre-clinical or clinical data (Table 1). A summary of the major features of each system is shown in Table 2.

Table 1.
Emerging and currently available master–slave robotic platforms with applications in urologic surgery.

Table 2.
Summary of major features of identified master–slave robotic platforms.
da Vinci Surgical System
The da Vinci surgical system is a master–slave laparoscopic robotic platform, designed and owned by Intuitive Surgical (Sunnyvale, United States) (Figure 2). It has played a crucial role in enhancing minimally invasive surgery and was Food and Drug Administration (FDA) approved in the year 1998, originally for laparoscopic cholecystectomies, and received the Conformitè Europëenne (CE) mark in 2017. One of its early competitors, Zeus (Computer Motion), was discontinued following the company’s merger with Intuitive Surgical in 2003. Iterations of the da Vinci have been in use for over 2 decades, and its applications include general, cardiac, colorectal, otolaryngology, neurosurgery, thoracic, gynecologic and urologic surgery. Its features include a 3-dimensional (3-D) high- definition (HD) camera with a binocular view, and up to 3 instrument arms, which articulate at the wrist of the instrument with 7 degrees of freedom (DOF). There several available series, including the da Vinci S, Si, X, Xi, and SP (single port), with the newest versions having haptic feedback for the operator. Although modifications are aimed at improvement in currently available technology, reports suggest that changing from one model to another still poses some challenges for the surgeons [11]. However, certain models may be better suited for certain procedures, for example, the Xi resulted in a 48 minute shorter operative time for nephroureterectomies [12]. Intuitive has also made advances in the field of LESS, where in addition to the SP platform, they have developed software to allow for same-sided hand–eye control of the instruments that enables the surgeon’s right hand to control the screen right instrument even though the instrument is in the left robotic arm and vice versa [13]. LESS using the da Vinci SP system allows a smaller incision with superior cosmesis and non-inferior oncologic and surgical outcomes, although there appears to be a great learning curve [6,7,14,15,16,17]. Additionally, side docking for urologic procedures has been made possible with the da Vinci S and Si systems, allowing better access to the perineum and the urethra throughout the procedure [18]. Successful use of the da Vinci has been reported for surgeries including radical prostatectomy, simple enucleation of the prostate, radical cystectomy with intra or extra-corporeal ileal conduit or neobladder, radical and simple nephrectomy, live donor nephrectomy, pyeloplasty, adrenalectomy, sural nerve grafting, vaso-vasostomy, ureteral reimplant, and renal transplantation [14,19,20,21,22,23,24,25]. In addition to surgery on adults, da Vinci has also been successfully used in pediatrics using 5mm instruments as well as a LESS approach with no conversion to open procedure and with high success rates [26,27]. There are few direct comparisons of the costs of robot-assisted laparoscopy in urologic surgery, although previous reports dating back nearly a decade suggested use of the da Vinci surgical platform was more expensive secondary to capital cost, maintenance of the robot, and limited life of the instruments [28]. Intuitive Surgical has grown its da Vinci Surgical System installed base to 6142 systems as of March 31, 2021, an increase of 8% compared with 5669 as of the end of the first quarter of 2020, continuing its near monopoly on the field of master–slave surgical platforms globally.

Figure 2.
da Vinci Surgical System (Credit: Intuitive Surgical).
Senhance Surgical System
The Senhance surgical system is a master–slave robotic platform designed by TransEnterix (Figure 3). It was renamed from the Alf – X in 2016, developed by SOFAR SpA (Milan, Italy), which was used to perform its first clinical cases in 2015 [29,30]. This system and the da Vinci by Intuitive are the 2 master–slave robotic platforms that have both a CE mark (2014) and FDA approval (2017) [31]. Its features include a seated-open concept control centre with haptic handles, a 2-D or 3-D HD monitor, depending on surgeon preference, an infrared eye-tracking system, a keyboard and touch pad, a single pedal, up to 4 detached and independent robotic arms, and reusable 5mm endoscopic instru- ments [32,33]. The major benefit of the open structure is reduced cost, as it allows for use of conventional laparoscopic equipment and operating theaters. Criticisms include a larger size, restricting space in the operating room, and longer time to dock the robotic arms [30]. Plans for the Senhance Surgical System include a “machine vision system,” a form of augmented intelligence whereby the system moves the camera on the basis of prior procedures and the movements of the surgeon’s instruments. Initial report of the first 40 extra-peritoneal radical prostatectomies performed by Kaštelan et al. showed higher than expected length of hospital stay and indwelling catheter time; however, this was thought to be related to the learning curve of using a new platform [34]. Samalavicius and colleagues performed 31 radical prostatectomies using Senhance, with few complications and no conversion to open procedures [35]. A subsequent case series reported decreases in positive surgical margin rate and lower rates of postoperative incontinence in patients undergoing radical prostatectomy, and expanded the application of the Senhance robotic platform to adrenalectomies, nephrectomy, kidney cyst fenestration, and pyeloplasty with success [36]. The aforementioned developments and clinical data showing the success of the Senhance platform will make it a strong competitor in the field of master–slave surgical robots.
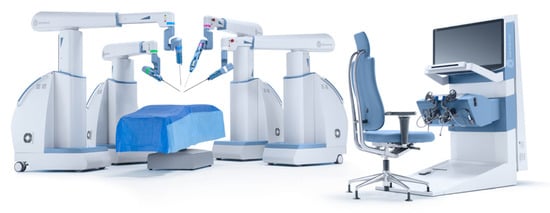
Figure 3.
Senhance Surgical System(Credit: Asensus Surgical, TransEnterix).
Versius Surgical Robotic System
The Versius system is a master–slave surgical platform developed by Cambridge Medical Robotics Limited (CMR Ltd.) Surgical in the United Kingdom (Figure 4). It received the CE Mark in 2019 and Therapeutic Goods Administration (Australia) approval in 2020. The platform is ergonomic, with an open console that enables both sitting and standing for the operator, with HD-3D visual aid. The surgeon can use up to 5 lightweight robotic arms, each as a solitary robotic unit for greater freedom of port placement. V-wrist technology allows 360-degree wrist motion, 7 DOF, with haptic feedback [37]. Although not performed with the final product, pre-clinical trials using Versius were completed, with a total of 24 surgeries performed (radical nephrectomy, radical prostatectomy, and pelvic lymph node dissection), with no device or non-device intraoperative complications [38]. Human clinical trials using the platform have been limited to gynecologic and general surgery procedures [39].
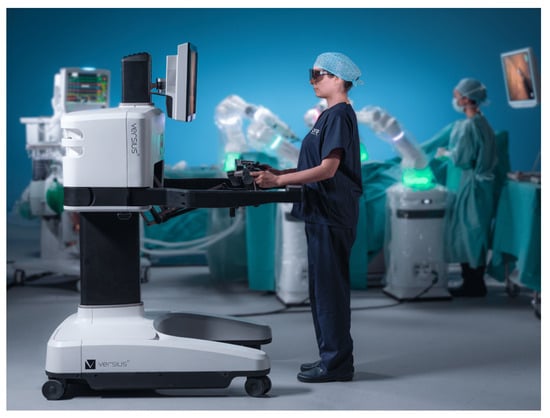
Figure 4.
Versius Surgical Robotic System (Credit: CMR Surgical).
Enos Surgical System
The Enos surgical system, rebranded in 2020 from the single-port orifice robotic technology (SPORT) surgical system is master–slave robotic platform created by the Canadian company, Titan Medical (Toronto, Canada). Enos does not have FDA approval or CE mark to date. The surgeon workstation is a seated-open design, with 3D-HD visualization. It has a single-arm mobile patient cart to allow for LESS, and a multi-articulating endoscope and instruments that provide 6 DOF. In 2018, Seeliger and colleagues showed promising feasibility and operator improvement with the SPORT platform by completing 12 minimally invasive procedures in porcine and human cadaveric pre-clinical trial procedures [5]. There are currently no pre-clinical or clinical data specifically on the Enos platform since it was rebranded.
Revo-I Model MSR-5000
Meere Company Incorporated, a Korean company, began development on the Revo-I Model MSR-5000, a master–slave robotic surgical platform, in 2006. The Revo-I Model MSR-5000 received approval for commercial use from the Korean government in August 2017, but it has not received FDA or CE Mark to date. The Revo-I Model MSR-5000 (Figure 5) consists of a seated-closed surgeon control console, a 4-arm robotic operation cart, a 3D-HD vision cart, and reusable endoscopic instruments [40,41]. Previous models of the robotic platform had a seated-open surgical console [42]. The Revo-I Model MSR-5000 robotic instruments also offer the greatest f lexibility with 12 DOF compared with the 7 DOF built into its contemporaries. Chang and colleagues successfully completed 17 Retzius- sparing robotic prostatectomy using the Revo-I Model MSR-5000 with no conversion to open or laparoscopic procedures or systems failures. One major limitation of this study, however, was that no pelvic lymph node dissections were performed for fear of intraoperative pelvic vessel injury [41]. Despite these successes, there has not been widespread use of the Revo-I Model MSR- 5000 outside Korea.
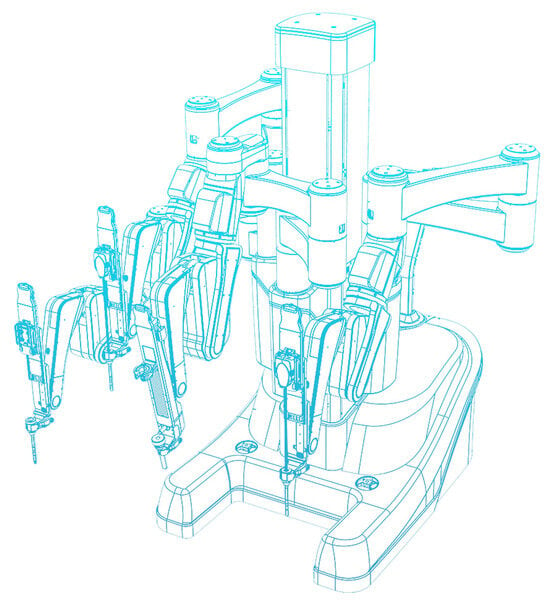
Figure 5.
Revo-I Model MSR-5000 (Credit: Revo-i, robotic surgical system (meerecompany)).
MiroSurge
The DLR (Deutsches Zentrum für Luft- und Raumfahrt e.V) telesurgery MiroSurge is a master–slave robotic platform made by German Aerospace Center. It currently does not have any regulatory body approval. The DLR MiroSurge (Figure 6) is a modular system that combines several robotic components, including 3 robot arms (DLR MIRO) and at least 2 instruments (DLR MICA). It offers a seated-open console with a 3-D HD video display, with instruments with 3 DOF and haptic feedback. One of the arms guides the laparoscopic view while the video screen updates the status of currently used instruments and prevents collision of instruments via force feedback [43]. No current pre-clinical or clinical data are available; the last publication was in 2011.
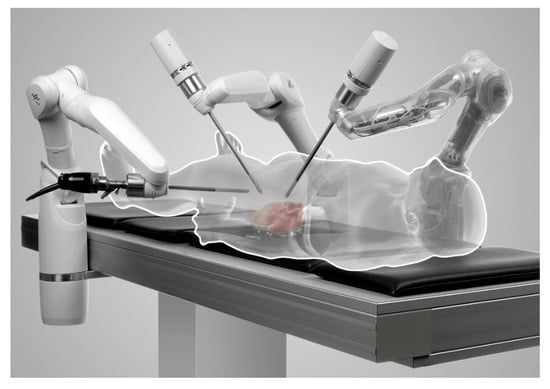
Figure 6.
MiroSurge (Credit: MiroSurge, German Aerospace Center).
Avatera System
The Avatera system (Figure 7) is a master–slave robotic platform which has been in development since 2011 as a joint venture between Avateramedical (Jena, Germany) and Force Dimension (Nyon, Switzerland). It received European CE Mark approval in 2019. It offers a seated- closed console, with 4 robotic arms mounted on a single cart, forceps-like handles with haptic feedback, single- use 5mm instruments with 7 DOF, and an HD-3D camera. Unique features include the absence of external fans, which decreases the noise level, and a space-saving compact design. No clinical data on the use of the Avatera System have been published to date.
Figure 7.
Avatera System (Credit: Avateramedical).
Hugo RAS System
The Hugo RAS system is a master–slave robotic platform created by Medtronic, following the acquisition of German-based robotic system MiroSurge as part of the acquisition of Covidien in 2014. It does not currently have regulatory approval in Europe. The surgeon console employs a seated, semi-open design, requiring 3-D glasses with HD visualization. Each robotic arm is attached to an individualized cart, allowing robotic arms to be split up and used for different procedures, each with 7 DOF. Currently, no pre-clinical or clinical data are available.
Ottava
Ottava, previously the Verb master–slave robotic platform, is in development, and is a joint venture by Ethicon, Johnson & Johnson (J & J), and Verily (a life sciences research organization within Google, Inc). In addition, in 2019, J & J acquired Auris Health who had developed the MONARCH platform for endoscopy. The platform does not have any regulatory body approval. Initial reports suggest that there may be 6 robotic arms directly attached to the operating table, but there is no credible information about the robotic platform currently available and no identified pre-clinical or clinical data on the Ottava platform.
Hinotori
The Hinotori master–slave surgical platform was developed through a joint venture begun in 2013 between 2 Japanese companies, Kawasaki Heavy industries, Ltd. and Sysmex Company. The Hinotori surgical system, which received Japanese regulatory approval in August 2020, is advertised as an easy docking system, with 4 robotic arms attached to the cart, and instruments with 8 DOF. The surgeon wears polarized glasses, using a semi-open console with a microscope-like ocular lens. Although a simulation system is in development for use on this platform, no pre-clinical or clinical data have been published to date.
Conclusions
We provide a scoping review of identified master– slave robotic surgical platforms with applications in urology. Despite increasing use of these platforms in surgery, there is still a paucity of published literature comparing different robotic platforms, many of which are still awaiting regulatory approval. Cost comparisons are currently not possible as many of these emerging platforms are not yet commercially available. Further research with direct comparisons of robotic platforms will be necessary to assess clinical outcomes, surgeon preference, and economic and environmental sustainability. To this point, the Intuitive Surgical da Vinci series has dominated the field, but this will likely change as other systems are approved.
Conflicts of Interest
None declared.
Abbreviations
| DOF | degrees of freedom |
| HD | high definition |
| LESS | laparoendoscopic single-site surgery |
| MIS | minimally invasive surgery |
| N/A | not available |
References
- Challacombe, B.J.; Khan, M.S.; Murphy, D.; Dasgupta, P. The history of robotics in urology. World J Urol. 2006, 24, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Choussein, S.; Srouji, S.S.; Farland, L.V.; Wietsma, A.; Missmer, S.A.; Hollis, M.; et al. Robotic assistance confers ambidexterity to laparoscopic surgeons. J Minim Invasive Gynecol. 2018, 25, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Atug, F.; Castle, E.P.; Woods, M.; Davis, R.; Thomas, R. Robotics in urologic surgery: An evolving new technology. Int J Urol. 2006, 13, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Abiri, A.; Pensa, J.; Tao, A.; Ma, J.; Juo, Y.Y.; Askari, S.J.; et al. Multi-modal haptic feedback for grip force reduction in robotic surgery. Sci Rep. 2019, 9, 5016. [Google Scholar] [CrossRef]
- Seeliger, B.; Diana, M.; Ruurda, J.P.; Konstantinidis, K.M.; Marescaux, J.; Swanstrom, L.L. Enabling single-site laparoscopy: The SPORT platform. Surg Endosc. 2019, 33, 3696–3703. [Google Scholar] [CrossRef] [PubMed]
- Gaboardi, F.; Pini, G.; Suardi, N.; Smelzo, S.; Passaretti, G.; Rosso, M.; et al. Robotic laparoendoscopic single-site (r-LESS) radical prostatectomy: IDEAL phase 1. Eur Urol. 2016, 15, eV12. [Google Scholar] [CrossRef]
- Billah, M.S.; Stifelman, M.; Munver, R.; Tsui, J.; Lovallo, G.; Ahmed, M. Single port robotic assisted reconstructive urologic surgery-with the da Vinci SP surgical system. Transl Androl Urol. 2020, 9, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Goonewardene, S.S.; Cahill, D. The da Vinci Xi and robotic radical prostatectomy-an evolution in learning and technique. J Robot Surg. 2017, 11, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.N.; Hemal, A.K. Does advancing technology improve outcomes? Comparison of the da Vinci Standard/S/Si to the Xi Robotic Platforms during robotic nephroureterectomy. J Endourol. 2018, 32, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Autorino, R.; Kaouk, J.H.; Stolzenburg, J.U.; Gill, I.S.; Mottrie, A.; Tewari, A.; et al. Current status and future directions of robotic single-site surgery: A systematic review. Eur Urol. 2013, 63, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Won Lee, J.; Arkoncel, F.R.; Rha, K.H.; Choi, K.H.; Yu, H.S.; Chae, Y.; et al. Urologic robot-assisted laparoendoscopic single-site surgery using a homemade single-port device: A single-center experience of 68 cases. J Endourol. 2011, 25, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, A.; Belba, A.; Dasgupta, P. Robotic single-port transumbilical radical prostatectomy: Our first experience using the gel-port system. BJU Int. 2012, 130. [Google Scholar]
- Kim, K.H.; Song, W.; Yoon, H.; Lee, D.H. Single-port robot-assisted radical prostatectomy with the da Vinci SP system: A single surgeon’s experience. Investig Clin Urol. 2020, 61, 173–179. [Google Scholar] [CrossRef]
- Kaouk, J.; Aminsharifi, A.; Sawczyn, G.; Kim, S.; Wilson, C.A.; Garisto, J.; et al. Single-port robotic urological surgery using purpose-built single-port surgical system: Single-institutional experience with the first 100 cases. Urology. 2020, 140, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Yee, C.H.; Lo, K.L.; Chan, C.K.; Hou, S.M.; Ng, C.F. Side- docking technique for robot-assisted urologic pelvic surgery. Urology. 2013, 82, 1300–1303. [Google Scholar] [CrossRef]
- Chan, S.Y.; Hou, S.M.; Wong, W.S.; Ng, C.F. Robotic urological surgery: Prospects for Hong Kong. Surg Pract. 2007, 11, 154–158. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, W.; Choi, Y.D.; Chung, B.H.; Hong, S.J.; Rha, K.H. Yonsei experience in robotic urologic surgery—Application in various urological procedures. Yonsei Med J. 2008, 49, 897–900. [Google Scholar] [CrossRef]
- Fareed, K.; Zaytoun, O.M.; Autorino, R.; White, W.M.; Crouzet, S.; Yakoubi, R.; et al. Robotic single port suprapubic transvesical enucleation of the prostate (R-STEP): Initial experience. BJU Int. 2012, 110, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Yuh, B.; Yu, X.; Raytis, J.; Lew, M.; Fong, Y.; Lau, C. Use of a mobile tower- based robot—The initial Xi robot experience in surgical oncology. J Surg Oncol. 2016, 113, 5–7. [Google Scholar] [CrossRef]
- Osmonov, D.; Prell, F.; Kalz, A.; Junemann, K.P. VS-1-9 Da Vinci robot-assisted vasovasostomy and vasoepididymostomy. J Sex Med. 2020, 17 (Suppl. 2), S149. [Google Scholar] [CrossRef]
- Mah, L.; Noel, O.; Rothschild, J.; Dall’Era, M.; Canvasser, N. Right robotic intracorporeal ileal ureter with single-position port placement using da Vinci Xi. J Urol. 2019, 201 (Suppl. 1), e401. [Google Scholar] [CrossRef]
- Garisto, J.; Bertolo, R.; Kaouk, J. Transperineal Approach for intracorporeal ileal conduit urinary diversion using a purpose-built single-port robotic system: Step-by-step. Urology. 2018, 122, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Paradise, H.J.; Huang, G.O.; Elizondo Saenz, R.A.; Baek, M.; Koh, C.J. Robot- assisted laparoscopic pyeloplasty in infants using 5-mm instruments. J Pediatr Urol. 2017, 13, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Jang, W.S.; Kim, S.W.; Kim, S.H.; Han, S.W.; Lee, Y.S. Robot- assisted laparoscopic single-port pyeloplasty using the da Vinci SP® system: Initial experience with a pediatric patient. J Pediatr Urol. 2019, 15, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Ibrahim, A.; Wang, T.T.; Khan, N.; Challacombe, B.; Khan, M.S.; et al. Assessing the cost effectiveness of robotics in urological surgery—A systematic review. BJU Int. 2012, 110, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, G.; Gidaro, S.; Taverna, G. Robot-assisted laparoscopic partial nephrectomy with the ALF-X robot on pig models. Eur Urol. 2016, 69, 376–377. [Google Scholar] [CrossRef]
- Falavolti, C.; Gidaro, S.; Ruiz, E.; Altobelli, E.; Stark, M.; Ravasio, G.; et al. Experimental nephrectomies using a novel telesurgical system: (The Telelap ALF-X)-a pilot study. Surg Technol Int. 2014, 25, 37–41. [Google Scholar]
- Brodie, A.; Vasdev, N. The future of robotic surgery. Ann R Coll Surg Engl. 2018, 100 (Suppl. 7), 4–13. [Google Scholar] [CrossRef] [PubMed]
- deBeche-Adams, T.; Eubanks, W.S.; de la Fuente, S.G. Early experience with the Senhance(R)-laparoscopic/robotic platform in the US. J Robot Surg. 2019, 13, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, Ž.; Kneževic’, N.; Hudolin, T.; Kuliš, T.; Penezic´, L.; Goluža, E.; et al. Extraperitoneal radical prostatectomy with the Senhance Surgical System robotic platform. Croat Med J. 2019, 60, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Kastelan, Z.; Hudolin, T.; Kulis, T.; Penezic, L.; Gidaro, S.; Bakula, M.; et al. Extraperitoneal radical prostatectomy with the Senhance Robotic Platform: First 40 cases. Eur Urol. 2020, 78, 932–934. [Google Scholar] [CrossRef]
- Samalavicius, N.E.; Janusonis, V.; Siaulys, R.; Jasenas, M.; Deduchovas, O.; Venckus, R.; et al. Robotic surgery using Senhance® robotic platform: Single center experience with first 100 cases. J Robot Surg. 2020, 14, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Kastelan, Z.; Hudolin, T.; Kulis, T.; Knezevic, N.; Penezic, L.; Maric, M.; et al. Upper urinary tract surgery and radical prostatectomy with Senhance® robotic system: Single center experience-first 100 cases. Int J Med Robot. 2021, 17, e2269. [Google Scholar] [CrossRef] [PubMed]
- Haig, F.; Medeiros, A.C.B.; Chitty, K.; Slack, M. Usability assessment of Versius, a new robot-assisted surgical device for use in minimal access surgery. BMJ Surg Interv Health Technol. 2020, 2, e000028. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.C.; Slack, M.; Hussain, M.; Barber, N.; Pradhan, A.; Dinneen, E.; et al. Preclinical evaluation of the Versius Surgical System, a new robot-assisted surgical device for use in minimal access renal and prostate surgery. Eur Urol Focus. 2021, 7, 444–452. [Google Scholar] [CrossRef]
- Kelkar, D.; Borse, M.A.; Godbole, G.P.; Kurlekar, U.; Slack, M. Interim safety analysis of the first-in-human clinical trial of the Versius surgical system, a new robot-assisted device for use in minimal access surgery. Surg Endosc. 2020. [CrossRef]
- Kim, D.K.; Park, D.W.; Rha, K.H. Robot-assisted partial nephrectomy with the REVO-I Robot Platform in porcine models. Eur Urol. 2016, 69, 541–542. [Google Scholar] [CrossRef]
- Chang, K.D.; Abdel Raheem, A.; Choi, Y.D.; Chung, B.H.; Rha, K.H. Retzius- sparing robot-assisted radical prostatectomy using the Revo-i robotic surgical system: Surgical technique and results of the first human trial. BJU Int. 2018, 122, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Abdel Raheem, A.; Troya, I.S.; Kim, D.K.; Kim, S.H.; Won, P.D.; Joon, P.S.; et al. Robot-assisted Fallopian tube transection and anastomosis using the new REVO-I robotic surgical system: Feasibility in a chronic porcine model. BJU Int. 2016, 118, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Hagn, U.; Konietschke, R.; Tobergte, A.; Nickl, M.; Jorg, S.; Kubler, B.; et al. DLR MiroSurge: A versatile system for research in endoscopic telesurgery. Int J Comput Assist Radiol Surg. 2010, 5, 183–193. [Google Scholar] [CrossRef] [PubMed]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2021 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.