Classification of Molecular Biomarkers
Abstract
:Introduction
Biomarkers by Source
Blood
Serum and plasma
Cellular fractions
Urine
Ejaculate and Prostatic Secretions
Tissue
Biomarkers by Type
Genomic biomarkers
Factors affecting genomic biomarkers
DNA
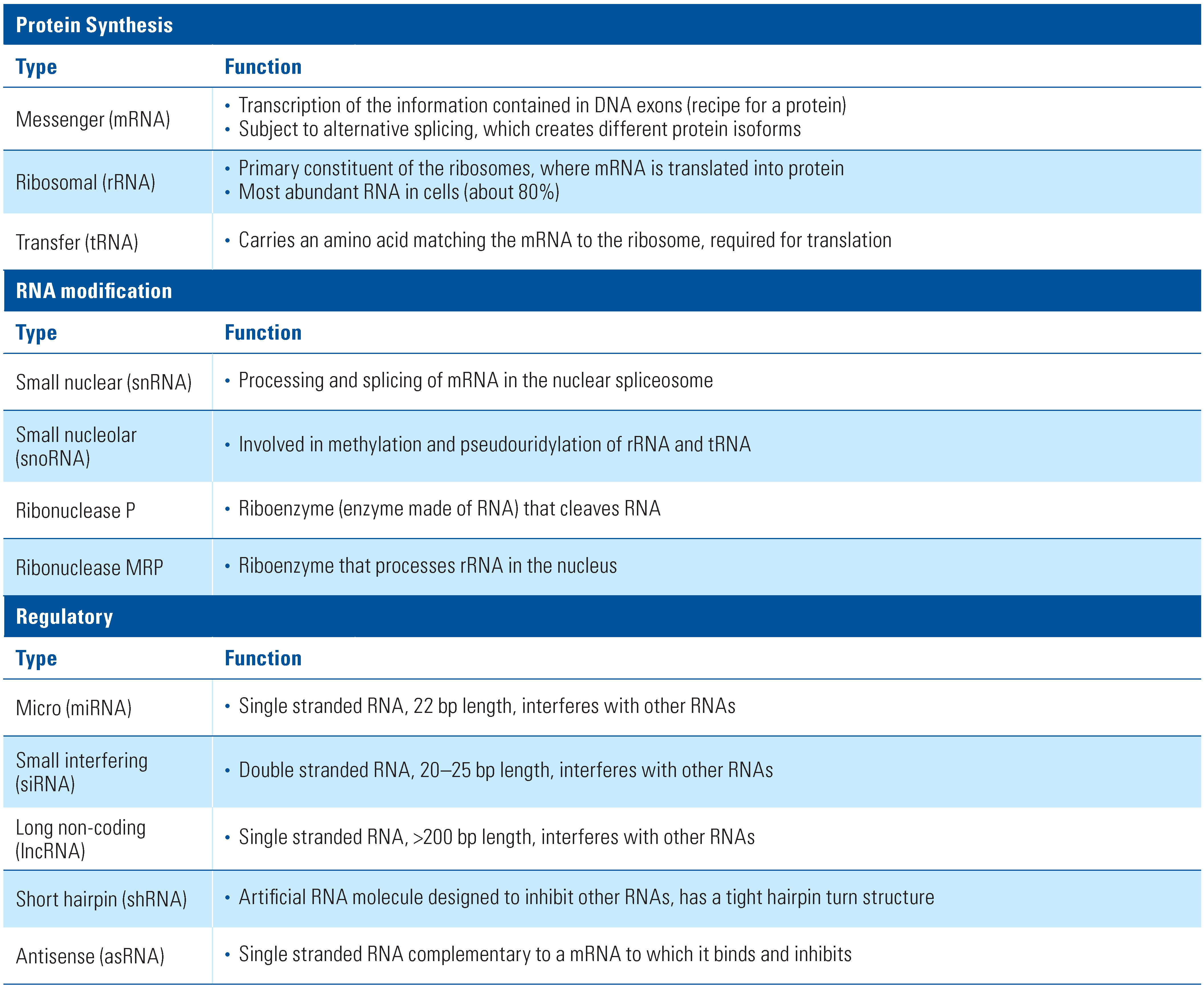
RNA
Protein
Glycans
Lipids
Imaging
Pathology
Conclusions
Conflicts of Interest
Abbreviations
| DNA | deoxyribonucleic acid |
| DNase | deoxyribonuclease |
| FFPE | formalin-fixed, paraffin-embedded |
| RNA | ribonucleic acid |
| RNase | ribonuclease |
References
- Vandekerkhove, G.; Struss, W.J.; Annala, M.; et al. Circulating Tumor DNA Abundance and Potential Utility in De Novo Metastatic Prostate Cancer. Eur. Urol. 2019, 75, 667–675. [Google Scholar] [CrossRef]
- How Kit, A.; Nielsen, H.M.; Tost, J. DNA methylation based biomarkers: Practical considerations and applications. Biochimie 2012, 94, 2314–2337. [Google Scholar] [CrossRef] [PubMed]
- Loke, S.Y.; Lee, A.S.G. The future of blood-based biomarkers for the early detection of breast cancer. Eur. J. Cancer 2018, 92, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska, M.; Wlosowicz, A.; Gawin, M.; Widlak, P. MS-Based Proteomic Analysis of serum and plasma: Problem of high abundant components and lights and shadows of albumin removal. Adv. Exp. Med. Biol. 2019, 1073, 57–76. [Google Scholar] [CrossRef]
- O’Connell, G.C.; Treadway, M.B.; Petrone, A.B.; et al. Leukocyte dynamics influence reference gene stability in whole blood: Data-driven qRT-PCR normalization is a robust alternative for measurement of transcriptional biomarkers. Lab. Med. 2017, 48, 346–356. [Google Scholar] [CrossRef]
- Matomaki, P.; Kainulainen, H.; Kyrolainen, H. Corrected whole blood biomarkers—The equation of Dill and Costill revisited. Physiol. Rep. 2018, 6, e13749. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.L.; Yasui, Y.; Li, C.I.; Fitzpatrick, A.L.; Lampe, P.D. Impact of freeze-thaw cycles and storage time on plasma samples used in mass spectrometry based biomarker discovery projects. Cancer Inform. 2005, 1, 98–104. [Google Scholar] [CrossRef]
- Scaramuzzino, D.A.; Schulte, K.; Mack, B.N.; Soriano, T.F.; Fritsche, H.A. Five-year stability study of free and total prostate-specific antigen concentrations in serum specimens collected and stored at −70 degrees C or less. Int. J. Biol. Markers 2007, 22, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Elliot, G. Preservation of biologics in a dry state: Advances in isothermal vitrification technology. Cryobiology 2013, 67, 428. [Google Scholar] [CrossRef]
- Kluge, J.A.; Li, A.B.; Kahn, B.T.; Michaud, D.S.; Omenetto, F.G.; Kaplan, D.L. Silk-based blood stabilization for diagnostics. Proc. Natl. Acad. Sci. USA 2016, 113, 5892–5897. [Google Scholar] [CrossRef]
- Jackson, D.H.; Banks, R.E. Banking of clinical samples for proteomic biomarker studies: A consideration of logistical issues with a focus on pre-analytical variation. Proteom. Clin. Appl. 2010, 4, 250–270. [Google Scholar] [CrossRef] [PubMed]
- Dittadi, R.; Fabricio, A.S.C.; Rainato, G.; et al. Preanalytical stability of [-2]proPSA in whole blood stored at room temperature before separation of serum and plasma: Implications to Phi determination. Clin. Chem. Lab. Med. 2019, 57, 521–531. [Google Scholar] [CrossRef]
- Cantiello, F.; Russo, G.I.; Vartolomei, M.D.; et al. Systemic inflammatory markers and oncologic outcomes in patients with high-risk non-muscle-invasive urothelial bladder cancer. Eur. Urol. Oncol. 2018, 1, 403–410. [Google Scholar] [CrossRef]
- Bartlett, E.K.; Flynn, J.R.; Panageas, K.S.; et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer 2020, 126, 76–85. [Google Scholar] [CrossRef]
- Okita, K.; Hatakeyama, S.; Tanaka, T.; et al. Impact of disagreement between two risk group models on prognosis in patients with metastatic renal-cell carcinoma. Clin. Genitourin. Cancer 2019, 17, e440–e6. [Google Scholar] [CrossRef]
- Zhao, L.; He, R.; Long, H.; et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat. Med. 2018, 24, 1536–1544. [Google Scholar] [CrossRef]
- Atkins, C.G.; Buckley, K.; Blades, M.W.; Turner, R.F.B. Raman Spectroscopy of blood and blood components. Appl. Spectrosc. 2017, 71, 767–793. [Google Scholar] [CrossRef]
- Riedhammer, C.; Halbritter, D.; Weissert, R. Peripheral blood mononuclear cells: Isolation, freezing, thawing, and culture. Methods Mol. Biol. 2016, 1304, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Ramcharitar, S.; Christeff, N.; Nisbett-Brown, E.; Nunez, E.; Malkin, A. Effect of anticoagulants in vitro on the viability of lymphocytes and content of free fatty acids in plasma. In Vitro Cell Dev. Biol. 1991, 27, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Buhl, T.; Legler, T.J.; Rosenberger, A.; Schardt, A.; Schon, M.P.; Haenssle, H.A. Controlled-rate freezer cryopreservation of highly concentrated peripheral blood mononuclear cells results in higher cell yields and superior autologous T-cell stimulation for dendritic cell-based immunotherapy. Cancer Immunol. Immunother. 2012, 61, 2021–2031. [Google Scholar] [CrossRef]
- McKinnon, K.M. Flow cytometry: An overview. Curr. Protoc. Immunol. 2018, 120, 5–1. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Jeronimo, C.; Henrique, R.; et al. Biomarkers in bladder cancer: A metabolomic approach using in vitro and ex vivo model systems. Int. J. Cancer 2016, 139, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, H.; Palma-Duran, S.A.; et al. Influence of storage conditions and preservatives on metabolite fingerprints in urine. Metabolites 2019, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.L.; Rood, I.M.; Deegens, J.K.J.; Klein, J.B. Isolation and characterization of urinary extracellular vesicles: Implications for biomarker discovery. Nat. Rev. Nephrol. 2017, 13, 731–749. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Di Lorenzo, V.F.; Krol, S.; Paradiso, A.V. Urinary exosomal shuttle RNA: Promising cancer diagnosis biomarkers of lower urinary tract. Int. J. Biol. Markers 2019, 34, 101–107. [Google Scholar] [CrossRef]
- Roberts, M.J.; Richards, R.S.; Gardiner, R.A.; Selth, L.A. Seminal fluid: A useful source of prostate cancer biomarkers? Biomark Med. 2015, 9, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, T.; Straus, J.; Ritter, M.A.; Jarrard, D.F.; Huang, W. Semen AMACR protein as a novel method for detecting prostate cancer. Urol. Oncol. 2018, 36, 532.e1–532.e7. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Maccaferri, M.; Mandrioli, M.; et al. Seminal cell-free DNA assessment as a novel prostate cancer biomarker. Pathol. Oncol. Res. 2018, 24, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; de la Taille, A. The role of prostate cancer antigen 3 (PCA3) in prostate cancer detection. Expert. Rev. Anticancer Ther. 2018, 18, 1013–1020. [Google Scholar] [CrossRef]
- Goessl, C.; Muller, M.; Heicappell, R.; Krause, H.; Miller, K. DNA-based detection of prostate cancer in blood, urine, and ejaculates. Ann. N. Y. Acad. Sci. 2001, 945, 51–8. [Google Scholar] [CrossRef]
- Moschini, M.; Spahn, M.; Mattei, A.; Cheville, J.; Karnes, R.J. Incorporation of tissue-based genomic biomarkers into localized prostate cancer clinics. BMC Med. 2016, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.L.; Bailey, S.; Beer, T.M.; et al. Molecular Testing in Patients With Castration-Resistant Prostate Cancer and Its Impact on Clinical Decision Making. JCO Precis Oncol. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, S.; Nodin, B.; Leandersson, K.; Boman, K.; Jirstrom, K. Clinical impact of T cells, B cells and the PD-1/PD-L1 pathway in muscle invasive bladder cancer: A comparative study of transurethral resection and cystectomy specimens. Oncoimmunology 2019, 8, e1644108. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, L.; Xia, Y.; et al. Tumor infiltrating mast cells determine oncogenic HIF-2alpha-conferred immune evasion in clear cell renal cell carcinoma. Cancer Immunol. Immunother. 2019, 68, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Salgado, R.; Gevaert, T.; et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- Cole, L.M.; Clench, M.R.; Francese, S. Sample treatment for tissue proteomics in cancer, toxicology, and forensics. Adv. Exp. Med. Biol. 2019, 1073, 77–123. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S. Sample processing considerations for detecting copy number changes in formalin-fixed, paraffin-embedded tissues. Cold Spring Harb. Protoc. 2012, 2012, 1195–1202. [Google Scholar] [CrossRef]
- Jacobs, S. Data analysis considerations for detecting copy number changes in formalin-fixed, paraffin-embedded tissues. Cold Spring Harb Protoc. 2012, 2012, 1203–1209. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Topic E15. Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories. 2007. Available online: https://ema.europa.eu/en/documents/scientific-guideline/ich-e-15-establish-definitions-genomic-biomarkers-pharmacogenomics pharmacogenetics-genomic-data_en.pdf (accessed on 10 August 2020).
- Grizzle, W.E.; Otali, D.; Sexton, K.C.; Atherton, D.S. Effects of cold ischemia on gene expression: A review and commentary. Biopreserv. Biobank. 2016, 14, 548–558. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sedmak, D.; Jewell, S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol. 2002, 161, 1961–1971. [Google Scholar] [CrossRef]
- Evers, D.L.; Fowler, C.B.; Cunningham, B.R.; Mason, J.T.; O’Leary, T.J. The effect of formaldehyde fixation on RNA: Optimization of formaldehyde adduct removal. J. Mol. Diagn. 2011, 13, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, Y.; Nakamura, T.; Satonaka, K.; et al. Fundamental study on the mechanism of DNA degradation in tissues fixed in formaldehyde. J. Clin. Pathol. 1990, 43, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Groelz, D.; Viertler, C.; Pabst, D.; Dettmann, N.; Zatloukal, K. Impact of storage conditions on the quality of nucleic acids in paraffin embedded tissues. PLoS ONE. 2018, 13, e0203608. [Google Scholar] [CrossRef] [PubMed]
- King, I.B.; Satia-Abouta, J.; Thornquist, M.D.; et al. Buccal cell DNA yield, quality, and collection costs: Comparison of methods for large-scale studies. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1130–1133. [Google Scholar]
- Hansen, T.V.; Simonsen, M.K.; Nielsen, F.C.; Hundrup, Y.A. Collection of blood, saliva, and buccal cell samples in a pilot study on the Danish nurse cohort: Comparison of the response rate and quality of genomic DNA. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 2072–6. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Li, T.; Huang, Y.; et al. RNA biomarkers: Frontier of precision medicine for cancer. Noncoding RNA 2017, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.M. Are samples in your freezer still good for biomarker discovery? Am. J. Clin. Pathol. 2013, 140, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.; Shaffer, D.R.; Philip, J.; Chaparro, C.A.; Erdjument-Bromage, H.; Olshen, A.B.; et al. Differential exoprotease activities confer tumor-specific serum peptidome patterns. J Clin Investig. 2006, 116, 271–284. [Google Scholar] [CrossRef]
- Thomas, C.E.; Sexton, W.; Benson, K.; Sutphen, R.; Koomen, J. Urine collection and processing for protein biomarker discovery and quantification. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 953–9. [Google Scholar] [CrossRef]
- Harpole, M.; Davis, J.; Espina, V. Current state of the art for enhancing urine biomarker discovery. Expert. Rev. Proteom. 2016, 13, 609–626. [Google Scholar] [CrossRef]
- Khoury, G.A.; Baliban, R.C.; Floudas, C.A. Proteome-wide post-translational modification statistics: Frequency analysis and curation of the swiss-prot database. Sci. Rep. 2011, 1. [Google Scholar] [CrossRef]
- Kailemia, M.J.; Park, D.; Lebrilla, C.B. Glycans and glycoproteins as specific biomarkers for cancer. Anal. Bioanal. Chem. 2017, 409, 395–410. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cheng, X.L.; Lin, R.C. Lipidomics applications for discovering biomarkers of diseases in clinical chemistry. Int. Rev. Cell Mol. Biol. 2014, 313, 1–26. [Google Scholar] [CrossRef]
- Stephenson, D.J.; Hoeferlin, L.A.; Chalfant, C.E. Lipidomics in translational research and the clinical significance of lipid-based biomarkers. Transl. Res. 2017, 189, 13–29. [Google Scholar] [CrossRef] [PubMed]
- White paper on imaging biomarkers. Insights Imaging 2010, 1, 42–45. [CrossRef]
- Medical imaging in personalised medicine: A white paper of the research committee of the European Society of Radiology (ESR). Insights Imaging 2011, 2, 621–630. [CrossRef] [PubMed]
- Schwartz, L.H.; Litiere, S.; de Vries, E.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–7. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.H. An introduction to the clinical practice of theranostics in oncology. Br. J. Radiol. 2018, 91, 20180440. [Google Scholar] [CrossRef]
- Janowczyk, A.; Madabhushi, A. Deep learning for digital pathology image analysis: A comprehensive tutorial with selected use cases. J. Pathol. Inform. 2016;7, 7, 29. [Google Scholar] [CrossRef]
- Janowczyk, A.; Zuo, R.; Gilmore, H.; Feldman, M.; Madabhushi, A. HistoQC: An open-source quality control tool for digital pathology slides. JCO Clin. Cancer Inform. 2019, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2020 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Shah, A.; Grimberg, D.C.; Inman, B.A. Classification of Molecular Biomarkers. Soc. Int. Urol. J. 2020, 1, 8-15. https://doi.org/10.48083/AKUI6936
Shah A, Grimberg DC, Inman BA. Classification of Molecular Biomarkers. Société Internationale d’Urologie Journal. 2020; 1(1):8-15. https://doi.org/10.48083/AKUI6936
Chicago/Turabian StyleShah, Ankeet, Dominic C. Grimberg, and Brant A. Inman. 2020. "Classification of Molecular Biomarkers" Société Internationale d’Urologie Journal 1, no. 1: 8-15. https://doi.org/10.48083/AKUI6936
APA StyleShah, A., Grimberg, D. C., & Inman, B. A. (2020). Classification of Molecular Biomarkers. Société Internationale d’Urologie Journal, 1(1), 8-15. https://doi.org/10.48083/AKUI6936




