Abstract
Objectives: We aimed to study the incidence and predictors of upper tract urothelial cancer (UTUC) in patients with high-risk non-muscle invasive bladder cancer (HR-NMIBC). Methods: Patients who had HR-NMIBC were reviewed to identify those who subsequently developed UTUC. Complete transurethral resection was performed, and biopsies were collected for histopathology followed by intravesical chemoimmunotherapy. Patients were screened annually by computed tomography (CT) for UTUC. Results: Data for 1501 patients were reviewed. UTUC developed in 59 (4%) after a median of 20 months after HR-NMIBC. Most patients were symptomatic, but UTUC was discovered on routine follow-up imaging in 28%. On bivariate analysis, only multiple bladder tumors and the number of bladder recurrences were predictors for UTUC (P = 0.01 and P = 0.008, respectively). Multiple bladder tumors and ≥ 3 bladder recurrences remained significant on multivariable analysis. Conclusion: UTUC after HR-NMIBC is uncommon (4%). Despite routine follow-up CT imaging, recurrence was detected due to symptoms in most patients, and based on imaging only in 28%. Imaging surveillance can be prioritized in patients with multiple bladder tumors and those with ≥ 3 bladder recurrences. For the other patients, the benefit of imaging surveillance has to be weighed against the risks.
Introduction
In patients with primary upper tract urothelial cancer (UTUC), secondary bladder recurrence is high (30% to 45%)[1], and routine follow-up can be performed easily with a flexible cystoscope during an office visit. However, secondary UTUC post non-muscle invasive urinary bladder cancer (NMIBC) is different; it has a low incidence (1% to 4%)[2,3,4], and the follow-up requires lifelong contrast-enhanced imaging.
Whether routine annual computed tomography (CT) imaging should be performed on all NMIBC patients or if CT should be individualized is still an open issue. Some guidelines still recommend routine upper tract imaging[5]. However, IVU/CT is reported to have poor sensitivity for the detection of UTUC, which is usually low grade and low stage[6]. Moreover, most of the UTUC patients are symptomatic, and the follow-up procedure requires lifelong CT with contrast administration.
In 2 large separately published studies that involved more than 1500 and 1000 NMIBC patients, the high-risk (HR-NMIBC) group constituted the majority of the patients[7,8]. Regarding the incidence of secondary UTUC, a statistically significant difference was reported between low- and high-risk groups (0.6, 4% and 1, 9.8%, respectively)[3,4]. Moreover, it was reported that UTUC has low incidence in the low- and intermediate-risk groups (LE: 2b)[9]. New studies were therefore necessary to focus on the high-risk group alone.
We aimed to study the predictors of secondary UTUC and survival rates in patients with HR-NMIBC. The study may help in stratifying patients to determine who requires strict routine upper tract imaging and in whom the benefits could outweigh the risks. Our data will help in validating the existing knowledge and in patient counseling.
Materials and Methods
After institutional board approval (R/18.04.217), a retrospective analysis was conducted between January 2004 and December 2018. The data of patients with non-muscle invasive urothelial bladder cancers who were managed with transurethral resection (TUR) were reviewed. Patients’ files were further reviewed to identify those who had developed UTUC on follow-up.
Patients were classified into 3 risk groups according to European Association of Urology guidelines[9]: low, intermediate, and high. The low-risk group included all of the following: primary, solitary, Ta, G1 tumor < 3 cm, and no CIS. The high-risk group included any of the following: T1, G3, CIS, and multiple and recurrent and large (> 3 cm) Ta G1G2 tumors. The intermediate-risk group included tumors that were not identified in the other 2 groups.
- Inclusion and exclusion criteria: The high-risk group was the only group included in our study, and we eliminated those with low- and intermediate-risk NMIBC. Additionally, patients who had previous and/or concomitant UTUC were eliminated.
- Preoperative workup: This included medical history, physical examination, and routine laboratory and imaging workup. Office flexible cystoscopy was used as an initial tool for the diagnosis of patients who had hematuria, persistent irritative lower urinary tract symptoms, or any suspicious bladder mass on ultrasound.
- Operative details: Cystoscopy and bladder tumor resection were performed under spinal anesthesia, and general anesthesia was used if the spinal failed or was contraindicated, or if obturator jerk occurred, jeopardizing the resection. Instillation of a single dose of intravesical chemotherapy (epirubicin, 50 mg) was standard practice as a part of a prospective randomized 4-year study in our hospital. Its aim was to evaluate the effectiveness of the therapy in intermediate- and high- risk groups[10].
- Postoperative care: All patients were kept in the hospital, and the urethral catheter was kept in place for 48 hours unless other recommendations were given in cases of deep resection or suspected bladder perforation.
Routine second look TUR for all high-risk patients was introduced at our institute in 2010; before 2010, it was performed based on surgeon’s recommendations.
Eligible patients received adjuvant intravesical instillation of chemotherapy or immunotherapy (bacillus Calmette-Guérin, BCG) according to European Association of Urology guidelines. All patients underwent routine flexible cystoscopy every 3 months for the first 2 years, every 6 months for the next 3 years, and then annually. Patients with recurrent tumors were scheduled for repeat TUR followed by resumption of intravesical immunotherapy. Patients were screened annually by CT for any UTUC recurrence.
For tumor grading, the 3-tiered World Health Organization grading system[11] was used to determine the pathologic grade by different pathologists. The tumor was staged according to the 2009 TNM classification.
- The primary outcome was development of secondary UTUC (dependent variable) in patients who had non-muscle invasive bladder tumor that was correlated with possible risk factors (independent variables) and to study its clinical implementation on routine imaging.
- Statistical analysis: The data were collected using IBM SPSS version 21 (Armonk, NY: IBM Corp.). For univariate analysis, frequency and percentage were used to express nominal and ordinal variables. Mean and standard deviation were used to express scale variables with normally distributed data. Median and range were used for non-normally distributed data. For bivariate analysis, chi-square test was used for nominal variables. Multivariate analysis with a logistic regression model in a forward LR-selection strategy was generated for significant variables in bivariate analysis. In all tests, the P value was 2-sided, and significance was set at P < 0.05.
Results
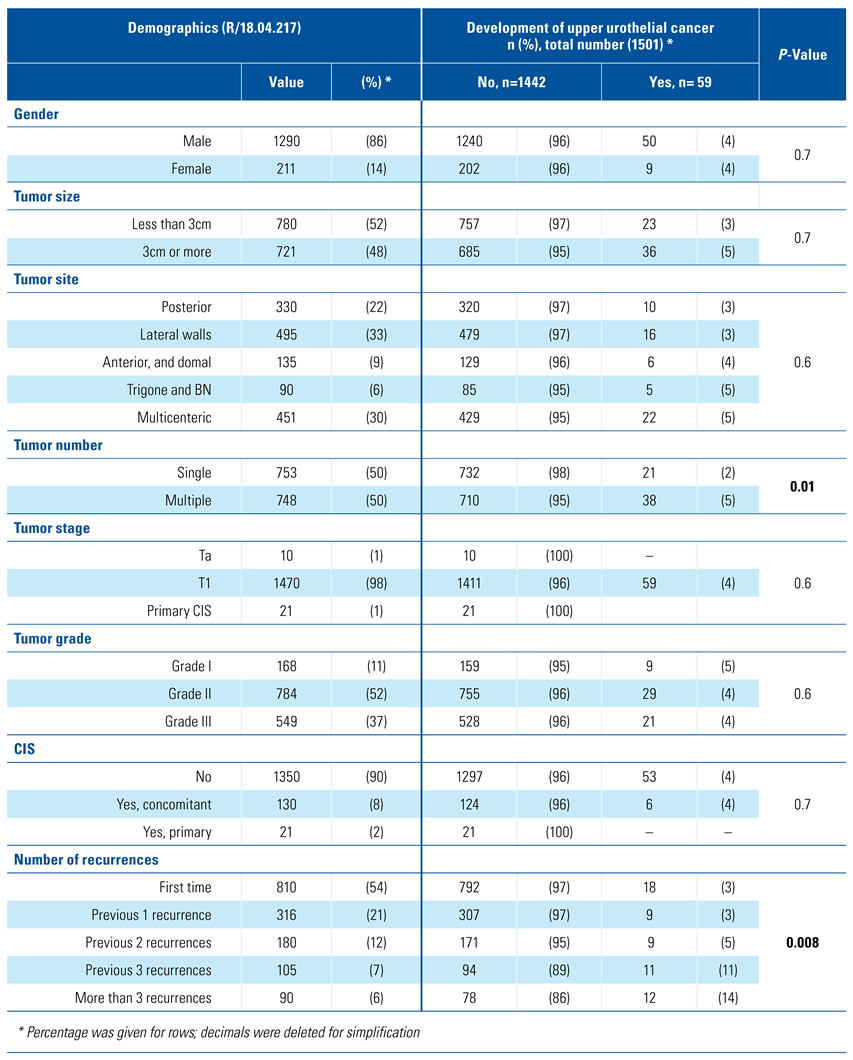
Of 1565 patients, 64 were not compliant to upper tract imaging and were eliminated from the analysis, leaving 1501 patients eligible for analysis. The mean age was 58±11, 90% (1354/1501) were male, and median follow-up was 21 months (6 to 210). Nearly half of the patients (51%) had a single bladder tumor, and (53%) had tumors less than 3cm in diameter and of GII. Upper tract urothelial cancer developed in 59 patients (4%). Demographic information and NMIBC tumor criteria for the remaining patients are shown in Table 1.

Table 1.
Demographics of 1501 patients and bivariate analysis of risk factors for development of upper urothelial cancer in patients with non-muscle invasive bladder cancer.
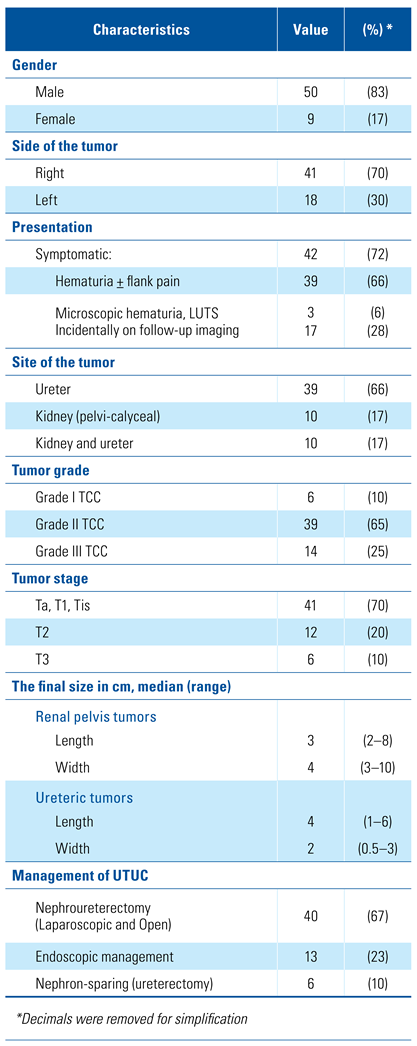
Most of the secondary UTUC were in the ureter: alone (39/59 = 66%), also in the kidney (10/59 = 17%), or in the kidney alone (10/59 = 17%). In the ureteral tumor group (n = 49), distal ureteral tumors were more common (30/49 = 61%) than multicentric or proximal ureter tumors (11/49 = 23% and 8/49 = 16%, respectively). The median time for the development of UTUC was 20 months (6 to 106 months). Hematuria was the most common symptom in this cohort (64%), while UTUC was discovered on follow-up imaging in 28% of the patients. Cytology was used in detecting UTUC with overall diagnostic accuracy of 75%. The other UTUC characteristics are shown in Table 2.

Table 2.
Tumor characteristics of 59 patients who had secondary UTUC post-surgical management of NMIBC.
Bivariate analysis of the risk factors showed that none of the following were predictors for the development of secondary UTUC: gender, tumor size, site, tumor stage or grade; only bladder tumor number (single or multiple) and the number of bladder recurrences were the predictors for secondary UTUC (P = 0.01 and P = 0.008, respectively), Table 1.
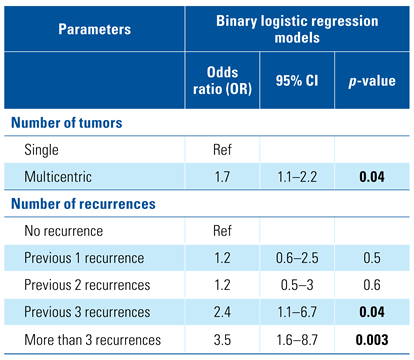
On multivariable analysis, multiple tumors remained significant when compared with the presence of a single tumor (P = 0.04). Also, as the number of previous recurrences increased, the chance of UTUC increased; previous recurrences (1 to 2) were not predictors (P = 0.5, and P = 0.6, respectively), but 3 or more previous recurrences were strongly significant predictors for secondary UTUC (P = 0.04 and P = 0.003, respectively) Table 3.

Table 3.
Multivariate logistic regression analysis of the possible risk factors.
Discussion
Urothelial cancer is a field-change disease. The multiple foci of urothelial cancers on different anatomical sites in the urothelium is a common feature of the disease[12]. Recurrence, either synchronous or metachronous, is an inherent behavior of urothelial cancer. In treating NMIBC, which is quite a common tumor, synchronous UTUC can be detected at the time of initial evaluation of bladder tumor, but metachronous UTUC is of greater concern. It requires not only routine radiation exposure but also contrast administration that may increase the risk of contrast-induced nephropathy, especially in the elderly and in those with borderline renal function. Moreover, metachronous recurrence has a lifelong risk and could present after many years (7 to 15 years)[13]. In addition, patients with metachronous recurrence require repeated contrast administration, which increases the odds of contrast-induced nephropathy. Because of these issues, our study and other published studies have aimed to identify those at high risk and to determine whether the risk outweighs the benefit.
The incidence of secondary UTUC in our series is 4%, which is consistent with the majority of published data (1% to 4%)[2,3]. This small range in incidence can be explained by the different bladder tumor characteristics between series, in terms of bladder tumor grade, stage, CIS, and recurrence.
The ureter is the site most frequently affected in secondary UTUC. In our series, 80% of the cases were in the ureter, either alone or with involvement of the renal pelvis. Others have reported similar results regarding ureteral involvement, especially in the pelvic ureter and intramural ureter[4,14]. This observation can be explained by vesicoureteral reflux; however, this theory cannot explain UTUC recurrence post radical cystectomy that was reported with similar incidence (3.9%) in a study of 1420 patients[15], or an even higher incidence (6.4%) in a large meta-analysis[16]. The theory of the panurethral nature of UTUC is therefore more widely accepted than the theory that it is due to reflux.
Of 59 patients who had secondary UTUC in our series, 72%, were symptomatic, and hematuria, either microscopic or macroscopic, was the most common complaint. Patients in this situation usually seek medical attention and undergo imaging, so UTUC is discovered at an early stage. Nevertheless, the issue remains for the 28% (17/59) who had no symptoms but were discovered incidentally with routine imaging to the upper tract. Sternberg et al., in a series of 935 patients of NMIBC, and Picozzi et al., in a large meta-analysis of UTUC recurrence post radical cystectomy in more than 13 000 patients, reported similar percentages (30% and 38%)of asymptomatic patients[6,16].
On bivariate analysis, multiple bladder tumors at the time of TURBT and bladder recurrences were the only predictors for secondary UTUC. This was consistent with the findings of Millan-Rodriguez et al.[4] and with a series of 375 patients all of whom had Ta bladder tumors[2]. The natural behavior of urothelial cancer, urothelial instability, field-change disease, and multicentricity all explain our findings and those of other studies. The results of Cox regression multivariable analysis solidified this. Although this was not calculated in our series, the recurrence time of bladder tumor could indicate more bladder instability. Recurrence within 12 months increases the risk of UTUC recurrence by 4.5-fold, as reported by Canales et al.[2]. Other predictors were reported in a few studies: tumor grade, CIS, and BCG failure[5], tumor morphology (being non-papillary increases the risk)[14], and in some cases, no predictors were found[17]. A statistically significant difference in the incidence of UTUC between low- and high-risk NMIBC patients was reported by Hurle et al. (0.6% and 4%) and Millan-Rodriguez et al. (1% and 9.8%), respectively[3,4]. For that reason, we focused only on the high-risk group in our series.
We aimed to address the debate in the literature regarding the necessity and frequency of upper tract imaging post management of NMIBC. The majority of published studies do not support its routine use for many reasons (low incidence, low grade and stage, lifelong risk), and many patients who developed UTUC are symptomatic. Moreover, this imaging requires contrast administration with known side effects on the kidney. Contrast nephropathy is one of the reasons for hospital-acquired renal insufficiency[18], and this is in addition to the radiation exposure and its side effects. Stenberg et al. reported more than 3 thousand routine imaging scans were conducted for an overall efficiency of 0.49%. In our series, more than 4 thousand were performed to detect UTUC in 59 patients (4000/59 = 68), only 17 of whom were asymptomatic (4000/17 = 235) [5]. Therefore, 235 patients were exposed to imaging in order to diagnose 1 asymptomatic patient. Most of the comments in the literature recommend imaging for symptomatic patients, those with abnormal cytology, or those with new urinary tract obstruction.
Urine cytology can be taken through regular surveillance by outpatient cystoscopy. It would be considered an excellent way to monitor the urothelium, and unexplained positive for high grade cells should warrant imaging of the UT. Instead of routine imaging, Sternberg et al. recommended a combination of history and urine cytology with lower side effects[6]. Holmang et al. and others recommended routine imaging in the case of tumor progression[17,19].
We agree that patients could be more stratified to detect those in whom the benefits of routine imaging with contrast would outweigh the risks. In the 17 asymptomatic patients who developed UTUC in our series, 9 had 2 or more intravesical recurrences, while the remaining 8 presented with silent hydronephrosis. Patients with recurrent bladder tumors may require routine ultrasonography surveillance for the upper tract, while those having 3 or more recurrences require a stricter follow-up. These data require more validation with prospective studies.
The treatment of recurrent UTUC, post management of NMIBC, follows the same standards as primary UTUC. Nephroureterectomy, whether open or laparoscopic and with excision of the bladder cuff, was the primary surgical modality in our series. However, distal ureterectomy and ureterovesical reimplantation was performed in select patients with localized tumor and no other upper urothelial foci of cancers.
The conventional 3-tiered WHO grading system was used in our series. There is still limited data regarding intraobserver and interobserver variability differences between the WHO 1973 and 2004 classification systems. The European Association of Urology currently recommends reporting both WHO 1973 and WHO 2004/2016 classifications[20].
We preferred spinal over general anesthesia for many reasons: the procedure is brief, and the patient is awake and can describe any abdominal pain for early detection of bladder perforation.
A limitation of our study is its retrospective nature; however, with the low incidence of secondary UTUC, it is difficult to conduct prospective studies. Also, it has been reported that peripheral blood levels of neutrophil to lymphocyte ratio are associated with an increased risk of disease recurrence in patients who have undergone TURBT for NMIBC[21]. Our data lacked information on white blood cell count. Also, we did not include the time of recurrence of the UTUC in BCG unresponsive and refractory populations because the dates of this group were absent for many patients and could not be retrieved. Despite these limitations, our study included a large HR-NMIBC cohort of patients who were treated at a single tertiary urology institute.
Conclusion
UTUC post management of high-risk NMIBC is uncommon (4%). UTUC was discovered on routine follow-up CT in 28% of patients; all others were symptomatic. Imaging surveillance should be performed in patients with multiple bladder tumors and those with 3 or more bladder tumor recurrences. For the other patients, the benefits of imaging surveillance have to be weighed against the risks. The optimum protocol and frequency for upper tract imaging is to be determined by future prospective studies.
Author Contributions
Ebrahim Elsaeed Abouelenein: data collection; Mohamed Elawdy: project development, data analysis; Diaa-Eldin Taha: data collection; Yasser Osman: manuscript writing; Bedeir Ali-El Dein: manuscript reviewing; Ahmed Mosbah: manuscript reviewing.
Conflicts of Interest
None declared.
Abbreviations
| BCG bacillus Calmette-Guérin |
| CT computed tomography |
| IVU intravenous urography |
| NMIBC non-muscle invasive urinary bladder cancer |
| TUR transurethral resection |
| UTUC upper tract urothelial cancer |
References
- Elawdy, M.M.; Taha, D.E.; Elbaset, M.A.; Abouelkheir, R.T.; Osman, Y. Histopathologic characteristics of upper tract urothelial carcinoma with an emphasis on their effect on cancer survival: a single- institute experience with 305 patients with long-term follow-up. Clin. Genitourin. Cancer 2016, 14, e609–e615. [Google Scholar] [CrossRef]
- Canales, B.K.; Anderson, J.K.; Premoli, J.; Slaton, J.W. Risk factors for upper tract recurrence in patients undergoing long-term surveillance for stage ta bladder cancer. J. Urol. 2006, 175, 74–77. [Google Scholar] [CrossRef]
- Hurle, R.; Losa, A.; Manzetti, A.; Lembo, A. Upper urinary tract tumors developing after treatment of superficial bladder cancer: 7-year follow-up of 591 consecutive patients. Urology 1999, 53, 1144–1148. [Google Scholar] [CrossRef]
- Millan-Rodriguez, F.; Chechile-Toniolo, G.; Salvador-Bayarri, J.; Huguet- Perez, J.; Vicente-Rodriguez, J. Upper urinary tract tumors after primary superficial bladder tumors: prognostic factors and risk groups. J. Urol. 2000, 164, 1183–1187. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; et al. European Association of Urology guidelines on non-muscle- invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Sternberg, I.A.; Keren Paz, G.E.; Chen, L.Y.; Herr, H.W.; Donat, S.M.; Bochner, B.H.; et al. Upper tract imaging surveillance is not effective in diagnosing upper tract recurrence in patients followed for nonmuscle invasive bladder cancer. J. Urol. 2013, 190, 1187–1191. [Google Scholar] [CrossRef]
- Ali-El-Dein, B.; Sooriakumaran, P.; Trinh, Q.D.; Barakat, T.S.; Nabeeh, A.; Ibrahiem el, H.I. Construction of predictive models for recurrence and progression in >1000 patients with non-muscle-invasive bladder cancer (NMIBC) from a single centre. BJU Int. 2013, 111, E331–E341. [Google Scholar] [CrossRef]
- Millán-Rodríguez, F.; Chéchile-Toniolo, G.; Salvador-Bayarri, J.; Palou, J.; Vicente-Rodríguez, J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J. Urol. 2000, 163, 73–78. [Google Scholar] [CrossRef]
- Babjuk, M.; Oosterlinck, W.; Sylvester, R.; Kaasinen, E.; Böhle, A.; Palou- Redorta, J.; et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur. Urol. 2011, 59, 997–1008. [Google Scholar] [CrossRef]
- Elsawy, A.A.; El-Assmy, A.M.; Bazeed, M.A.; Ali-El-Dein, B. The value of immediate postoperative intravesical epirubicin instillation as an adjunct to standard adjuvant treatment in intermediate and high- risk non-muscle-invasive bladder cancer: a preliminary results of randomized controlled trial. Urol. Oncol. 2019, 37, 179.e179–179.e118. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Reuter, V.R.; Mostofi, F.K. The World Health Organization/ International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am. J. Surg. Pathol. 1998, 22, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Cookson, M.S.; Soloway, S.M. Upper tract tumors in patients with primary bladder cancer followed for 15 years. J. Urol. 1996, 156, 1286–1287. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Hotta, H.; Takahashi, A.; Yanase, M.; Itoh, N.; Tachiki, H.; et al. Upper tract urothelial carcinoma following intravesical bacillus Calmette-Guerin therapy for nonmuscle- invasive bladder cancer: results from a multi-institutional retrospective study. Urol. Oncol. 2018, 36, 306.e309–306.e315. [Google Scholar] [CrossRef] [PubMed]
- Volkmer, B.G.; Schnoeller, T.; Kuefer, R.; Gust, K.; Finter, F.; Hautmann, R.E. Upper urinary tract recurrence after radical cystectomy for bladder cancer--who is at risk? J. Urol. 2009, 182, 2632–2637. [Google Scholar] [CrossRef] [PubMed]
- Picozzi, S.; Ricci, C.; Gaeta, M.; Ratti, D.; Macchi, A.; Casellato, S.; et al. Upper urinary tract recurrence following radical cystectomy for bladder cancer: a meta-analysis on 13,185 patients. J. Urol. 2012, 188, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Hession, P.; Flynn, P.; Paul, N.; Goodfellow, J.; Murthy, L.N. Intravenous urography in urinary tract surveillance in carcinoma of the bladder. Clin. Radiol. 1999, 54, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.; Hafeez, A.; Hou, S. Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 2002, 39, 930–936. [Google Scholar] [CrossRef]
- Holmang, S.; Hedelin, H.; Anderstrom, C.; Holmberg, E.; Johansson, S.L. Long-term followup of a bladder carcinoma cohort: routine followup urography is not necessary. J. Urol. 1998, 160, 45–48. [Google Scholar] [CrossRef]
- Compérat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Rouprêt, M.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus. 2019, 5, 457–466. [Google Scholar] [CrossRef]
- Vartolomei, M.D.; Porav-Hodade, D.; Ferro, M.; Mathieu, R.; Abufaraj, M.; Foerster, B.; et al. Prognostic role of pretreatment neutrophil- to-lymphocyte ratio (NLR) in patients with non-muscle-invasive bladder cancer (NMIBC): A systematic review and meta-analysis. Urol. Oncol. 2018, 36, 389–399. [Google Scholar] [CrossRef] [PubMed]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2021 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.