Unraveling the Genetic Threads of History: mtDNA HVS-I Analysis Reveals the Ancient Past of the Aburra Valley
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Ancient DNA Extraction
2.3. PCR Amplification and Sequencing
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aceituno Bocanegra, F.J.; Uriarte González, A. Conectando un territorio: Simulación de rutas de movilidad entre cazadores-recolectores y primeros cultivadores. El caso del Cauca medio (Macizo Volcánico 2019, Colombia). Trab. Prehist. 2019, 76, 219–235. [Google Scholar] [CrossRef]
- Correal, G.; Van der Hammen, T. Supervivencia de Mastodontes, Megaterios y presencia del Hombre en el Valle del Magdalena (Colombia) entre 6000 y 5000 AP. Rev. Acad. Colomb. De Cienc. 2003, 27, 159–164. [Google Scholar] [CrossRef]
- Díaz-Matallana, M.; Gómez, A.; Briceño, I.; Rodríguez, J.V. Genetic analysis of Paleo-Colombians from Nemocón, Cundinamarca provides insights on the early peopling of Northwestern South America. Rev. La Acad. Colomb. Cienc. Exactas Físicas Nat. 2016, 40, 461–483. [Google Scholar] [CrossRef]
- Delgado, M.; Rodríguez, F.; Kassadjikova, K.; Fehren-Schmitz, L. A paleogenetic perspective of the Sabana de Bogotá (Northern South America) population history over the Holocene (9000–550 cal BP). bioRxiv 2020. bioRxiv:2020.01.24.918425. [Google Scholar] [CrossRef]
- Gustavo, V.S.; Marín, C.A.M.; Salas, L.V.C. Alteration of tropical forest vegetation from the Pleistocene–Holocene transition and plant cultivation from the end of early Holocene through Middle Holocene in Northwest Colombia. Quat. Int. 2015, 363, 28–42. [Google Scholar]
- Aristizábal Espinosa, P. Los Aburráes. In Tras Los Rastros de Nuestros Ancestros, 1st ed.; Secretaría de Cultura Ciudadana, Alcaldía de Medellín: Medellín, Colombia, 2015. [Google Scholar]
- Santos Vecino, G. Las prácticas funerarias prehispánicas de la región central de Antioquia. In Catálogo Región Andina; Universidad de Antioquia: Medellín, Colombia, 2017; pp. 41–71. [Google Scholar]
- Cooper, A.; Poinar, H. Ancient DNA: Do it right or not at All. Science 2000, 289, 1139. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.; Barrell, B.; de Bruijn, M.; Coulson, A.; Drouin, J.; Eperon, I.; Nierlich, D.; Roe, B.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Fehren-Schmitz, L.; Warnberg, O.; Reindel, M.; Seidenberg, V.; Tomasto-Cagigao, E.; Isla-Cuadrado, J.; Hummel, S.; Herrmann, B. Diachronic investigations of mitochondrial and Y-chromosomal genetic markers in Pre-Columbian Andean highlanders from South Peru. Ann. Hum. Genet. 2011, 75, 266–283. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Phillip, M.; Briceño, I.; Gómez, A.; Devor, E.; Bernal, J.E.; Crawford, M. Biological relationship between central and South American Chibchan speaking populations: Evidence from mtDNA. Am. J. Phys. Anthropol. 2007, 133, 753–770. [Google Scholar] [CrossRef]
- Claudia, N.S.M.; Anderson, C.E.; Uricoechea, D.; Durán, C.; Briceño-Balcázar, I.; Villegas, J.B. Mitochondrial DNA analysis suggests a Chibchan migration into Colombia. Univ. Sci. 2015, 20, 261–278. [Google Scholar] [CrossRef]
- Casas-Vargas, A.; Romero, L.; Usaquén, W.; Zea, S.; Silva, M.; Briceño, I.; Gómez, A.; Rodríguez, J.V. Diversidad del ADN mitocondrial en restos óseos prehispánicos. Biomédica 2017, 37, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Raff., A.J.; Bolnick, D.; Tackney, J.; O’Rourke, D. Ancient DNA perspectives on American colonization and population history. Am. J. Phys. Anthropol. 2011, 146, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Starikovskaya, Y.B.; Sukernik, R.I.; Schurr, T.G.; Kogelnik, A.M.; Wallace, D.C. mtDNA diversity in Chukchi and Siberian Eskimos: Implications for the genetic history of Ancient Beringia and the peopling of the New World. Am. J. Hum. Genet. 1998, 63, 1473–1491. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Hayes, M.G.; Cabana, G.S.; Huff, C.; Coltrain, J.B.; O’rourke, D.H. Inferring population continuity versus replacement with aDNA: A cautionary tale from the Aleutian Islands. Hum. Biol. 2009, 81, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Raff, J.; Tackney, J.; O’Rourke, D.H. South from Alaska: A pilot aDNA study of genetic history on the Alaska Peninsula and the eastern Aleutians. Hum. Biol. 2010, 82, 677–693. [Google Scholar] [CrossRef]
- Malhi, R.S.; Kemp, B.M.; Eshleman, J.A.; Cybulski, J.; Smith, D.G.; Cousins, S.; Harry, H. Mitochondrial haplogroup M discovered in prehistoric North Americans. J. Archaeol. Sci. 2007, 34, 642–648. [Google Scholar] [CrossRef]
- Monsalve, M.V.; Stone, A.C.; Lewis, C.M.; Rempel, A.; Richards, M.; Straathof, D.; Devine, D.V. Brief communication: Molecular analysis of the Kwäday Dän Ts’ finchi ancient remains found in a glacier in Canada. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2002, 119, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.G. Paleogenetic Assessments of Human Migration and Population Replacement in North American Arctic Prehistory; The University of Utah: Salt Lake City, UT, USA, 2002. [Google Scholar]
- Gilbert, M.T.P.; Kivisild, T.; Grønnow, B.; Andersen, P.K.; Metspalu, E.; Reidla, M.; Tamm, E.; Axelsson, E.; Campos, P.F.; Willerslev, E.; et al. Paleo-Eskimo mtDNA genome reveals matrilineal discontinuity in Greenland. Science 2008, 320, 1787–1789. [Google Scholar] [CrossRef]
- Malhi, R.S.; Mortensen, H.M.; Eshleman, J.A.; Kemp, B.M.; Lorenz, J.G.; Kaestle, F.A.; Johnson, J.R.; Gorodezky, C.; Smith, D.G. Native American mtDNA prehistory in the American southwest. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2003, 120, 108–124. [Google Scholar] [CrossRef]
- Kaestle, F.A.; Horsburgh, K.A. Ancient DNA in anthropology: Methods, applications, and ethics. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2002, 119, 92–130. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.C.; Stoneking, M. mtDNA analysis of a prehistoric Oneota population: Implications for the peopling of the New World. Am. J. Hum. Genet. 1998, 62, 1153–1170. [Google Scholar] [CrossRef]
- Snow, M.H.; Durand, K.R.; Smith, D.G. Ancestral Puebloan mtDNA in context of the greater southwest. J. Archaeol. Sci. 2010, 37, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Carlyle, S.W.; Parr, R.L.; Hayes, M.G.; O’Rourke, D.H. Context of maternal lineages in the Greater Southwest. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2000, 113, 85–101. [Google Scholar] [CrossRef]
- LeBlanc, S.A.; Cobb Kreisman, L.S.; Kemp, B.M.; Smiley, F.E.; Carlyle, S.W.; Dhody, A.N.; Benjamin, T. Quids and aprons: Ancient DNA from artifacts from the American Southwest. J. Field Archaeol. 2007, 32, 161–175. [Google Scholar] [CrossRef]
- Schultz Shook, B.A.; Smith, D.G. Using ancient mtDNA to reconstruct the population history of northeastern North America. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2008, 137, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.A.; Smith, D.G. Migration and social structure among the Hopewell: Evidence from ancient DNA. Am. Antiq. 2007, 72, 627–644. [Google Scholar] [CrossRef]
- Raff, J.A. An Ancient DNA Perspective on the Prehistory of the Lower Illinois Valley. Doctoral Dissertation, Indiana University, Bloomington, Indiana, 2008. [Google Scholar]
- Mills, L.A. Mitochondrial DNA Analysis of the Ohio Hopewell of the Hopewell Mound Group; The Ohio State University: Columbus, OH, USA, 2003. [Google Scholar]
- Kemp, B.M.; Reséndez, A.; Román Berrelleza, J.A.; Malhi, R.S.; Smith, D.G. An analysis of ancient Aztec mtDNA from Tlatelolco: Pre-Columbian relations and the spread of Uto-Aztecan. Biomol. Archaeol. Genet. Approaches Past 2005, 32, 22–42. [Google Scholar]
- González-Oliver, A.; Márquez-Morfín, L.; Jiménez, J.C.; Torre-Blanco, A. Founding amerindian mitochondrial DNA lineages in ancient Maya from Xcaret, Quintana Roo. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2001, 116, 230–235. [Google Scholar] [CrossRef]
- Merriwether, D.A.; Reed, D.M.; Ferrell, R.E. Ancient and Contemporary Mitochondrial DNA Variation in the Maya; Smithsonian Institute Press: Washington, DC, USA, 1997; pp. 208–217. [Google Scholar]
- Lalueza-Fox, C.; Calderon, F.L.; Calafell, F.; Morera, B.; Bertranpetit, J. MtDNA from extinct Tainos and the peopling of the Caribbean. Ann. Hum. Genet. 2001, 65, 137–151. [Google Scholar] [CrossRef]
- Lalueza-Fox, C.; Gilbert, M.T.P.; Martínez-Fuentes, A.J.; Calafell, F.; Bertranpetit, J. Mitochondrial DNA from pre-Columbian Ciboneys from Cuba and the prehistoric colonization of the Caribbean. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2003, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Torroni, A.; Sukernik, R.I.; Schurr, T.G.; Starikorskaya, Y.B.; Cabell, M.F.; Crawford, M.H.; Comuzzie, A.G.; Wallace, D.C. mtDNA variation of aboriginal Siberians reveals distinct genetic affinities with Native Americans. Am. J. Hum. Genet. 1993, 53, 591. [Google Scholar] [PubMed]
- Santos, M.; Ward, R.H.; Barrantes, R. mtDNA variation in the Chibcha Amerindian Huetar from Costa Rica. Hum. Biol. 1994, 66, 963–977. [Google Scholar] [PubMed]
- Kolman, C.J.; Bermingham, E.; Cooke, R.; Ward, R.H.; Arias, T.D.; Guionneau-Sinclair, F. Reduced mtDNA diversity in the Ngöbé Amerinds of Panamá. Genetics 1995, 140, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Batista, O.; Kolman, C.J.; Bermingham, E. Mitochondrial DNA diversity in the Kuna Amerinds of Panama. Hum. Mol. Genet. 1995, 4, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Casas-Vargas, A.; Gómez, A.; Briceño, I.; Díaz-Matallana, M.; Bernal, J.E.; Rodríguez, J.V. High genetic diversity on a sample of pre-Columbian bone remains from Guane territories in northwestern Colombia. Am. J. Phys. Anthropol. 2011, 146, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Uricoechea Patiño, D.; Collins, A.; García, O.J.R.; Santos Vecino, G.; Cuenca, J.V.R.; Bernal, J.E.; Benavides Benítez, E.; Vergara Muñoz, S.; Briceño Balcázar, I. High Mitochondrial Haplotype Diversity Found in Three Pre-Hispanic Groups from Colombia. Genes 2023, 14, 1853. [Google Scholar] [CrossRef]
- Fernandez, C. La Arqueología Molecular Aplicada a la Solución de Problemas Prehistóricos: Análisis de ADN Mitocondrial en Momias y Restos Óseos Prehispánicos. Undergraduate Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 1999. [Google Scholar]
- Jara, N.P.; Díaz, M.; Villegas, V.; López de Mesa, C.; Torres, D.; Bernal, J.; Gómez, A.; Briceño, I. Aplication of authenticity criteria in mitochondrial studies on archaic bone remains from a prehispanic Muisca population. Colomb. Médica 2010, 41, 306–314. [Google Scholar]
- Sánchez, C. Secuenciación de ADN Mitocondrial a Partir de Fragmentos Óseos Prehispánicos Hallados en el Sector de Candelaria La Nueva en Bogotá; Pontificia Universidad Javeriana: Bogotá, Colombia, 2007. [Google Scholar]
- Noguera-Santamaría, M.C.; Rivera-Sandoval, J.; Martín, J.G.; Briceño-Balcázar, I.; Gómez-Gutiérrez, A. Análisis genético de restos humanos precolombinos del Bajo Magdalena sugiere una ruta migratoria y continuidad genética matrilineal en el norte de Suramérica. Rev. La Acad. Colomb. Cienc. Exactas Físicas Nat. 2020, 44, 704–715. [Google Scholar] [CrossRef]
- Romero Murillo, L.M.; Silva Montaña, V.M.; Zea Montoya, S.; Usaquén Martínez, W.; Briceño Balcázar, I.; Gómez Gutiérrez, A.; Casas Vargas, L.A. Diversidad del ADN Mitocondrial en Restos óseos Prehispánicos Asociados al Templo del Sol en los Andes Orientales Colombianos; Pontificia Universidad Javeriana: Bogotá, Colombia, 2017. [Google Scholar]
- Silva, A.; Briceño, I.; Burgos, J.; Torres, D.; Villegas, V.; Gómez, A.; Rodríguez Cuenca, J.V. Mitochondrial DNA analysis on pre-Columbian bone remains of the Herrera period. Biomédica 2008, 28, 569–577. [Google Scholar] [CrossRef]
- Miller, M.J.; Agarwal, S.C.; Aristizabal, L.; Langebaek, C. The daily grind: Sex-and age-related activity patterns inferred from cross-sectional geometry of long bones in a pre-Columbian muisca population from Tibanica, Colombia. Am. J. Phys. Anthropol. 2018, 167, 311–326. [Google Scholar] [CrossRef]
- Monsalve, M.V.; Cardenas, F.; Guhl, F.; Delaney, A.D.; Devine, D.V. Phylogenetic analysis of mtDNA lineages in South American mummies. Ann. Hum. Genet. 1996, 60, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Usme-Romero, S.; Alonso, M.; Hernandez-Cuervo, H.; Yunis, E.J.; Yunis, J.J. Genetic differences between Chibcha and Non-Chibcha speaking tribes based on mitochondrial DNA (mtDNA) haplogroups from 21 Amerindian tribes from Colombia. Genet. Mol. Biol. 2013, 36, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, J.W.; Fonseca, O. Goldwork and Chibchan identity: Endogenous change and diffuse unity in the Isthmo-Colombian area. In Gold and Power in Ancient Costa Rica, Panama, and Colombia; Dumbarton Oaks Research Library and Collection: Washington, DC, USA, 2003; pp. 49–89. [Google Scholar]
- Ward, R.H.; Redd, A.; Valencia, D.; Frazier, B.; Pääbo, S. Genetic and linguistic differentiation in the Americas. Proc. Natl. Acad. Sci. USA 1993, 90, 10663–10667. [Google Scholar] [CrossRef] [PubMed]
- Keyeux, G.; Rodas, C.; Gelvez, N.; Carter, D. Possible migration routes into South America deduced from mitochondrial DNA studies in Colombian Amerindian populations. Hum. Biol. 2002, 74, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Schurr, T.G.; Ballinger, S.W.; Gan, Y.Y.; Hodge, J.A.; Merriwether, D.A.; Lawrence, D.N.; Knowler, W.C.; Weiss, K.M.; Wallace, D.C. Amerindian mitochondrial DNAs have rare Asian mutations at high frequencies, suggesting they derived from four primary maternal lineages. Am. J. Hum. Genet. 1990, 46, 613. [Google Scholar] [PubMed]
- Xavier, C.; Builes, J.J.; Gomes, V.; Ospino, J.M.; Aquino, J.; Parson, W.; Amorim, A.; Gusmão, L.; Goios, A. Admixture and genetic diversity distribution patterns of non-recombining lineages of Native American ancestry in Colombian populations. PLoS ONE 2015, 10, e0120155. [Google Scholar] [CrossRef] [PubMed]
- Shimada, I.; Shinoda, K.; Farnum, J.; Corruccini, R.; Watanabe, H. An integrated analysis of pre-hispanic mortuary practices: A middle Sicn case study. Curr. Anthropol. 2004, 45, 369–402. [Google Scholar] [CrossRef]
- Shinoda, K.I.; Adachi, N.; Guillen, S.; Shimada, I. Mitochondrial DNA analysis of ancient Peruvian highlanders. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2006, 131, 98–107. [Google Scholar] [CrossRef]
- Kemp, B.M.; Tung, T.A.; Summar, M.L. Genetic continuity after the collapse of the Wari empire: Mitochondrial DNA profiles from Wari and post-Wari populations in the ancient Andes. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2009, 140, 80–91. [Google Scholar] [CrossRef]
- Luciani, S.; Fornaciari, G.; Rickards, O.; Labarga, C.M.; Rollo, F. Molecular characterization of a pre-Columbian mummy and in situ coprolite. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2006, 129, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Valverde, G.; Barreto Romero, M.I.; Flores Espinoza, I.; Cooper, A.; Fehren-Schmitz, L.; Llamas, B.; Haak, W. Ancient DNA analysis suggests negligible impact of the Wari empire expansion in Peru’s central coast during the Middle Horizon. PLoS ONE 2016, 11, e0155508. [Google Scholar] [CrossRef] [PubMed]
- Fehren-Schmitz, L.; Reindel, M.; Cagigao, E.T.; Hummel, S.; Herrmann, B. Pre-Columbian population dynamics in coastal southern Peru: A diachronic investigation of mtDNA patterns in the Palpa region by ancient DNA analysis. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2010, 141, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Buikstra, J.E.; Stone, A.C. Ancient DNA and genetic continuity in the South Central Andes. Lat. Am. Antiq. 2007, 18, 145–160. [Google Scholar] [CrossRef]
- Moraga, M.; Santoro, C.M.; Standen, V.G.; Carvallo, P.; Rothhammer, F. Microevolution in prehistoric Andean populations: Chronologic mtDNA variation in the desert valleys of northern Chile. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2005, 127, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Carnese, F.R.; Mendisco, F.; Keyser, C.; Dejean, C.B.; Dugooujon, J.-M.; Bravi, C.M.; Ludes, B.; Crubézy, E. Paleogenetical study of pre-Columbian samples from Pampa Grande (Salta Argentina). Am. J. Phys. Anthropol. 2010, 141, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Manríquez Soto, G.R.; Moraga Vergara, M.; Santoro, C.; Aspillaga Fontaine, E.; Arriaza, B.T.; Rothhammer Engel, F. Morphometric and mtdna analyses of archaic skeletal remains from southwestern South America. Chungara Rev. De Antropol. Chil. 2011, 43, 283–292. [Google Scholar] [CrossRef]
- Ginther, C.; Corach, D.; Penacino, G.A.; Rey, J.A.; Carnese, F.R.; Hutz, M.H.; Anderson, A.; Just, J.; Salzano, F.M.; King, M.C. Genetic variation among the Mapuche Indians from the Patagonian region of Argentina: Mitochondrial DNA sequence variation and allele frequencies of several nuclear genes. In DNA Fingerprinting: State of the Science; Springer: Berlin/Heidelberg, Germany, 1993; pp. 211–219. [Google Scholar]
- Lewis, C.M.; Lizárraga, B.; Tito, R.Y.; López, P.W.; Iannacone, G.C.; Medina, A.; Martínez, R.; Polo, S.I.; Augusto, F.; Cáceres, A.M.; et al. Mitochondrial DNA and the peopling of South America. Hum. Biol. 2007, 79, 159–178. [Google Scholar] [CrossRef]
- Wang, S.; Lewis, C.M., Jr.; Jakobsson, M.; Ramachandran, S.; Ray, N.; Bedoya, G.; Rojas, W.; Parra, M.V.; Molina, J.A.; Ruiz-Linares, A. Genetic variation and population structure in Native Americans. PLoS Genet. 2007, 3, e185. [Google Scholar] [CrossRef]
- Torroni, A.; Schurr, T.G.; Cabell, M.F.; Brown, M.D.; Neel, J.V.; Larsen, M.; Smith, D.G.; Vullo, C.M.; Wallace, D.C. Asian affinities and continental radiation of the four founding Native American mtDNAs. Am. J. Hum. Genet. 1993, 53, 563. [Google Scholar]
- Ward, R.H.; Salzano, F.M.; Bonatto, S.L.; Hutz, M.H.; Coimbra, C.E.A., Jr.; Santos, R.V. Mitochondrial DNA polymorphism in three Brazilian Indian tribes. Am. J. Hum. Biol. Off. J. Hum. Biol. Assoc. 1996, 8, 317–323. [Google Scholar] [CrossRef]
- Huber, N.; Parson, W.; Dür, A. Next generation database search algorithm for forensic mitogenome analyses. Forensic Sci. International. Genet. 2018, 37, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, M.R.; Aram, B.; Raveane, A.; Migliore, N.R.; Colombo, G.; Ongaro, L.; Rivera, J.; Mendizábal, T.; Hernández-Mora, I.; Achilli, A.; et al. Archaeogenomic distinctiveness of the Isthmo-Colombian area. Cell 2021, 184, 1706–1723. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Perego, U.A.; Bravi, C.M.; Coble, M.D.; Kong, Q.P.; Woodward, S.R.; Salas, A.; Torroni, A.; Bandelt, H.J. The phylogeny of the four pan-American MtDNA haplogroups: Implications for evolutionary and disease studies. PLoS ONE 2008, 3, e1764. [Google Scholar] [CrossRef] [PubMed]
- Duggan, A.T.; Harris, A.J.T.; Marciniak, S.; Marshall, I.; Kuch, M.; Kitchen, A.; Renaud, G.; Southon, J.; Fuller, B.; Young, J.; et al. Genetic Discontinuity between the Maritime Archaic and Beothuk Populations in Newfoundland, Canada. Curr. Biol. CB 2017, 27, 3149–3156.e11. [Google Scholar] [CrossRef] [PubMed]
- Lindo, J.; Achilli, A.; Perego, U.A.; Archer, D.; Valdiosera, C.; Petzelt, B.; Mitchell, J.; Worl, R.; Dixon, E.J.; Malhi, R.S.; et al. Ancient individuals from the North American Northwest Coast reveal 10,000 years of regional genetic continuity. Proc. Natl. Acad. Sci. USA 2017, 114, 4093–4098. [Google Scholar] [CrossRef] [PubMed]
- Perego, U.A.; Lancioni, H.; Tribaldos, M.; Angerhofer, N.; Ekins, J.E.; Olivieri, A.; Woodward, S.R.; Pascale, J.M.; Cooke, R.; Motta, J.; et al. Decrypting the mitochondrial gene pool of modern Panamanians. PLoS ONE 2012, 7, e38337. [Google Scholar] [CrossRef]
- Gayden, T.; Perez, A.; Persad, P.J.; Bukhari, A.; Chennakrishnaiah, S.; Simms, T.; Maloney, T.; Rodriguez, K.; Herrera, R.J. The Himalayas: Barrier and conduit for gene flow. Am. J. Phys. Anthropol. 2013, 151, 169–182. [Google Scholar] [CrossRef]
- Kumar, S.; Ravuri, R.R.; Koneru, P.; Urade, B.P.; Sarkar, B.N.; Chandrasekar, A.; Rao, V.R. Reconstructing Indian-Australian phylogenetic link. BMC Evol. Biol. 2009, 9, 173. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Kumar, S.; Sreenath, J.; Sarkar, B.N.; Urade, B.P.; Mallick, S.; Bandopadhyay, S.S.; Barua, P.; Barik, S.S.; Rao, V.R.; et al. Updating phylogeny of mitochondrial DNA macrohaplogroup m in India: Dispersal of modern human in South Asian corridor. PLoS ONE 2009, 4, e7447. [Google Scholar] [CrossRef]
- Bhandari, S.; Zhang, X.; Cui, C.; Bianba Liao, S.; Peng, Y.; Zhang, H.; Xiang, K.; Shi, H.; Qi, X.; Su, B.; et al. Genetic evidence of a recent Tibetan ancestry to Sherpas in the Himalayan region. Sci. Rep. 2015, 5, 16249. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Song, I.; Ha, E.; Cho, S.B.; Yang, W.I.; Shin, K.J. mtDNAmanager: A Web-based tool for the management and quality analysis of mitochondrial DNA control-region sequences. BMC Bioinform. 2008, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Malyarchuk, B.A.; Derenko, M.V. Evaluating the role of selection in the evolution of mitochondrial genomes of aboriginal peoples of Siberia. Vavilov J. Genet. Breed. 2023, 27, 218. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Siddiqi, M.H.; Ali, S.; Ali, S.; Sabar, M.F. Mitochondrial DNA control region variants analysis in Balti population of Gilgit-Baltistan, Pakistan. Meta Gene 2020, 23, 100630. [Google Scholar] [CrossRef]
- Starikovskaya, E.B.; Sukernik, R.I.; Derbeneva, O.A.; Volodko, N.V.; Ruiz-Pesini, E.; Torroni, A.; Brown, M.D.; Lott, M.T.; Hosseini, S.H.; Wallace, D.C.; et al. Mitochondrial DNA diversity in indigenous populations of the southern extent of Siberia, and the origins of Native American haplogroups. Ann. Hum. Genet. 2005, 69, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Der Sarkissian, C.; Brotherton, P.; Balanovsky, O.; Templeton, J.E.; Llamas, B.; Soubrier, J.; Moiseyev, V.; Khartanovich, V.; Cooper, A.; Genographic Consortium; et al. Mitochondrial genome sequencing in Mesolithic North East Europe Unearths a new sub-clade within the broadly distributed human haplogroup C1. PLoS ONE 2014, 9, e87612. [Google Scholar]
- Russo, M.G.; Dejean, C.B.; Avena, S.A.; Seldes, V.; Ramundo, P. Mitochondrial lineage A2ah found in a pre-Hispanic individual from the Andean region. Am. J. Hum. Biol. 2018, 30, e23134. [Google Scholar] [CrossRef]
- Russo, M.G.; Arencibia, V.; Emery, M.; Bettera Marcat, G.; Seldes, V.; Mercolli, P.; Soria, S.; Maldonado, L.; Kamenetzky, L.; Stone, A.C.; et al. Ancient mitochondrial genome diversity in South America: Contributions from Quebrada del Toro, Northwestern Argentina. Am. J. Biol. Anthropol. 2023, 181, 597–610. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Herrnstadt, C.; Yao, Y.G.; Kong, Q.P.; Kivisild, T.; Rengo, C.; Scozzari, R.; Richards, M.; Villems, R.; Macaulay, V.; et al. Identification of Native American founder mtDNAs through the analysis of complete mtDNA sequences: Some caveats. Ann. Hum. Genet. 2003, 67, 512–524. [Google Scholar] [CrossRef]
- Sala, A.; Caputo, M.; Ginart, S.; Theiler, G.; Parolin, M.L.; Carnese, R.F.; Fainboim, L.; Corach, D. Historical records under the genetic evidence: “Chiriguano” tribe genesis as a test case. Mol. Biol. Rep. 2018, 45, 987–1000. [Google Scholar] [CrossRef]
- Cui, Y.; Lindo, J.; Hughes, C.E.; Johnson, J.W.; Hernandez, A.G.; Kemp, B.M.; Ma, J.; Cunningham, R.; Petzelt, B.; Mitchell, J.; et al. Ancient DNA Analysis of Mid-Holocene Individuals from the Northwest Coast of North America Reveals Different Evolutionary Paths for Mitogenomes. PLoS ONE 2013, 8, e66948. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.R.; Sturk-Andreaggi, K.; Ring, J.D.; Huber, N.; Bodner, M.; Crawford, M.H.; Parson, W.; Marshall, C. Resolving mitochondrial haplogroups B2 and B4 with next-generation mitogenome sequencing to distinguish Native American from Asian haplotypes. Forensic Sci. Int. Genet. 2019, 43, 102143. [Google Scholar] [CrossRef] [PubMed]
- Flores-Huacuja, M.; Snow, M.; Ramos-Madrigal, J.; Contreras-Cubas, C.; Barajas-Olmos, F.; Gonzalez-Oliver, A.; Mendoza-Caamal, E.; Ciceron-Arellano, I.; Centeno-Cruz, F.; Orozco, L.; et al. Whole mitogenome analysis highlights demographic history and shared connections among distal Indigenous groups of Mexico Complete mitogenome sequencing from 60 Mexican Native American groups. bioRxiv 2023. bioRxiv:2023–09. [Google Scholar]
- Aqil, A.; Gill, S.; Gokcumen, O.; Malhi, R.S.; Reese, E.A.; Smith, J.L.; Heaton, T.T.; Lindqvist, C. A paleogenome from a Holocene individual supports genetic continuity in Southeast Alaska. Iscience 2023, 26. [Google Scholar] [CrossRef] [PubMed]
- Malhi, R.S.; Breece, K.E.; Shook, B.A.S.; Kaestle, F.A.; Chatters, J.C.; Hackenberger, S.; Smith, D.G. Patterns of mtDNA diversity in northwestern North America. Hum. Biol. 2004, 76, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Mannis, O.; Kayser, M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum. Mutat. 2009, 30, E386–E394. [Google Scholar] [CrossRef]
- Taboada-Echalar, P.; Alvarez-Iglesias, V.; Heinz, T.; Vidal-Bralo, L.; Gómez-Carballa, A.; Catelli, L.; Pardo-Seco, J.; Pastoriza, A.; Carracedo, A.; Torres-Balanza, A.; et al. The genetic legacy of the pre-colonial period in contemporary Bolivians. PLoS ONE 2013, 8, e58980. [Google Scholar] [CrossRef]
- Behar, D.M.; Rosset, S.; Blue-Smith, J.; Balanovsky, O.; Tzur, S.; Comas, D.; Mitchell, R.J.; Quintana-Murci, L.; Tyler-Smith, C.; Genographic Consortium; et al. The Genographic Project public participation mitochondrial DNA database. PLoS Genet. 2007, 3, e104. [Google Scholar] [CrossRef]
- Kong, Q.P.; Yao, Y.G.; Sun, C.; Bandelt, H.J.; Zhu, C.L.; Zhang, Y.P. Phylogeny of East Asian mitochondrial DNA lineages inferred from complete sequences. Am. J. Hum. Genet. 2003, 73, 671–676. [Google Scholar] [CrossRef]
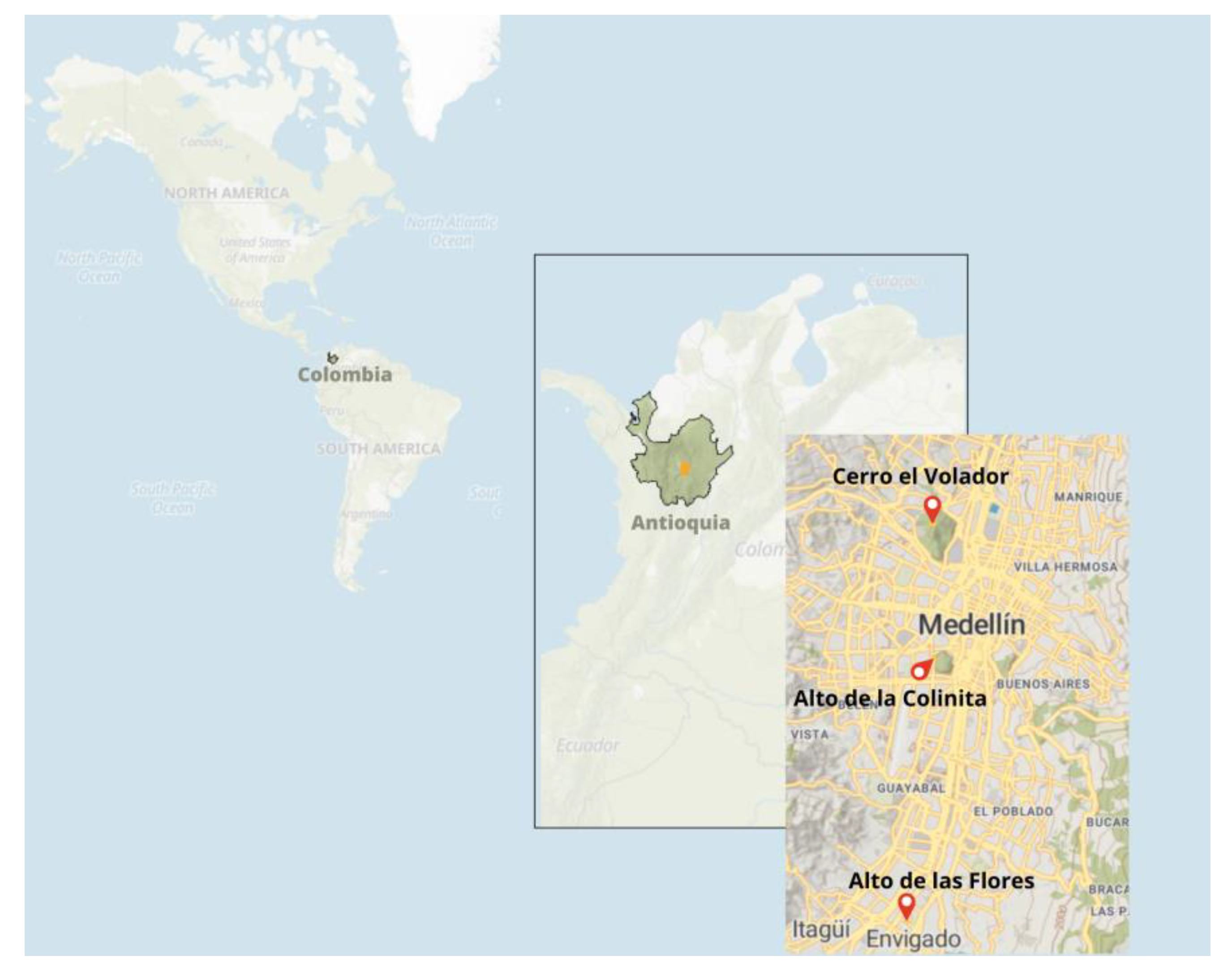
| Place | Region | Dates BP Not Calibrated |
|---|---|---|
| Tocaima-Cundinamarca | Pubenza | 16,400 ± 420 |
| Zipaquirá-Cundinamarca | El Abra | 12,400 ± 160 |
| Tocancipá-Cundinamarca | Tibitó 1 | 11,740 ± 110 |
| Tequerdama-Cundinamarca | Tequendama 1 y 2 | 9740 ± 135 |
| Nemocón-Cundinamarca | Checua | 8500–3000 |
| Roncesvalles -Tolima | Jordan | 12.910 ± 60–9760 ± 160 |
| Porce 045-Antioquia | Porce | 7.080 ± 130 |
| La Morena Envigado-Antioquia | Porce River High Basin | 10,060 ± 60–9680 ± 60 |
| Porce, Primavera II-Antioquia | Porce River Low Basin | 7730 ± 170 |
| Medellín Guayabal-Antioquia | La Colinita | 880 ± 30 |
| Medellín, Robledo-Antioquia | Cerro El Volador T10 | 950 ± 70 |
| Medellín, Robledo-Antioquia | Cerro El Volador T5 | 530 ± 80 |
| Medellín, Robledo-Antioquia | Cerro El Volador T13 | 480 ± 60 |
| Medellín, Robledo-Antioquia | Cerro El Volador T9 | 420 ± 50 |
| Medellín, Robledo-Antioquia | Cerro El Volador T8 | 330 ± 60 |
| Envigado, Loma del Barro-Antioquia | Alto de las Flores | 140 ± 30 |
| Sample | Gender | Site | Description | Range | Haplogroup | Quality | Polymorphisms |
|---|---|---|---|---|---|---|---|
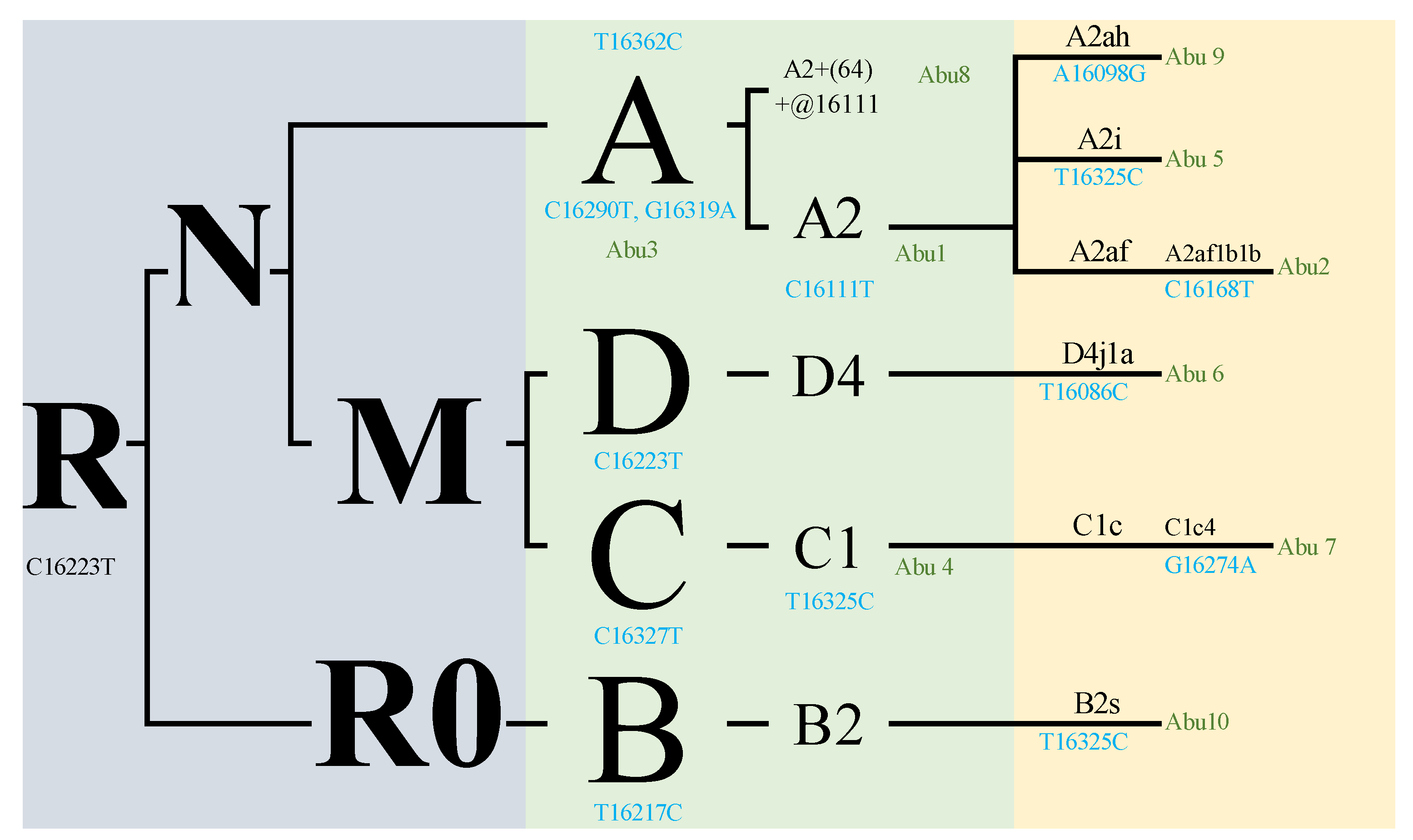
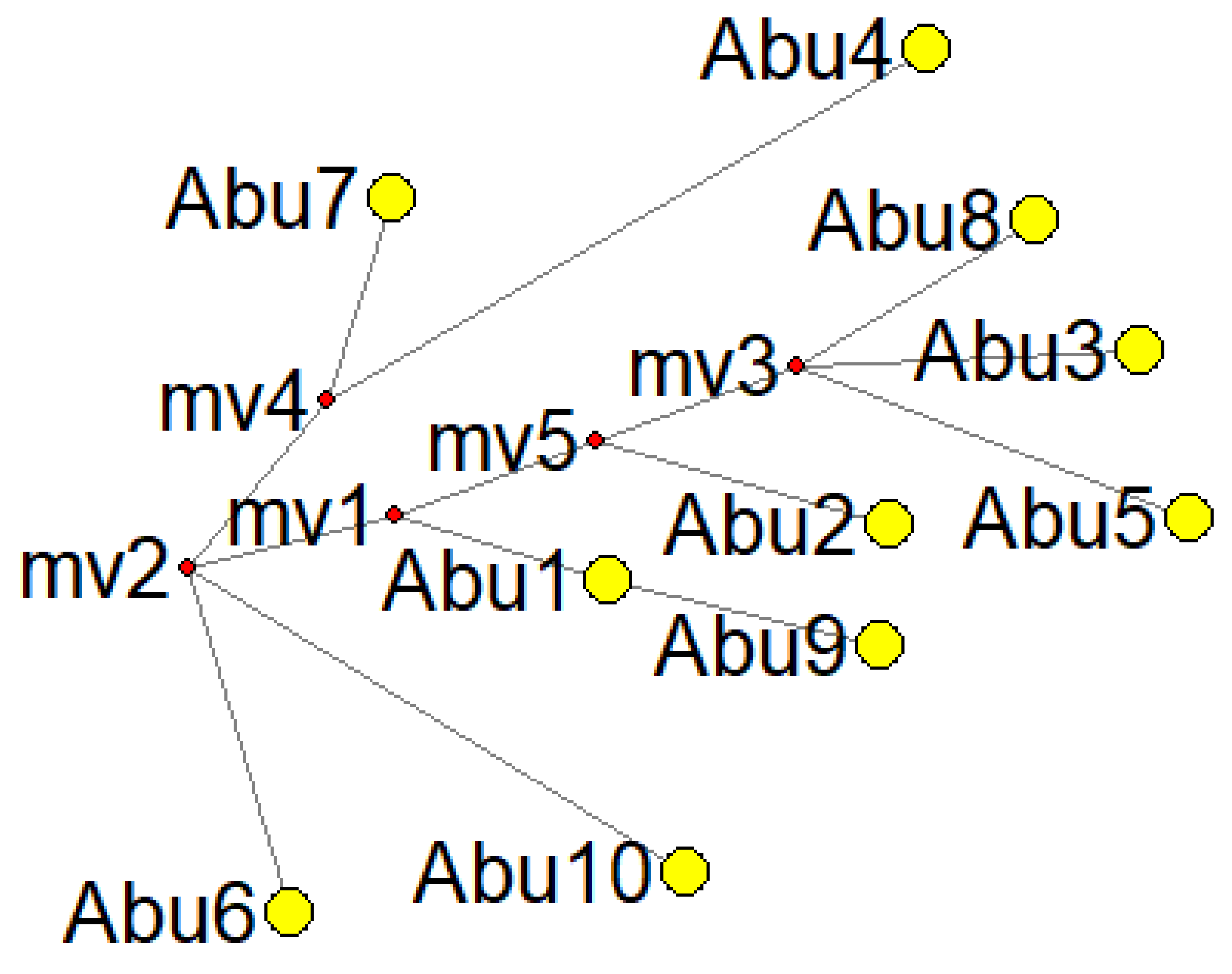
| Abu 1 | Indet. | La Colinita | Molar | 16,024–16,569 | A, A2 | 86% | 16111T 16223T 16290T |
| Abu 2 | Indet. | La Colinita | Molar | 16,024–16,569; 1–576 | A, A2af1b1b | 60% | 16168T 16223T 16290T 16362C |
| Abu 3 | Indet. | La Colinita | Molar | 16,024–16,569 | A | 94% | 16129A 16223T 16290T 16319A 16362C |
| Abu 4 | Indet. | La Colinita | Molar | 16,024–16,569 | C | 97% | 16223T 16288C 16298C 16311C 16327T |
| Abu 5 | Indet. | La Colinita | Molar | 16,024–16,569 | A, A2i | 100% | 16111T 16223T 16290T 16319A 16325C 16362C |
| Abu 6 | Indet. | La Colinita | Molar | 16,024–16,569; 1–576 | D, D4j1a | 65% | 16086C 16183C 16223T |
| Abu 7 | Indet. | La Colinita | Molar | 16,024–16,569; | C, C1c4 | 94% | 16223T 16274A 16327Y |
| Abu 8 | Indet. | La Colinita | Molar | 16,024–16,569 | A, A2+(64)+ @16111 | 82% | 16290T 16319A 16362C |
| Abu 9 | Female | Cerro el Volador | Bone remains | 16,024–16,569; 1–576 | A, A2ah | 81% | 16098G 16111T 16223T 16290T |
| Abu 10 | Indet. | Alto de las Flores | Bone remains | 16,024–16,569; 1–576 | B2, B2s | 62% | 16217C 16325C |
| Population | K | H | π | Tajima’s | p-Value |
|---|---|---|---|---|---|
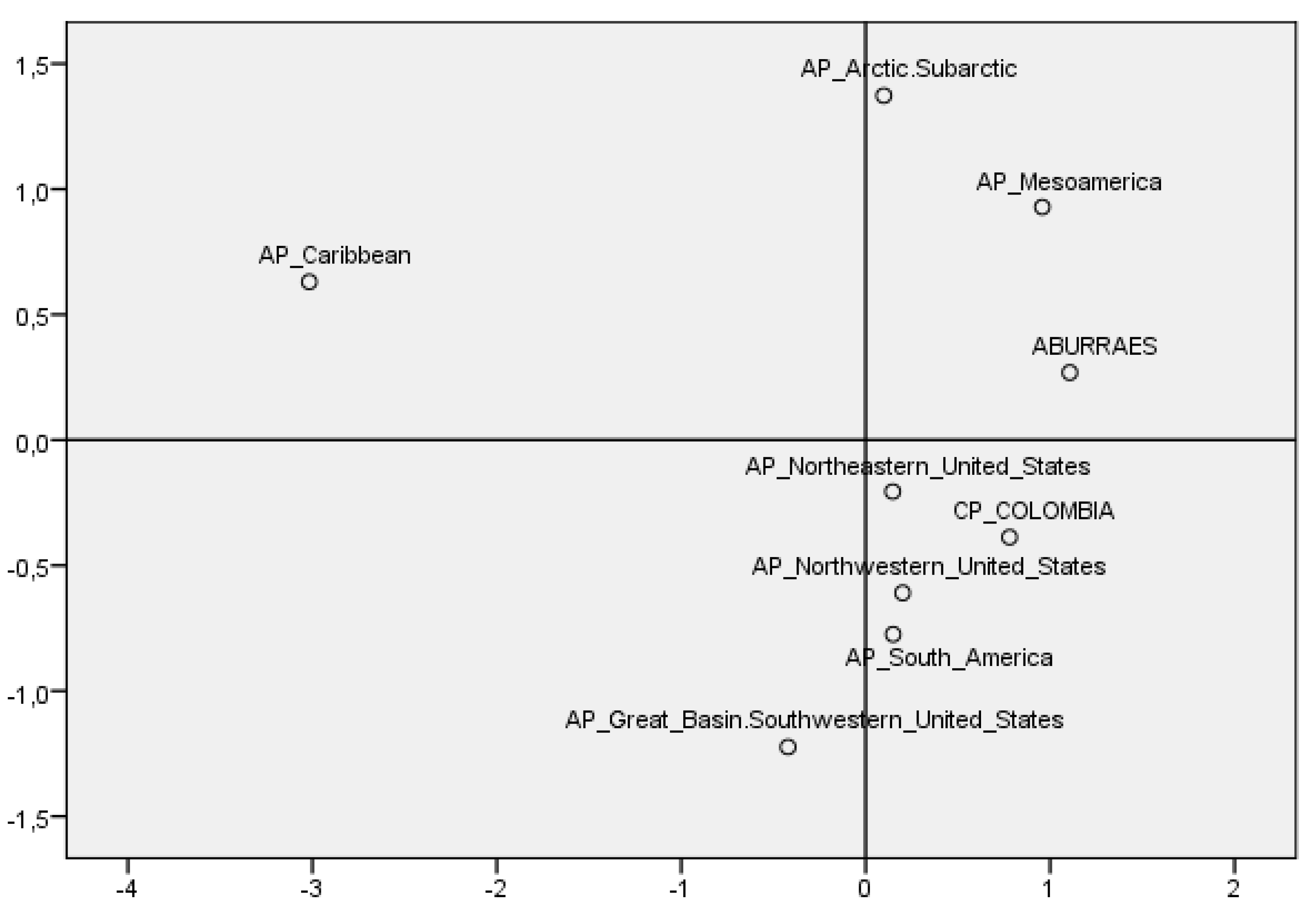
| Aburráes | 10 | 0.644 | 0.012 | −0.72937 | 0.254 |
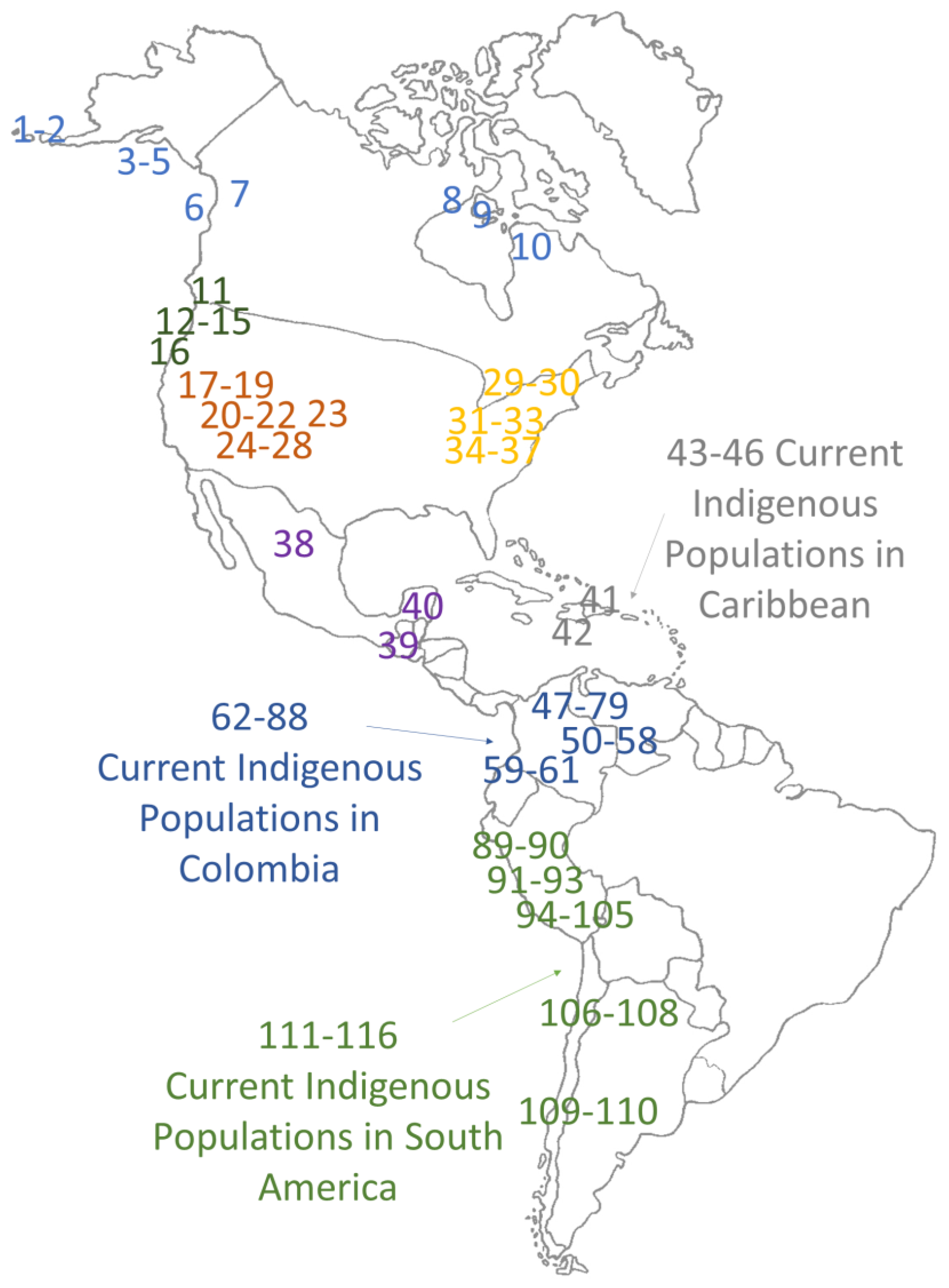
| Position on the Map | Geographic Zone | Archaeological Site | AP | N | A | B | C | D | X | M | H | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Arctic, Subarctic | Early Aleuts | 3500–1200 | 11 | 8 | 0 | 0 | 3 | 0 | 0 | 0.436 | [17] |
| 2 | Arctic, Subarctic | Late Pre-Contact Aleuts | 1000–400 | 52 | 12 | 0 | 0 | 40 | 0 | 0 | 0.362 | [17] |
| 3 | Arctic, Subarctic | Brooks River | 938–1318 | 8 | 5 | 2 | 0 | 1 | 0 | 0 | 0.607 | [18] |
| 4 | Arctic, Subarctic | Hot Springs | 2070 | 3 | 1 | 0 | 0 | 2 | 0 | 0 | N.C. | [18] |
| 5 | Arctic, Subarctic | On-Your-Knees Cave | 10,300 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | N.C. | [19] |
| 6 | Arctic, Subarctic | Mink Island | 1029–1215 | 6 | 1 | 0 | 0 | 5 | 0 | 0 | 0.333 | [18] |
| 7 | Arctic, Subarctic | Tatshenshini-Alsek Glacier | 550 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | N.C. | [20] |
| 8 | Arctic, Subarctic | Thule | 1130–628 | 15 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | [21] |
| 9 | Arctic, Subarctic | Sadlermiut | 977–682 | 18 | 10 | 0 | 0 | 8 | 0 | 0 | 0.523 | [21] |
| 10 | Arctic, Subarctic | Dorset | 2260–1216 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | N.C. | [21] |
| 11 | Northwest United States | China Lake and Big Bar Lake | 4975 | 3 | 1 | 0 | 0 | 0 | 0 | 2 | N.C. | [19] |
| 12 | Northwest United States | Plateau Salish | 200 | 11 | 3 | 6 | 1 | 1 | 0 | 0 | 0.673 | [19] |
| 13 | Northwest United States | Plateau Sahaptian | 200 | 8 | 0 | 4 | 2 | 2 | 0 | 0 | 0.714 | [19] |
| 14 | Northwest United States | Wishram | 200 | 33 | 7 | 17 | 0 | 9 | 0 | 0 | 0.635 | [19] |
| 15 | Northwest United States | Vantage | 500–1500 | 7 | 2 | 0 | 1 | 3 | 1 | 0 | 0.81 | [19] |
| 16 | Northwest United States | Paisley 5 Mile Point Caves | 14,000 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | N.C. | [22] |
| 17 | Great Basin, Southwest United States | Cecil | 3600–2860 | 16 | 0 | 1 | 9 | 6 | 0 | 0 | 0.575 | [23] |
| 18 | Great Basin, Southwest United States | Cook | 2000 | 23 | 1 | 2 | 10 | 10 | 0 | 0 | 0.64 | [23] |
| 19 | Great Basin, Southwest United States | Applegate | 1765–2055 | 6 | 0 | 2 | 4 | 0 | 0 | 0 | 0.533 | [23] |
| 20 | Great Basin, Southwest United States | Pyramid Lake | 860–5905 | 18 | 2 | 6 | 0 | 10 | 0 | 0 | 0.601 | [24] |
| 21 | Great Basin, Southwest United States | Pyramid Lake (Wizards Beach) | 9200 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | N.C. | [24] |
| 22 | Great Basin, Southwest United States | Stillwater Marsh | 290–3290 | 21 | 1 | 8 | 0 | 12 | 0 | 0 | 0.552 | [24] |
| 23 | Great Basin, Southwest United States | Hourglass Cave | 8000 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | N.C. | [25] |
| 24 | Great Basin, Southwest United States | Tommy Site | 850–1150 | 36 | 1 | 25 | 5 | 5 | 0 | 0 | 0.492 | [26] |
| 25 | Great Basin, Southwest United States | Mine Canyon | 650–850 | 12 | 7 | 4 | 1 | 0 | 0 | 0 | 0.591 | [26] |
| 26 | Great Basin, Southwest United States | Fremont | 500–1500 | 30 | 0 | 24 | 4 | 2 | 0 | 0 | 0.349 | [27] |
| 27 | Great Basin, Southwest United States | Anasazi | 1010–2010 | 38 | 4 | 27 | 7 | 0 | 0 | 0 | 0.462 | [27] |
| 28 | Great Basin, Southwest United States | Western Basketmaker II | 2500–1300 | 23 | 3 | 18 | 1 | 1 | 0 | 0 | 0.383 | [27,28] |
| 29 | Northeast United States | Great Western Park | 800 | 6 | 2 | 0 | 4 | 0 | 0 | 0 | 0.533 | [29] |
| 30 | Northeast United States | Glacial Kame | 2900 | 18 | 3 | 11 | 3 | 1 | 0 | 0 | 0.601 | [29] |
| 31 | Northeast United States | Morse | 2700 | 9 | 1 | 3 | 5 | 0 | 0 | 0 | 0.639 | [29] |
| 32 | Northeast United States | Orendorf | 800 | 11 | 5 | 0 | 3 | 3 | 0 | 0 | 0.709 | [29] |
| 33 | Northeast United States | Norris Farms | 700 | 108 | 34 | 13 | 46 | 9 | 6 | 0 | 0.702 | [25] |
| 34 | Northeast United States | Pete Klunk Mound Group | 1825 | 39 | 9 | 5 | 19 | 5 | 1 | 0 | 0.694 | [30] |
| 35 | Northeast United States | Schild Mississippian | 900 | 47 | 18 | 6 | 11 | 4 | 8 | 0 | 0.762 | [31] |
| 36 | Northeast United States | Schild Late Woodland | 1200 | 19 | 5 | 1 | 4 | 1 | 8 | 0 | 0.743 | [31] |
| 37 | Northeast United States | Ohio Hopewell Mound Group | 1700 | 34 | 14 | 3 | 10 | 7 | 0 | 0 | 0.715 | [32] |
| 38 | Mesoamerica | Tlatelolco Post-Classic Aztec | 500–675 | 23 | 15 | 3 | 1 | 4 | 0 | 0 | 0.574 | [33] |
| 39 | Mesoamerica | Maya (Xcaret) | 480–1400 | 24 | 20 | 1 | 2 | 0 | 0 | 0 | 0.236 | [34] |
| 40 | Mesoamerica | Maya (Copan) | 750–1300 | 9 | 0 | 0 | 8 | 1 | 0 | 0 | 0.222 | [35] |
| 41 | Caribbean | La Caleta (Tainos) | 1330–320 | 24 | 0 | 0 | 18 | 6 | 0 | 0 | 0.391 | [36] |
| 42 | Caribbean | Cuba (Ciboneys) | 4700–1620 | 15 | 1 | 0 | 9 | 5 | 0 | 0 | 0.562 | [37] |
| 43 | Caribbean | Boruca | 0 | 14 | 3 | 10 | 0 | 1 | 0 | 0 | 0.473 | [38] |
| 44 | Caribbean | Huetar | 0 | 27 | 19 | 1 | 0 | 7 | 0 | 0 | 0.453 | [39] |
| 45 | Caribbean | Ngöbe | 0 | 46 | 31 | 15 | 0 | 0 | 0 | 0 | 0.449 | [40] |
| 46 | Caribbean | Tainos | 0 | 19 | 0 | 0 | 15 | 4 | 0 | 0 | 0.351 | [41] |
| 47 | Colombia | La Purnia (Guane) | 1090 | 17 | 6 | 7 | 0 | 4 | 0 | 0 | 0.875 | [42] |
| 48 | Colombia | Jerico (Lache) | 700 | 5 | 2 | 3 | 0 | 0 | 0 | 0 | 0.600 | [43] |
| 49 | Colombia | Andes Orientales Colombianos (Muisca, Lache, Guane, Checua, Aguazuque) | 1200–700 | 13 | 1 | 7 | 4 | 1 | 0 | 0 | 0.654 | [44] |
| 50 | Colombia | Madrid (Muisca) | 2000 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | [45] |
| 51 | Colombia | Candelaria (muisca) | 900 | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | [46] |
| 52 | Colombia | Antioquia (Aburraes) | 800 | 10 | 6 | 1 | 2 | 1 | 0 | 0 | 0.644 | [43] |
| 53 | Colombia | Malambo | 3000 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | [47] |
| 54 | Colombia | Sogamoso (Muisca) | 800–1500 | 13 | 9 | 3 | 1 | 0 | 0 | 0 | 0.500 | [48] |
| 55 | Colombia | Nemocon (Checua) | 8000 | 5 | 3 | 1 | 1 | 0 | 0 | 0 | 0.700 | [3] |
| 56 | Colombia | Herrera (Muisca) | 1400–100 | 7 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | [49] |
| 57 | Colombia | Tibanica (Muisca) | 800 | 18 | 12 | 2 | 0 | 4 | 0 | 0 | 0.523 | [50] |
| 58 | Colombia | Agroalfarero (early Muisca) | 900 | 24 | 13 | 6 | 4 | 1 | 0 | 0 | 0.641 | [51] |
| 59 | Colombia | Páez 2 | 0 | 36 | 10 | 3 | 10 | 12 | 1 | 0 | 0.747 | [52] |
| 60 | Colombia | Valle del Cauca (Calima) | 800–1200 | 17 | 5 | 8 | 2 | 2 | 0 | 0 | 0.706 | [43] |
| 61 | Colombia | Ovando (Quimbaya) | 1500–700 | 7 | 0 | 6 | 0 | 1 | 0 | 0 | 0.286 | [53] |
| 62 | Colombia | Desano | 0 | 20 | 3 | 3 | 9 | 5 | 0 | 0 | 0.726 | [52] |
| 63 | Colombia | Curripaco | 0 | 22 | 1 | 9 | 8 | 3 | 1 | 0 | 0.710 | [52] |
| 64 | Colombia | Wayuu | 0 | 30 | 8 | 8 | 13 | 0 | 1 | 0 | 0.692 | [12,54] |
| 65 | Colombia | Guane | 0 | 17 | 6 | 7 | 0 | 4 | 0 | 0 | 0.691 | [42] |
| 66 | Colombia | Waunana | 0 | 40 | 2 | 19 | 11 | 7 | 1 | 0 | 0.682 | [55] |
| 67 | Colombia | Ingano | 0 | 48 | 19 | 17 | 11 | 1 | 0 | 0 | 0.679 | [52] |
| 68 | Colombia | Tule-Cuna | 0 | 30 | 15 | 8 | 6 | 0 | 1 | 0 | 0.660 | [55,56] |
| 69 | Colombia | Tucano | 0 | 14 | 1 | 3 | 1 | 8 | 1 | 0 | 0.659 | [52] |
| 70 | Colombia | Embera | 0 | 21 | 2 | 11 | 6 | 2 | 0 | 0 | 0.657 | [52] |
| 71 | Colombia | Antioquia | 0 | 38 | 18 | 10 | 0 | 10 | 0 | 0 | 0.654 | [57] |
| 72 | Colombia | Maya | 0 | 27 | 14 | 6 | 4 | 2 | 0 | 0 | 0.652 | [56] |
| 73 | Colombia | Embera | 0 | 21 | 7 | 10 | 1 | 2 | 0 | 0 | 0.647 | [55] |
| 74 | Colombia | Tatuyo | 0 | 10 | 4 | 0 | 5 | 1 | 0 | 0 | 0.644 | [52] |
| 75 | Colombia | Zenu | 0 | 34 | 5 | 11 | 17 | 1 | 0 | 0 | 0.642 | [55] |
| 76 | Colombia | Chibcha | 0 | 15 | 6 | 2 | 4 | 2 | 1 | 0 | 0.781 | [52] |
| 77 | Colombia | Puinave | 0 | 61 | 5 | 31 | 20 | 4 | 1 | 0 | 0.633 | [52] |
| 78 | Colombia | Cauca | 0 | 60 | 11 | 5 | 38 | 5 | 1 | 0 | 0.560 | [57] |
| 79 | Colombia | Páez 1 | 0 | 31 | 18 | 2 | 11 | 0 | 0 | 0 | 0.551 | [55] |
| 80 | Colombia | Guane-Butar | 0 | 33 | 4 | 21 | 0 | 8 | 0 | 0 | 0.538 | [55] |
| 81 | Colombia | Cubeo | 0 | 24 | 8 | 2 | 7 | 6 | 1 | 0 | 0.764 | [52] |
| 82 | Colombia | Guambiano | 0 | 80 | 5 | 6 | 59 | 10 | 0 | 0 | 0.436 | [57] |
| 83 | Colombia | Arsario | 0 | 28 | 20 | 0 | 8 | 0 | 0 | 0 | 0.423 | [12] |
| 84 | Colombia | Kuna | 0 | 63 | 45 | 18 | 0 | 0 | 0 | 0 | 0.415 | [12] |
| 85 | Colombia | Kogui | 0 | 21 | 17 | 0 | 4 | 0 | 0 | 0 | 0.324 | [12,54] |
| 86 | Colombia | Arhuaco | 0 | 21 | 19 | 1 | 1 | 0 | 0 | 0 | 0.186 | [52] |
| 87 | Colombia | Ijka | 0 | 31 | 28 | 1 | 2 | 0 | 0 | 0 | 0.185 | [12] |
| 88 | Colombia | Chimila | 0 | 56 | 50 | 1 | 1 | 2 | 1 | 0 | 0.174 | [52] |
| 89 | South America | North Peruvian Coast | 1000 | 21 | 6 | 7 | 1 | 7 | 0 | 0 | 0.729 | [58] |
| 90 | South America | Peruvian Highlanders | 550–450 | 35 | 3 | 23 | 8 | 1 | 0 | 0 | 0.523 | [59] |
| 91 | South America | Conchapata | 1400–1200 | 14 | 4 | 7 | 2 | 1 | 0 | 0 | 0.692 | [60] |
| 92 | South America | Cuzco | 1020–830 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | N.C. | [61] |
| 93 | South America | Huari | 900–600 | 18 | 3 | 4 | 10 | 1 | 0 | 0 | 0.647 | [62] |
| 94 | South America | Paracas (Peninsula) | 2800–2200 | 10 | 0 | 0 | 3 | 7 | 0 | 0 | 0.467 | [63] |
| 95 | South America | Chen Chen | 1215–1000 | 23 | 9 | 9 | 4 | 1 | 0 | 0 | 0.692 | [64] |
| 96 | South America | Paracas (Palpa) | 2800–2200 | 28 | 2 | 0 | 4 | 22 | 0 | 0 | 0.370 | [63] |
| 97 | South America | Nasca-Rural (Palpa) | 2200–1400 | 37 | 1 | 4 | 8 | 24 | 0 | 0 | 0.535 | [63] |
| 98 | South America | Nasca-Urban (Palpa) | 2200–1400 | 28 | 0 | 5 | 12 | 11 | 0 | 0 | 0.653 | [63] |
| 99 | South America | Middle Horizon (Palpa) | 1400–1000 | 11 | 0 | 3 | 4 | 4 | 0 | 0 | 0.727 | [63] |
| 100 | South America | Pacapaccari (Highlands) | 820 | 16 | 0 | 11 | 5 | 0 | 0 | 0 | 0.458 | [63] |
| 101 | South America | Yacotogia | 1187 | 25 | 1 | 14 | 10 | 0 | 0 | 0 | 0.547 | [63] |
| 102 | South America | Ocoro | 1400–1000 | 5 | 1 | 3 | 1 | 0 | 0 | 0 | 0.700 | [63] |
| 103 | South America | Botigiriayocc | 1000–600 | 12 | 0 | 8 | 4 | 0 | 0 | 0 | 0.485 | [63] |
| 104 | South America | Huayuncalla | 978 | 5 | 1 | 2 | 2 | 0 | 0 | 0 | 0.800 | [63] |
| 105 | South America | Layuni | 1400–1000 | 9 | 0 | 5 | 3 | 1 | 0 | 0 | 0.639 | [63] |
| 106 | South America | North Chile Late Archaic | 6000–3900 | 14 | 7 | 5 | 1 | 1 | 0 | 0 | 0.659 | [65] |
| 107 | South America | North Chile Middle Horizon | 1650–1000 | 19 | 6 | 8 | 5 | 0 | 0 | 0 | 0.690 | [65] |
| 108 | South America | North Chile Late Intermediate | 1000–500 | 15 | 3 | 8 | 3 | 1 | 0 | 0 | 0.676 | [65] |
| 109 | South America | Pampa Grande | 1600–1350 | 19 | 2 | 9 | 0 | 8 | 0 | 0 | 0.620 | [66] |
| 110 | South America | Baño nuevo, Tagua Tagua, Camarones | 9000–7500 | 30 | 9 | 10 | 9 | 2 | 0 | 0 | 0.729 | [67] |
| 111 | South America | Mapuche | 0 | 39 | 6 | 15 | 8 | 10 | 0 | 0 | 0.740 | [68] |
| 112 | South America | Yungay | 0 | 20 | 1 | 10 | 5 | 4 | 0 | 0 | 0.679 | [69] |
| 113 | South America | Quechua | 0 | 23 | 2 | 13 | 7 | 1 | 0 | 0 | 0.605 | [70] |
| 114 | South America | Yanomami | 0 | 53 | 0 | 5 | 31 | 15 | 3 | 0 | 0.593 | [71] |
| 115 | South America | Zoro | 0 | 29 | 6 | 0 | 4 | 18 | 1 | 0 | 0.571 | [72] |
| 116 | South America | Xavante | 0 | 24 | 4 | 20 | 0 | 0 | 0 | 0 | 0.290 | [72] |
| Sample ID | Locality | Haplogroup | Haplotype | Haplotype Global Distribution |
|---|---|---|---|---|
| Abu 1 | La Colinita | A2 | 16111T 16223T 16290T | Found across the entire continent of the Americas [73] |
| Abu 2 | La Colinita | A2af1b1b | 16168T 16223T 16290T 16362C | Specifically present in Panama and Costa Rica [74,75] |
| Abu 3 | La Colinita | A | 16129A 16223T 16290T 16319A 16362C | Found across the entire continent of the Americas [73] |
| Abu 4 | La Colinita | C | 16223T 16288C 16298C 16311C 16327T | Prevalent in most parts of the American continent [73] |
| Abu 5 | La Colinita | A2i | 16111T 16223T 16290T 16319A 16325C 16362C | Concentrated in central North America, including Nebraska, Minnesota, and South Dakota [73], found in ancient populations in Canada [76,77,78] |
| Abu 6 | La Colinita | D4j1a | 16086C 16183C 16223T | Found in a small number of individuals in Thailand and Tibet [73,79,80,81,82,83,84,85] |
| Abu 7 | La Colinita | C1c4 | 16223T 16274A 16327Y | Located in the southern region of Mexico [86,87] |
| Abu 8 | La Colinita | A2 | 16290T 16319A 16362C | Found across the entire continent of the Americas [73] |
| Abu 9 | Cerro el Volador | A2ah | 16098G 16111T 16223T 16290T | Present in North America but more concentrated in South America [73,88,89,90,91] |
| Abu 10 | Alto de las Flores | B2s | 16217C 16325C | Specifically found in North America [73,92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uricoechea Patiño, D.; Collins, A.; Romero García, O.J.; Santos Vecino, G.; Aristizábal Espinosa, P.; Bernal Villegas, J.E.; Benavides Benitez, E.; Vergara Muñoz, S.; Briceño Balcázar, I. Unraveling the Genetic Threads of History: mtDNA HVS-I Analysis Reveals the Ancient Past of the Aburra Valley. Genes 2023, 14, 2036. https://doi.org/10.3390/genes14112036
Uricoechea Patiño D, Collins A, Romero García OJ, Santos Vecino G, Aristizábal Espinosa P, Bernal Villegas JE, Benavides Benitez E, Vergara Muñoz S, Briceño Balcázar I. Unraveling the Genetic Threads of History: mtDNA HVS-I Analysis Reveals the Ancient Past of the Aburra Valley. Genes. 2023; 14(11):2036. https://doi.org/10.3390/genes14112036
Chicago/Turabian StyleUricoechea Patiño, Daniel, Andrew Collins, Oscar Julián Romero García, Gustavo Santos Vecino, Pablo Aristizábal Espinosa, Jaime Eduardo Bernal Villegas, Escilda Benavides Benitez, Saray Vergara Muñoz, and Ignacio Briceño Balcázar. 2023. "Unraveling the Genetic Threads of History: mtDNA HVS-I Analysis Reveals the Ancient Past of the Aburra Valley" Genes 14, no. 11: 2036. https://doi.org/10.3390/genes14112036







