Evaluation of the Guidelines for Penile Cancer Treatment: Overview and Assessment
Abstract
Introduction
Materials and Methods
Results
Level of evidence assessment and grading of recommendations
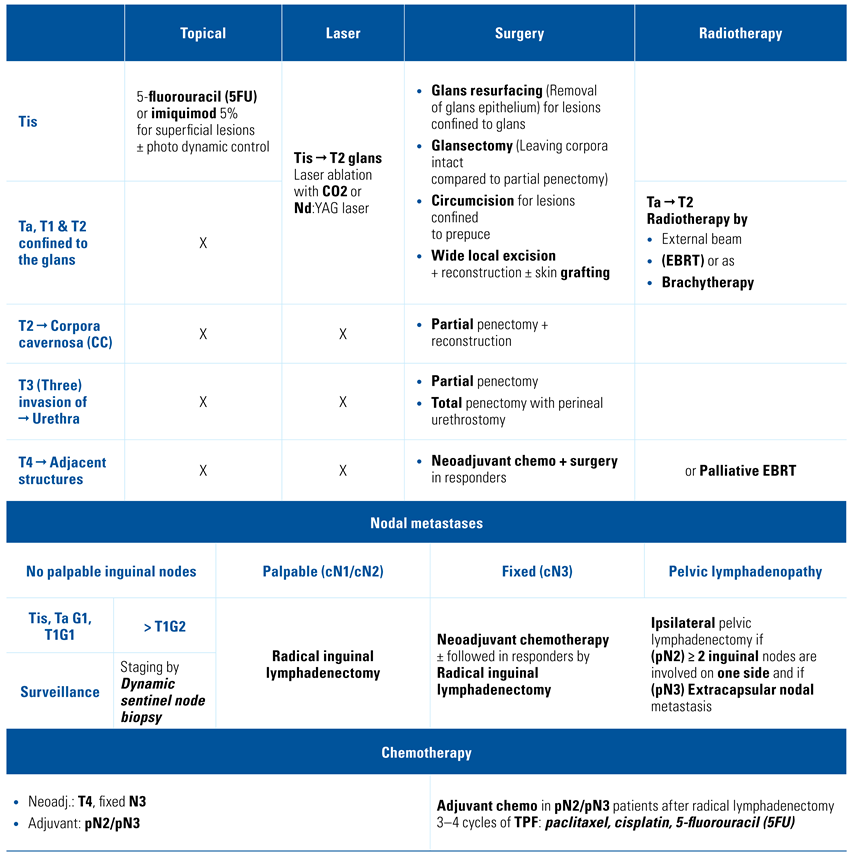
Treatment strategy according to stage
Organ-sparing treatment in Tis, Ta, and T1a tumors
- Summary of treatment recommendations: For patients with penile Tis or Ta, we recommend topical therapy[17,18] and excisional organ-sparing technique[19], a topical agent such as imiquimod (5%) or 5-fluorouracil (5FU) cream, circumcision and wide local excision, laser therapy, or complete glansectomy (Table 2).
Invasive disease treatment confined to the glans T1/T2
- Summary of treatment recommendations: Our recommendation for the treatment of invasive disease confined to the glans is a glansectomy with or without resurfacing with a partial thickness skin graft of the corporeal heads[20]. A partial amputation for patients who are not candidates for reconstructive surgery should be performed[21]. Radiotherapy may also be an option[22] (Table 2).
Treatment of invasive disease T3/T4
- Summary of treatment recommendations: For the treatment of cT3, we recommend glansectomy with corporectomy and reconstruction or partial penectomy with reconstruction as a standard of care[23,24]. Total penectomy with perineal urethrostomy is considered in selected cases. For cT4 disease, the recommended treatment remains a total penectomy with perineal urethrostomy[24]. Neoadjuvant chemotherapy for the locally advanced disease should be systematically considered and proposed[25,26] (Table 2).
Guidelines for treatment strategies for nodal metastases: cN0
- Summary of treatment recommendations: There are considerable discussions among researchers in the management of cN0 disease. Nonetheless, we believe that it is justified to recommend surveillance for Tis, Ta G1, and T1G1 if the patient is compliant[28]. In contrast, at least a dynamic sentinel node biopsy should be recommended to improve the outcome for ≥ T1G2 disease[29] (Table 2).
Guidelines for treatment strategies for nodal metastases: cN1/cN2
Guidelines for treatment strategies for nodal metastases: cN3
Enlarged pelvic lymph nodes
Guidelines for chemotherapy
- Summary of treatment recommendations: A neoadjuvant chemotherapy should be proposed systematically for patients with cN3 inguinal lymph nodes and discussed for all clinical lymph nodes ≥ 4cm. An adjuvant chemotherapy should be offered to patients with pN2/pN3 disease without previous systemic treatment. Three to 4 cycles of paclitaxel, cisplatin, 5-fluorouracil (5FU) are the recommended regimen[32,33,34] (Table 2).
Guidelines for Adjuvant Radiotherapy
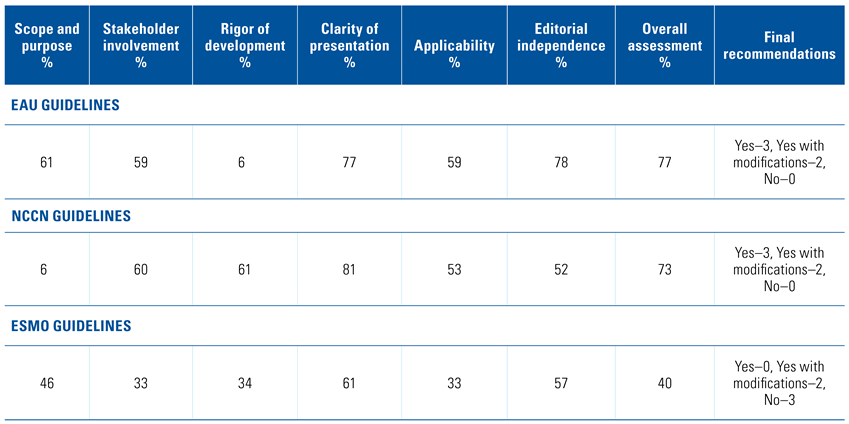
Assessment of the quality of the guidelines with the AGREE II instrument
Discussion
Conclusion
Conflicts of Interest
Abbreviations
| AGREE II Appraisal of Guidelines for Research and Evaluation II |
| NCCN National Comprehensive Cancer Network |
| EAU European Association of Urology |
| ESMO European Society for Medical Oncology |
| EBRT external beam radiation therapy |
| PLND pelvic lymph node dissection |
| LOE level of evidence |
| GOR grade of recommendation |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, M.C.; Heideman, D.A.M.; Snijders, P.J.F.; Horenblas, S.; Dillner, J.; Meijeret, C.L.M. Penile cancer: epidemiology, pathogenesis and prevention. World J. Urol. 2009, 27, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chipollini, J.; Tang, D.H.; Sharma, P.; Spiess, P.E. National trends and predictors of organ-sparing for invasive penile tumors: expanding the therapeutic window. Clin. Genitourin. Cancer 2018, 16, e383–e389. [Google Scholar] [CrossRef] [PubMed]
- Resch, I.; Abufaraj, M.; Hübner, N.A.; Shariat, S.F. An update on systemic therapy for penile cancer. Curr. Opin. Urol. 2020, 30, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Adashek, J.J.; Necchi, A.; Spiess, P.E. Updates in the molecular epidemiology and systemic approaches to penile cancer. Urol. Oncol. 2019, 37, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A. Systemic Therapy for penile cancer. Eur. Urol. Suppl. 2018, 17, 160–163. [Google Scholar] [CrossRef]
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; et al. AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010, 182, E839–842. [Google Scholar] [CrossRef] [PubMed]
- Practice, A.A.t.S.o. and Guidelines. AGREE website. Available online: https://www.agreetrust.org/ (accessed on 26 March 2021).
- National Comprehensive Cancer Network. Penile Cancer (Version 1.2020). 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf (accessed on 26 March 2021).
- Hakenberg, O.W.; Compérat, E.; Minhas, S.; Necchi, A.; Protzel, C.; Watkin, N.; Robinson, R. EAU Guidelines on Penile Cancer 2018, in European Association of Urology Guidelines. 2018 Edition. 2018, European Association of Urology Guidelines Office: Arnhem, The Netherlands. Available online: https://uroweb.org/guideline/penile-cancer/ (accessed on 26 March 2021).
- Van Poppel, H.; Watkin, N.A.; Osanto, S.; Moonen, L.; Horwich, A.; Kataja, V.; ESMO Guidelines Working Group. Penile cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi115–124. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; for the GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Vist, G.E.; Falck-Ytter, Y.; Schünemann, H.J.; GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? BMJ 2008, 336, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Dykewicz, C.A.; Centers for Disease Control and Prevention (U.S.); Infectious Diseases Society of America; American Society of Blood and Marrow Transplantation. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2001, 33, 139–144. [Google Scholar] [CrossRef]
- Raskin, Y.; Vanthoor, J.; Milenkovic, U.; Muneer, A.; Albersen, M. Organ- sparing surgical and nonsurgical modalities in primary penile cancer treatment. Curr. Opin. Urol. 2019, 29, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Choi, M.; Cho, K.H. A case of erythroplasia of queyrat treated with imiquimod 5% cream and excision. Ann. Dermatol. 2009, 21, 419–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schroeder, T.L.; Sengelmann, R.D. Squamous cell carcinoma in situ of the penis successfully treated with imiquimod 5% cream. J. Am. Acad. Dermatol. 2002, 46, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.S.; Mc Dougal, W.S. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J. Urol. 2011, 186, 1303–1307. [Google Scholar] [CrossRef]
- Azrif, M.; Logue, J.P.; Swindell, R.; Cowan, R.A.; Wylie, J.P.; Livsey, J.E. External-beam radiotherapy in T1–2 N0 penile carcinoma. Clin. Oncol. (R Coll Radiol) 2006, 18, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.; Hadway, P.; Biedrzycki, O.; Perry, M.J.A.; Corbishley, C.; Watkin, N.A. Reconstructive surgery for invasive squamous carcinoma of the glans penis. Eur. Urol. 2007, 52, 1179–1185. [Google Scholar] [CrossRef]
- Crook, J.; Ma, C.; Grimard, L. Radiation therapy in the management of the primary penile tumor: an update. World J. Urol. 2009, 27, 189–196. [Google Scholar] [CrossRef]
- Gotsadze, D.; Matveev, B.; Zak, B.; Mamaladze, V. Is conservative organ-sparing treatment of penile carcinoma justified? Eur. Urol. 2000, 38, 306–312. [Google Scholar] [CrossRef]
- Ornellas, A.A.; Kinchin, E.W.; Nóbrega, B.L.B.; Wisnescky, A.; Koifman, N.; Quirino, R. Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian National Cancer Institute long-term experience. J. Surg. Oncol. 2008, 97, 487–495. [Google Scholar] [CrossRef]
- Pizzocaro, G.; Piva, L. Adjuvant and neoadjuvant vincristine, bleomycin, and methotrexate for inguinal metastases from squamous cell carcinoma of the penis. Acta Oncol. 1988, 27, 823–824. [Google Scholar] [CrossRef] [PubMed]
- Leijte, J.A.; Kerst, J.M.; Bais, E.; Antonini, N.; Horenblas, S. Neoadjuvant chemotherapy in advanced penile carcinoma. Eur. Urol. 2007, 52, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.P.; Boon, T.A.; van Venrooij, G.E.; Wijburg, C.J. Long-term follow-up after laser therapy for penile carcinoma. Urology 2007, 69, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.J.; Liu, Z.-H.; Tang, L.-Y.; Wang, Y.-J.; Liang, J.-Y.; Zhang, R.-C.; et al. Radiocolloid-based dynamic sentinel lymph node biopsy in penile cancer with clinically negative inguinal lymph node: an updated systematic review and meta-analysis. Int. Urol. Nephrol. 2016, 48, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, P.K.; Dinney, C.P.; Pettaway, C.A. Controversies in ilioinguinal lymphadenectomy. Urol. Clin. North. Am. 2010, 37, 421–434. [Google Scholar] [CrossRef]
- Lughezzani, G.; Catanzaro, M.; Torelli, T.; Piva, L.; Biasoni, D.; Stagni, S.; et al. The relationship between characteristics of inguinal lymph nodes and pelvic lymph node involvement in penile squamous cell carcinoma: a single institution experience. J. Urol. 2014, 191, 977–982. [Google Scholar] [CrossRef]
- Noronha, V.; Patil, V.; Ostwal, V.; Tongaonkar, H.; Bakshi, G.; Prabhash, K.; et al. Role of paclitaxel and platinum-based adjuvant chemotherapy in high-risk penile cancer. Urol. Ann. 2012, 4, 150–153. [Google Scholar] [CrossRef]
- Giannatempo, P.; Paganoni, A.; Sangalli, L.; Colecchia, M.; Piva, L.; Torelli, M.C.T.; et al. Survival analyses of adjuvant or neoadjuvant combination of a taxane plus cisplatin and 5-fluorouracil (T-PF) in patients with bulky nodal metastases from squamous cell carcinoma of the penis (PSCC): Results of a single high-volume center. J. Clin. Oncol. 2014, 32 (Suppl. 4), 377. [Google Scholar] [CrossRef]
- Bandini, M.; Pederzoli, F.; Necchi, A. Neoadjuvant chemotherapy for lymph node-positive penile cancer: current evidence and knowledge. Curr. Opin. Urol. 2020, 30, 218–222. [Google Scholar] [CrossRef]
- Kulkarni, J.N.; Kamat, M.R. Prophylactic bilateral groin node dissection versus prophylactic radiotherapy and surveillance in patients with N0 and N1–2A carcinoma of the penis. Eur. Urol. 1994, 26, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Graafland, N.M.; Moonen, L.M.F.; van Boven, H.H.; van Werkhoven, E.; Kerst, J.M.; Horenblas, S. Inguinal recurrence following therapeutic lymphadenectomy for node positive penile carcinoma: outcome and implications for management. J. Urol. 2011, 185, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Franks, K.N.; Kancherla, K.; Sethugavalar, B.; Whelan, P.; Eardley, I.; Kiltie, A.E.; et al. Radiotherapy for node positive penile cancer: experience of the Leeds teaching hospitals. J. Urol. 2011, 186, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.R.; Kearns, J.T.; Holt, S.K.; Mossanen, M.; Lin, D.W.; Wright, J.L. Is there a benefit to adjuvant radiation in stage III penile cancer after lymph node dissection? Findings from the National Cancer Database. Urol. Oncol. 2018, 36, 92.e11–92.e16. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.H.; Djajadiningrat, R.; Diorio, G.; Chipollini, J.; Ma, Z.; Schaible, B.J.; et al. Adjuvant pelvic radiation is associated with improved survival and decreased disease recurrence in pelvic node-positive penile cancer after lymph node dissection: a multi-institutional study. Urol. Oncol. 2017, 35, 605.e17–605.e23. [Google Scholar] [CrossRef] [PubMed]
- Homesley, H.D.; Bundy, B.N.; Sedlis, A.; Adcock, L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet. Gynecol. 1986, 68, 733–740. [Google Scholar]
- Parthasarathy, A.; Cheung, M.K.; Osann, K.; Husain, A.; Teng, N.N.; Berek, J.S. The benefit of adjuvant radiation therapy in single-node-positive squamous cell vulvar carcinoma. Gynecol. Oncol. 2006, 103, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.M.; Ottenhof, S.R.; van der Heijden, M.S.; Pos, F.J.; Horenblas, S.; Brouwer, O.R. Management of the penile squamous cell carcinoma patient after node positive radical inguinal lymph node dissection: current evidence and future prospects. Curr. Opin. Urol. 2020, 30, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Crook, J. Radiotherapy approaches for locally advanced penile cancer: neoadjuvant and adjuvant. Curr. Opin. Urol. 2017, 27, 62–67. [Google Scholar] [CrossRef]
- Burt, L.M.; Shrieve, D.C.; Tward, J.D. Stage presentation, care patterns, and treatment outcomes for squamous cell carcinoma of the penis. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 94–100. [Google Scholar] [CrossRef]
- Ravi, R.; Chaturvedi, H.K.; Sastry, D.V. Role of radiation therapy in the treatment of carcinoma of the penis. Br. J. Urol. 1994, 74, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Demkow, T. The treatment of penile carcinoma: experience in 64 cases. Int. Urol. Nephrol. 1999, 31, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Chen, W.-C.; Wu, C.-T.; Chuang, C.-K.; Ng, K.-F.; Chang, J.T.-C. Contemporary management of penile cancer including surgery and adjuvant radiotherapy: an experience in Taiwan. World J. Urol. 2004, 22, 60–66. [Google Scholar] [CrossRef]
- Djajadiningrat, R.S.; Graafland, N.M.; van Werkhoven, E.; Meinhardt, W.; Bex, A.; van der Poel, H.G.; et al. Contemporary management of regional nodes in penile cancer-improvement of survival? J. Urol. 2014, 191, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Marconi, L.; MacPepple, E.; Hakenberg, O.W.; Watkin, N.; Yuan, Y.; et al. Risks and benefits of adjuvant radiotherapy after inguinal lymphadenectomy in node-positive penile cancer: a systematic review by the European Association of Urology Penile Cancer Guidelines Panel. Eur. Urol. 2018, 74, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ager, M.; Njoku, K.; Serra, M.; Robinson, A.; Pickering, L.; Afshar, M.; et al. Long-term multicentre experience of adjuvant radiotherapy for pN3 squamous cell carcinoma of the penis. BJU Int. Online ahead of print. 2020. [Google Scholar] [CrossRef]
| Treatment | EAU Guidelines | NCCN Guidelines | ESMO Guidelines | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Recommendation | Grade of recommendation | Level of evidence | Recommendationr | Grade of recommendation | Level of evidence | Recommendation | Grade of recommendation | Level of evidence | |
| STAGE Tis | |||||||||
| Topical treatment with 5-fluorouracil (5-FU) or imiquimod | 5-FU is an effective first-line treatment | Strong recommendation | Tis, Ta, and T1 penile cancer lesions may be amenable to conservative penile organ-sparing approaches, including topical therapy | Considered appropriate | 2A | Penile-preserving techniques, including topical therapy (5% 5-fluorouracil and 5% imiquimod cream) | C | IV | |
| Laser ablation | (Nd:YAG) or Carbon dioxide (CO2) laser is an effective treatment option | Strong recommendation | The use of therapeutic lasers to treat selected primary penile tumors has been reported with acceptable outcomes | Considered appropriate | 2B | Laser therapy using CO2 or Nd: YAG laser | C | III | |
| Glans resurfacing | Glans resurfacing, total or partial, can be a primary treatment for PeIN or a secondary | Strong recommendation | Glansectomy, removal of the glans penis, may be considered for patients with distal tumors | Considered appropriate | 2B | Partial/total glans resurfacing | C | III | |
| Wide local excision with circumcision | Glans resurfacing, total or partial, can be a primary treatment for PeIN or a secondary | Penile tumors of the shaft may be treated with wide local excision, with or without circumcision | Considered appropriate | 2A | Wide local excision and circumcision | C | IV | ||
| Mohs surgery | Historical technique | Mohs surgery is an alternative to wide local excision in select cases | 2B | ||||||
| STAGE Ta, T1a (G1, G2) | |||||||||
| Wide local excision with circumcision | Partial glansectomy or total glansectomy with reconstruction are surgical options | Strong recommendation | Penile tumors of the shaft may be treated with wide local excision, with or without circumcision | Considered appropriate | 2A | Penile-preserving techniques, including wide local excision plus reconstructive surgery | C | III | |
| Glans resurfacing | Partial glansectomy or total glansectomy with reconstruction are surgical options | Strong recommendation | Glansectomy may be considered for select patients with distal tumors | Considered appropriate | 2B | ||||
| Glansectomy with reconstruction | Partial glansectomy or total glansectomy with reconstruction are surgical options | Strong recommendation | Glansectomy is not recommended unless required to ensure complete tumor eradication with negative margins | Considered appropriate | 2A | ||||
| Radiotherapy | External beam radiotherapy or brachytherapy is radiotherapeutic options | Strong recommendation | 2B | Consider <4 cm: Brachytherapy or EBRT >4 cm: EBRT with chemotherapy | Considered appropriate | 2B | Radiotherapy delivered as EBRT or brachytherapy with interstitial implant | C | IV |
| Laser ablation | Small lesions can also be treated by laser therapy | Strong recommendation | The use of therapeutic lasers to treat selected primary penile tumors has been reported with acceptable outcomes | Considered appropriate | 2B | Laser therapy | C | IV | |
| Partial penectomy | Strong recommendation | Partial or total penectomy when invasion into the corpora cavernosum is necessary to achieve a negative margin | Considered appropriate | 2A | |||||
| Mohs surgery | Strong recommendation | Mohs surgery is an alternative to wide local excision in select cases. | Considered appropriate | 2B | |||||
| STAGE T1B (G3) AND T2 | |||||||||
| Wide local excision plus reconstruction | Local excision, partial glansectomy or total glansectomy with reconstruction are surgical options | Strong recommendation | Penile tumors of the shaft may be treated with wide local excision | Considered appropriate | 2A | If tumor <50% of the glans and no invasion of the corpora cavernosa | B | III | |
| Glansectomy with circumcision and reconstruction | Local excision, partial glansectomy or total glansectomy with reconstruction are surgical options | Strong recommendation | Glansectomy may be considered for select patients with distal tumors | Considered appropriate | 2A | If tumor <50% of the glans and no invasion of the corpora cavernosa | B | III | |
| Radiotherapy | External beam radiotherapy or brachytherapy is radiotherapeutic options | Strong recommendation | Consider <4 cm: Brachytherapy or EBRT >4 cm: EBRT with chemotherapy | Considered appropriate | 2B | <4 cm: Brachytherapy or EBRT >4 cm: EBRT with chemotherapy | III | ||
| Total penectomy OR Partial | Total glansectomy, with or without resurfacing of the corporeal heads, is recommended | Strong recommendation | Partial or total penectomy when invasion into the corpora cavernosum is necessary to achieve a negative margin | Considered appropriate | 2A | Tumors with invasion into corpora cavernosa | B | III | |
| STAGE T2 | |||||||||
| Total glansectomy | Total glansectomy, with or without resurfacing of the corporeal heads, is recommended | Strong recommendation | 3 | C | III | ||||
| Radiotherapy | Radiation therapy is an option | Strong recommendation | Consider <4 cm: Brachytherapy or EBRT >4 cm: EBRT with chemotherapy | Considered appropriate | 2B | <4 cm: Brachytherapy or EBRT >4 cm: EBRT with chemotherapy | D | ||
| Total penectomy OR Partial | Partial amputation should be considered in patients unfit for reconstructive surgery | Strong recommendation | Partial or total penectomy when invasion into the corpora cavernosum is necessary to achieve a negative margin | Considered appropriate | 2A | Tumors with invasion into corpora cavernosa | B | III | |
| STAGE T3 | |||||||||
| Partial amputation with reconstruction or total penectomy | Glansectomy with distal corporectomy and reconstruction or partial amputation with reconstruction are standard | Strong recommendation | Partial or total penectomy when invasion into the corpora cavernosum is necessary to achieve a negative margin | Considered appropriate | 2A | T3-4 or N+: circumcision followed by EBRT with chemotherapy | D | ||
| Radiotherapy | Radiation therapy is an option | Strong recommendation | EBRT with chemotherapy are treatment options | Considered appropriate | 3 | ||||
| STAGE T3 WITH INVASION OF THE URETHRA | |||||||||
| Partial penectomy or total penectomy | Glansectomy with distal corporectomy and reconstruction or partial amputation with reconstruction are standard | Strong recommendation | Partial or total penectomy when invasion into the corpora cavernosum is necessary to achieve a negative margin | Considered appropriate | 2A | T3-4 or N+: circumcision followed by EBRT with chemotherapy | D | ||
| Radiotherapy | Radiation therapy is an option | Strong recommendation | EBRT with chemotherapy are treatment options | Considered appropriate | 2B | ||||
| STAGE T4 | |||||||||
| Partial penectomy or total penectomy | Extensive partial amputation or total penectomy with perineal urethrostomy is the standard advisable treatment | Weak recommendation | Partial or total penectomy when invasion into the corpora cavernosum is necessary to achieve a negative margin | Considered appropriate | 2A | T3-4 or N+: circumcision followed by EBRT with chemotherapy | D | ||
| Radiotherapy | Palliative radiotherapy is an option | EBRT with chemotherapy are treatment options | Considered appropriate | 3 | |||||
| EAU Guidelines | NCCN Guidelines | ESMO Guidelines | ||||||
|---|---|---|---|---|---|---|---|---|
| Recommendation | Grade of recommendation | Level of evidence | Recommendation | Grade of recommendation | Level of evidence | Recommendation | Grade of recommendation | Grade of recommendation |
| STAGE CN0 | ||||||||
| Surveillance is only recommended in patients with pTis/pTa tumors | Strong recommendation | Most low-risk patients are followed with a surveillance as the probability of occult micro metastases in ILNs is low | Considered appropriate | 2A | Low- risk (Tis, Ta, T1G1) and intermediate-risk (T1G2) are followed with surveillance | B | ||
| > T1G2: invasive lymph node staging is recommended by either bilateral modified inguinal lymphadenectomy or dynamic sentinel node biopsy | Strong recommendation | 2B | For high-risk standard or modified ILND or DSNB is strongly recommended in high-risk | Considered appropriate | 2A | DSNB is recommended in patients with non-palpable inguinal lymph nodes T1G2 or greater | B | |
| STAGE CN1/CN2 | ||||||||
| A radical inguinal lymphadenectomy should be performed | Strong recommendation | 2B | Percutaneous lymph node biopsy is considered standard Positive findings warrant an immediate ILND | 2A | Fine-needle aspiration (FNA) of the LN is standard for these patients (omitting the procedure for high-risk tumors to avoid delay of ILND) | |||
| STAGE CN3 | ||||||||
| Multimodal treatment with neoadjuvant chemotherapy followed by radical lymphadenectomy in responders is recommended | Weak recommendation | Should receive neoadjuvant Chemotherapy followed by radical inguinal and PLND lymphadenectomy in responders | Considered appropriate | 2A | Patients with fixed nodes should be considered for neoadjuvant chemoradiotherapy | C | III | |
| Consider postoperative radiotherapy or chemoradiotherapy | 2B | Responders receive consolidation surgery (bilateral and deep ILND and ipsilateral PLND if possible) | ||||||
| STAGE PELVIC LYMPH NOD | ||||||||
| Patients with 2 or more inguinal lymph node metastases on one side and/or extracapsular lymph node extension need to undergo ipsilateral pelvic lymphadenectomy | Strong recommendation | 2B | PLND should be considered at the time or following ILND in patients with ≥ three positive inguinal nodes on the ipsilateral ILND site | Considered appropriate | 2A | Patients with fixed nodes should be considered for neoadjuvant chemoradiotherapy | C | III |
| Bilateral PLND should be considered either at the time or following ILND in patients with ≥4 positive inguinal nodes | Considered appropriate | 2A | Responders receive consolidation surgery (bilateral and deep ILND and ipsilateral PLND if possible) | |||||
| STAGE CHEMOTHERAPY | ||||||||
| Neoadjuvant chemotherapy using cisplatin- and the taxanebased triple combination should be used in patients with fixed, unresectable, nodal disease | Strong recommendation | 2A | Neoadjuvant chemotherapy with TIP (paclitaxel, ifosfamide, and cisplatin) is preferred (prior to ILND) in patients with ≥4 cm inguinal lymphnodes (fixed or mobile) | Considered appropriate | 2A | Neoadjuvant chemotherapy followed by radical surgery is advisable in unresectable or recurrent LN metastases | C | |
| Strong recommendation | 2B | Adjuvant chemotherapy it is reasonable to give four courses of TIP in the adjuvant setting if it was not given preoperatively and the pathology shows high-risk features | 2A | Adjuvant chemotherapy is recommended in pN2-3 patients | C | |||
| STAGE RADIOTHERAPY | ||||||||
| Not recommended for nodal disease except as a palliative option | Strong recommendation | Adjuvant EBRT or chemoradiotherapy can also be considered for patients with high-risk features | Considered appropriate | 2B | The role of adjuvant postoperative radiationis controversial | |||
| EAU Guidelines | NCCN Guidelines | ESMO Guidelines | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Grade of recommendation | Level of evidence | Treatment | Grade of recommendation | Level of evidence | Treatment | Grade of recommendation | Level of evidence |
| NEOADJUVANT CHEMOTHERAPY | ||||||||
| (4 cycles) cisplatin- and taxanebased regimen | Weak | 2A | (4 courses) TIP (paclitaxel, ifosfamide, and cisplatin) | 2A | Considered appropriate | (4 courses) TIP (paclitaxel, ifosfamide, and cisplatin) | C | III |
| ADJUVANT CHEMOTHERAPY | ||||||||
| (3 to 4 cycles) cisplatin, a taxane and 5-fluorouracil or ifosfamide | Strong | 2B | (4 courses) Preferred regimen is TIP (paclitaxel, ifosfamide, and cisplatin) Other recommended regimen is 5- fluorouracil + cisplatin | 2A | Considered appropriate | |||
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2021 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Aydh, A.; Shariat, S.F.; Motlagh, R.S.; Laukhtina, E.; Quhal, F.; Mori, K.; Mostafaei, H.; Necchi, A.; Pradere, B. Evaluation of the Guidelines for Penile Cancer Treatment: Overview and Assessment. Soc. Int. Urol. J. 2021, 2, 171-186. https://doi.org/10.48083/TKFP8406
Aydh A, Shariat SF, Motlagh RS, Laukhtina E, Quhal F, Mori K, Mostafaei H, Necchi A, Pradere B. Evaluation of the Guidelines for Penile Cancer Treatment: Overview and Assessment. Société Internationale d’Urologie Journal. 2021; 2(3):171-186. https://doi.org/10.48083/TKFP8406
Chicago/Turabian StyleAydh, Abdulmajeed, Shahrokh F. Shariat, Reza Sari Motlagh, Ekaterina Laukhtina, Fahad Quhal, Keiichiro Mori, Hadi Mostafaei, Andrea Necchi, and Benjamin Pradere. 2021. "Evaluation of the Guidelines for Penile Cancer Treatment: Overview and Assessment" Société Internationale d’Urologie Journal 2, no. 3: 171-186. https://doi.org/10.48083/TKFP8406
APA StyleAydh, A., Shariat, S. F., Motlagh, R. S., Laukhtina, E., Quhal, F., Mori, K., Mostafaei, H., Necchi, A., & Pradere, B. (2021). Evaluation of the Guidelines for Penile Cancer Treatment: Overview and Assessment. Société Internationale d’Urologie Journal, 2(3), 171-186. https://doi.org/10.48083/TKFP8406




