Highlights
What are the main findings?
- A plant-based diet prevented and reversed compromised lung endothelial nitric oxide synthase in hypertensive rats, despite not impacting systemic hypertension.
- A plant-based diet enhanced expression of epithelial junction proteins and atten-uated profibrotic proteins.
What are the implications of the main findings?
- These positive molecular effects are associated with improved lung function and reduced lung disease in pulmonary hypertension.
- A plant-based diet could be used as a treatment for pulmonary hypertension, but more research is needed.
Abstract
Background: Essential hypertension is associated with an increased risk of pulmonary hypertension (PH). PH is diagnosed more frequently in females. Little is known about the effects of a plant-based diet (PBD) in improving lung abnormalities in PH. Methods: We compared 28- and 40-week-old female normotensive Wistar Kyoto and spontaneously hypertensive rats (SHR), maintained from the age of 4 weeks on a control refined diet or a PBD, comprising 28% fruits, vegetables, nuts and legumes. A subset of control SHRs were switched to the PBD at 28 weeks of age. Lungs were taken for protein and histological analysis. Results: Relative to WKYs, SHRs consuming the control diet exhibited decreased lung endothelial nitric oxide synthase (eNOS). PBD consumption by SHRs prevented and reversed this phenotype. Expression of E-cadherin was also reduced in SHRs. This reduction was attenuated by PBD consumption treatment. The phosphorylation of extracellular signal-regulated kinase (ERK)1/2 in the lung was increased in SHRs and attenuated by PBD. The expression of activated transforming growth factor (TGF)-β1 was also attenuated by a PBD. Conclusions: The PBD favorably mediated hypertension-induced pulmonary molecular abnormalities in lung endothelium, epithelial junction and pro-fibrotic signaling. Future studies should assess the effects of a PBD in improving PH and lung function.
1. Introduction
Pulmonary hypertension (PH) results in a 21% increased risk of mortality within 3 years of diagnosis [1]. While PH can develop in essential hypertension due to left ventricular (LV) dysfunction, in those with normal LV function, essential hypertension increases the risk of PH development by 300% [2]. Moreover, sex plays a role, as PH afflicts a greater number of females, who are 1.8–3.6 times more likely to be diagnosed compared to males [3]. PH was initially thought to be a disease afflicting mostly younger women (mean age 35); however, recent evidence suggests that it afflicts older adults as well, with 64% of new diagnoses occurring in those who are >65 years of age [4].
PH can lead to worsening lung function [5], in part due to microvascular endothelial dysfunction within lung tissue [6], resulting in poor gas exchange. This vascular stress can increase transforming growth factor (TGF)-β1, reducing epithelial adherens junction proteins, such as E-cadherin, within the lung, which increases permeability, while also promoting mesenchymal transition, as evidenced by increased epithelial expression of α-smooth muscle actin (SMA) [7]. These abnormal cellular alterations can cause congestion, scarring and overt lung disease over time. Inflammation and oxidative stress play a large part in driving these abnormal molecular processes [8,9], especially in downregulating endothelial function [10]. Targeting these molecular pathways may be of relevance in the management of lung disease induced by PH.
While studies are limited, nutrition can play a role in improving PH [11]. In particular, plant-based diets (PBDs) may be beneficial. Indeed, plant-based dietary patterns are associated with preserved lung function [12]; a PBD improves endothelial function [13] and a PBD can treat essential hypertension [14]. This may in part be due to the bioactive properties of polyphenols, secondary metabolites produced exclusively by plants, which can target the underlying pathways of inflammation, oxidative stress and endothelial dysfunction [15,16,17]. Thus, the objective of this study was to assess whether a PBD could improve lung abnormalities at the molecular level, which relates to PH as part of a secondary analysis in a model of essential hypertension.
2. Materials and Methods
This investigation is a secondary analysis of an existing study which evaluated coronary microvascular dysfunction [18]. This animal study was approved by Georgia State University’s Institutional Animal Care and Use Committee (protocol #: A23025). All animals were euthanized by CO2 affixation, followed by decapitation at either week 24 or week 36. PH is more prevalent in women compared to men; thus, females are appropriate to use in this model. Spontaneously hypertensive rats (SHRs), while a model of essential hypertension, have been validated to develop PH by 14–18 weeks of age, along with subsequent lung histopathological abnormalities [19]. Thus, SHRs are appropriate models of PH.
Wistar Kyoto (WKY) rats served as normotensive controls. All animals were female and three weeks old upon arrival. Further, animals were purchased from Inotiv (West Lafayette, IN, USA), within their own in-bred colonies. Upon arrival, rats were housed in pairs in an environmentally controlled animal care facility (50 ± 5% relative humidity, 20–25 °C) and maintained on 12 h light/dark cycles. Rats were doubly housed, since single animals may have altered hemodynamics due to increased stress [20]. Rats were maintained on a purified control diet (Table S1) [18]. After one week, SHRs either continued the control diet or were switched to a PBD. WKYs consumed the control diet for the entirety of the study. The PBD comprised 28% (w/w) of seven different plant foods: walnuts, black beans, red bell pepper, sweet potato, blueberries, brussels sprouts and lemon (4% each). The diversity of foods was to better reflect a diverse human PBD. The selection of these foods was based on two criteria: (1) high polyphenol content [21] and (2) commonly consumed in the United States. While the control diet contained casein, the PBD used soy protein instead (Table S1). The control diet and PBD were nearly identically matched in nutritional composition, including protein, carbohydrates, fat and fiber (insoluble and soluble), as well as vitamins and minerals. As such, the main known difference between the PBD and control diet was the polyphenol content. Polyphenol intake in rats consuming a PBD was estimated to be ~2582 mg/kg BW, based on average food intake, body weight of animals and available polyphenol analysis data from Phenol Explorer [22,23]. This corresponds to ~96 mg/kg BW of polyphenols in human equivalents [24], or 5760 mg of polyphenols per 2000 Kcal. The control diet did not contain any whole plant foods, and can be considered a proxy for a Western dietary pattern, which lacks plant foods [25].
The prevention phase comprised animals consuming their respective diets for 24 weeks, starting from 4 weeks of age (Figure 1). For additional clinical relevance, we also utilized a treatment model, in which a subset of SHRs on the control diet that were hypertensive for 24 weeks were switched to a PBD for 12 additional weeks. Otherwise, rats continued the control diet for 12 weeks during the treatment phase. Figure 1 outlines the animal study design. At the time of sacrifice, SHRs, irrespective of diet, remained hypertensive (systolic BP: ~160 mmHg), while WKYs were normotensive (systolic BP: ~112 mmHg) [18].
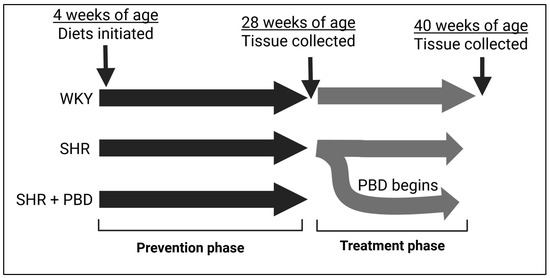
Figure 1.
Study design. Abbreviations: PBD, plant-based diet; SHR, spontaneously hypertensive rat and WKY, Wistar Kyoto.
2.1. Western Blot
The lung was excised, immediately frozen in liquid nitrogen, and stored at −80 °C for downstream protein analysis. Lung tissue (20–40) mg was homogenized in 400 µL RIPA (with protease and phosphatase inhibitors), using a glass Dounce homogenizer. Protein lysates from the tissue were centrifuged at 16,000× g for 20 min and supernatants were collected. The protein concentration of lysates was determined using the DC protein assay kit (BioRad Laboratories, Hercules, CA, USA). For Western blot, 60 µg of protein from tissue were separated in 8–15% SDS-PAGE gels (supplemented with 2-2-2-trichloroethanol, with a final concentration of 0.5% for total lane protein visualization) and transferred to polyvinylidene difluoride (PVDF) membranes, using Trans-Blot Turbo (BioRad Laboratories). Enhanced chemiluminescence (WBLUF0500, Millipore Sigma, Darmstadt, Germany) was used to determine the expression of proteins. The density of protein bands was quantified using Image Lab 6.0 (BioRad Laboratories, Hercules, CA, USA), which was normalized to total lane protein. Phosphorylated proteins were normalized to their respective total protein counterparts. If phosphorylated and total proteins were on different membranes, then each protein was first normalized to the total lane protein. Antibodies and their respective catalog numbers are listed in Table 1.

Table 1.
Antibodies used in this study for Western blot.
2.2. Histology
At sacrifice, lungs were stored in 10% formalin for 24 h at 4° C. After this, formalin was replaced with 70% ethanol and stored at room temperature. Tissue samples were dehydrated and embedded in paraffin for histological analysis. Cuts were made at 10 µm thickness. Morphology and fibrosis were assessed by hematoxylin and eosin (H&E) staining and Sirius red staining, respectively.
2.3. Statistical Analysis
GraphPad Prism (v10.6; San Diego, CA, USA) was used for all statistical analyses. Normality was assessed with a Shapiro–Wilk test, and all data were normally distributed. Pairwise comparisons were made using Student’s t-test between WKY and SHR or SHR and SHR + PBD. Values are represented as mean ± standard deviation (SD). Data were deemed significant if p ≤ 0.05. Raw p-values were reported in figures if p-value < 0.1.
3. Results
3.1. Impacts of a Plant-Based Diet on Lung Endothelial Nitric Oxide Synthase (eNOS) in Hypertension
As an indirect means of probing endothelial dysfunction, we measured the expression of endothelial nitric oxide synthase (eNOS), a nitric oxide-producing enzyme whose levels are associated with endothelial dysfunction [26,27,28]. The expression of endothelial nitric oxide synthase (eNOS) was significantly reduced in SHRs consuming the control diet relative to WKYs at week 24 (Figure 2A,B; 0.74 ± 0.09 vs. 1.00 ± 0.22), which progressively worsened by week 36 (Figure 2C,D; 0.56 ± 0.041 vs. 1.00 ± 0.17). A plant-based diet prevented this decline in eNOS (Figure 2A,B; 1.04 ± 0.12) and reversed it (Figure 2C,D; 0.94 ± 0.20).
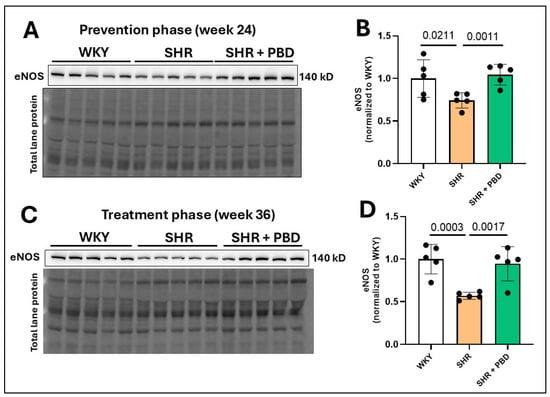
Figure 2.
Lung microvascular eNOS protein expression. At 4 weeks of age, spontaneously hypertensive rats (SHRs) consumed either a control purified diet or a plant-based diet (PBD) for 24 weeks. Wistar Kyoto (WKY) rats also consumed the control diet for 24 weeks. Animals were then euthanized (prevention phase). For the treatment phase, a subgroup of SHRs were switched from the control diet to a PBD after 24 weeks, while the remaining WKYs and SHRs continued the control diet for 12 additional weeks. Lung protein was isolated, and eNOS was assessed at (A,B) week 24 or (C,D) week 36 via Western blot. Statistical comparisons were made with Student’s t-test between WKY vs. SHR or SHR vs. SHR + PBD (n = 5/group). Data are expressed as mean ± SD. Abbreviations: eNOS, endothelial nitric oxide synthase; PBD, plant-based diet; SHR, spontaneously hypertensive rat and WKY, Wistar Kyoto.
3.2. Impacts of a Plant-Based Diet on Expression of Proteins Involved in Lung Epithelial Integrity and Fibrosis in Hypertension
Contrary to our hypothesis, there was no significant increase in vimentin expression in the lungs of SHRs consuming the control diet at weeks 24 (Figure 3A,B) and 36 (Figure 3E,F) compared to WKYs, suggesting that there was not a mesenchymal transition of epithelial cells. Nonetheless, E-cadherin, an adherens junction protein involved in epithelial integrity, was significantly reduced in SHRs consuming the control diet relative to WKYs at week 24 (Figure 3A,C, 0.82 ± 0.10 vs. 1.00 ± 0.18), which worsened at week 36 (Figure 3E,G; 0.75 ± 0.08 vs. 1.00 ± 0.11). While PBD supplementation did not improve E-cadherin during the prevention phase at week 24 (0.76 ± 0.05, p = 0.15), it did attenuate its decline during the treatment phase at week 36 (0.86 ± 0.08). The phosphorylation of extracellular signal-regulated kinase (ERK)1/2, which is closely tied to the loss of E-cadherin [29], was not significantly different between WKYs and SHRs (p = 0.22); however, its phosphorylation was reduced in SHR + PBD vs. SHR alone (Figure 3A,D, 0.81 ± 0.12 vs. 1.19 ± 0.42). At week 36, however, ERK1/2 phosphorylation continued to increase (Figure 3E,H) in SHRs consuming the control diet (1.74 ± 0.54), which was significantly greater than WKYs (1.00 ± 0.22) and SHR + PBD (1.18 ± 0.34).
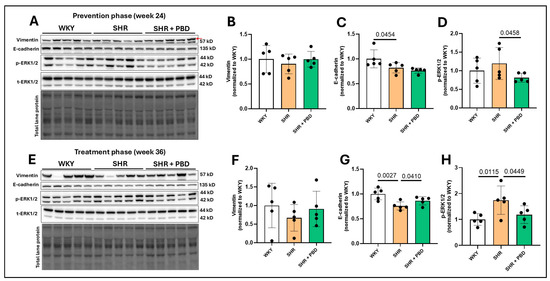
Figure 3.
Expression of mesenchymal transition and junction proteins. At 4 weeks of age, spontaneously hypertensive rats (SHRs) consumed either a control purified diet or a plant-based diet (PBD) for 24 weeks. Wistar Kyoto (WKY) rats also consumed the control diet for 24 weeks. Animals were then euthanized (prevention phase). For the treatment phase, a subgroup of SHRs were switched from the control diet to a PBD after 24 weeks, while the remaining WKYs and SHRs continued the control diet for 12 additional weeks. Lung protein was isolated and protein expression of (A,B,E,F) Vimentin, (A,C,E,G) E-cadherin and (A,D,E,H) phospho-ERK1/2 was assessed at (A–D) week 24 or (E–H) week 36, via Western blot. The red arrow in the vimentin blot for panel A points to the band of interest. Statistical comparisons were made with Student’s t-test between WKY vs. SHR or SHR vs. SHR + PBD (n = 5/group). Data are expressed as mean ± SD. Abbreviations: ERK, extracellular signal-regulated kinases; PBD, plant-based diet; SHR, spontaneously hypertensive rat and WKY, Wistar Kyoto.
The expression of matrix metalloproteinase (MMP)9, a protein typically increased in lung tissue in PH [30], was unchanged between animals in any experimental group (Figure 4A,B,G,H). The expression of α-SMA, a protein expressed in epithelial cells during mesenchymal transition and in activated fibroblasts, was significantly increased in SHRs vs. WKY at week 24 (Figure 4A,C, 1.46 ± 0.22 vs. 1.00 ± 0.13) and was attenuated by a PBD (1.11 ± 0.23). However, no significant differences in α-SMA were observed at week 36 between groups (Figure 4G,I), although a nonsignificant increase in SHR + PBD was observed vs. SHRs alone. TGF-β1, a protein involved in reducing E-cadherin, mesenchymal transition and promoting fibrosis, was significantly impacted by the presence of hypertension. The uncleaved and inactive form, pro-TGF-β1, was reduced in SHRs consuming the control diet vs. WKYs at week 24 (Figure 4A,D, 0.77 ± 0.32 vs. 1.00 ± 0.06), albeit not significantly (p = 0.0843), which was reduced to an even greater extent at week 36 (Figure 4G,J, 0.55 ± 0.13 vs. 1.00 ± 0.09) and significantly so (p = 0.0001). A PBD was as effective in WKY at attenuating pro-TGF-β1 cleavage vs. SHR alone. A reduction in uncleaved TGF-β1 implies that there is more cleaved and active TGF-β1. Indeed, at week 24 (Figure 4A,E), a significant increase in cleaved TGF-β1 was observed in SHRs alone vs. WKY (1.72 ± 0.49 vs. 1.00 ± 0.10). While a PBD did not significantly reduce the expression of cleaved TGF-β1 (1.41 ± 0.12, p = 0.11), the ratio of cleaved/uncleaved TGF-β1 (Figure 4A,F), an indicator of how much relative TGF-β1 that is produced is in its active form, was significantly reduced in SHR + PBD vs. SHR alone (1.86 ± 0.33 vs. 2.28 ± 0.34, p = 0.0401) and in WKY vs. SHR (1.00 ± 0.10, p <0.0001). At week 36, there were no significant differences between groups in the expression of cleaved TGF-β1 (Figure 4G,K); however, the ratio of cleaved/uncleaved TGF-β1 was significantly increased in SHRs consuming the control diet relative to WKY (Figure 4G,L, 2.06 ± 0.62 vs. 1.00 ± 0.42, p = 0.0069) and relative to SHR + PBD (1.31 ± 0.20, p = 0.0170).
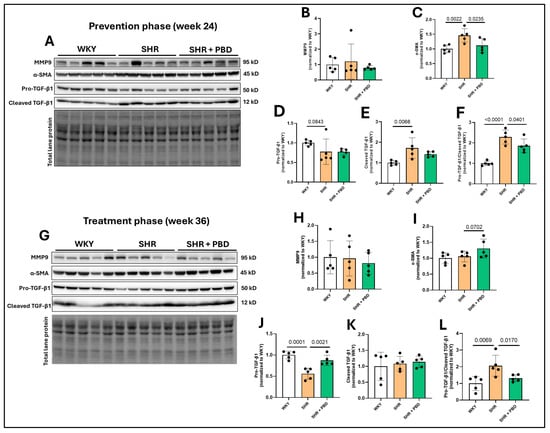
Figure 4.
Expression of proteins involved in fibrosis. At 4 weeks of age, spontaneously hypertensive rats (SHRs) consumed either a control purified diet or a plant-based diet (PBD) for 24 weeks. Wistar Kyoto (WKY) rats also consumed the control diet for 24 weeks. Animals were then euthanized (prevention phase). For the treatment phase, a subgroup of SHRs were switched from the control diet to a PBD after 24 weeks, while the remaining WKYs and SHRs continued the control diet for 12 additional weeks. Lung protein was isolated and protein expression of (A,B,G,H) MMP9, (A,C,G,I) α-SMA, (A,D,G,J) pro-TGF-β1, (A,E,G,K) cleaved TGF-β1 and (A,F,G,L) the ratio of cleaved/pro-TGF-β1, at (A–F) week 24 or (G–L) week 36 via Western blot. Statistical comparisons were made with Student’s t-test between WKY vs. SHR or SHR vs. SHR + PBD (n = 5/group). Data are expressed as mean ± SD. Abbreviations: MMP, matrix metalloproteinase; PBD, plant-based diet; SHR, spontaneously hypertensive rat; SMA, smooth muscle actin; TGF, transforming growth factor; WKY, Wistar Kyoto.
3.3. Impacts of a Plant-Based Diet on Lung Inflammatory Signaling Proteins in Hypertension
No significant differences in the immune cell infiltration marker, F4/80, were observed between groups at week 24 (Figure 5A,B) or week 36 (Figure 5G,H); nor was the phosphorylation of NF-κB significantly impacted (Figure 5A,C,G,I). However, the phosphorylation of p38, a mitogen-activated protein kinase (MAPK) involved in vascular remodeling [31], was increased in SHRs consuming the control diet vs. WKYs (1.86 ± 0.88 vs. 1.00 ± 0.54, p = 0.0504) and SHR + PBD vs. SHR alone (3.50 ± 1.2, p = 0.0210) at week 24 (Figure 5A,D). At week 36 (Figure 5G,J), differences in phospho-p38 between SHR and SHR + PBD were no longer significant (p = 0.2597); however, the expression of this protein remained elevated in SHRs consuming the control diet vs. WKYs (2.08 ± 0.69 vs. 1.00 ± 0.64, p = 0.0170). At week 24, the expression of phosphorylated stress-activated protein kinases (SAPK)/Jun amino-terminal kinases (JNK) (Figure 5A,E), a protein also involved in vascular remodeling [32], was increased (non-significantly) in SHRs consuming the control diet vs. WKYs (1.65 ± 0.82 vs. 1.00 ± 0.27, p = 0.0651) and vs. PBD (1.10 ± 0.30, p = 0.0980). This change, however, was significant in the phosphorylation of c-Jun (Figure 5A,F), a transcription factor directly activated by SAPK/JNK at week 24. No significant differences were observed in the phosphorylation of SAPK/JNK and c-Jun at week 36 (Figure 5G,K,L).
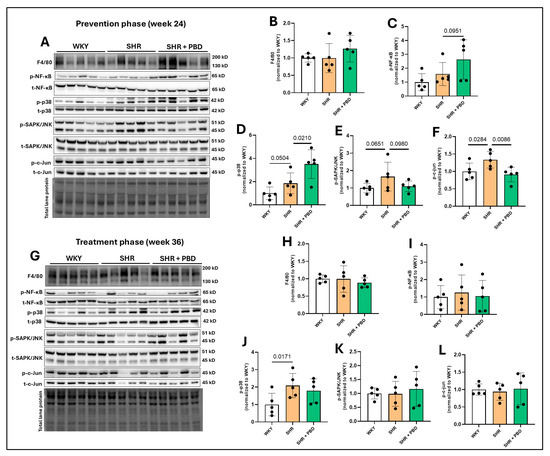
Figure 5.
Expression of inflammatory proteins. At 4 weeks of age, spontaneously hypertensive rats (SHRs) consumed either a control purified diet or a plant-based diet (PBD) for 24 weeks. Wistar Kyoto (WKY) rats also consumed the control diet for 24 weeks. Animals were then euthanized (prevention phase). For the treatment phase, a subgroup of SHRs were switched from the control diet to a PBD after 24 weeks, while the remaining WKY and SHRs continued the control diet for 12 additional weeks. Lung protein was isolated and protein expression of (A,B,G,H) F4/80, (A,C,G,I) p-NF-κB, (A,D,G,J) p-p38, (A,E,G,K) p-SAPK/JNK and (A,F,G,L) p-c-Jun, at (A–F) week 24 or (G–L) week 36 via Western blot. Statistical comparisons were made with Student’s t-test between WKY vs. SHR or SHR vs. SHR + PBD (n = 5/group). Data are expressed as mean ± SD. Abbreviations: NF-κB, nuclear Factor kappa-light-chain-enhancer of activated B cells; PBD, plant-based diet; SAPK/JNK, stress-activated protein kinases/Jun amino-terminal kinases; SHR, spontaneously hypertensive rat and WKY, Wistar Kyoto.
3.4. Impacts of a Plant-Based Diet in Pulmonary Redox Proteins
The expression of xanthine oxidase (XO), a producer of superoxide, was significantly increased at week 24 (Figure 6A,B) in WKY vs. SHR (1.00 ± 0.17 vs. 0.67 ± 0.25, p = 0.0227) and SHR vs. SHR + PBD (0.67 ± 0.25 vs. 0.44 ± 0.05, p = 0.0419). At week 36, the expression of XO (Figure 6F,G) was no longer significantly different between WKY and SHR (p = 0.25), while PBD supplementation non-significantly reduced XO expression vs. SHR alone (1.40 ± 1.11 vs. 0.55 ± 0.38, p = 0.0732). The expression of the NADPH-oxidase 2 subunit, p47phox, involved in the production of superoxide, was significantly reduced at week 24 (Figure 6A,C) and week 36 (Figure 6F,H) in SHRs consuming the control diet vs. WKYs, with no effect of PBD supplementation. The expression of the NADPH-oxidase subunit p22phox, which is ubiquitous among NADPH-oxidase isoforms, was not changed between groups at week 24 (Figure 6A,D), but was significantly increased in SHRs consuming the control diet vs. WKYs (1.17± 0.05 vs. 1.00 ± 0.05, p = 0.0185) at week 36 (Figure 6F,I). As a proxy for oxidative stress, the expression of 3-nitrotyrosine (3-NT) was not changed between WKY and SHR at week 24 (Figure 6A,E). In contrast, PBD supplementation significantly reduced 3-NT expression vs. SHRs consuming the control diet (0.54 ± 0.05 vs. 0.95 ± 0.28, p = 0.0068). At week 36, however, during the treatment phase, the expression of 3-NT (Figure 6F,J) was non-significantly reduced in SHRs consuming the control diet vs. WKYs (0.88 ± 0.12 vs. 1.00 ± 0.10, p = 0.0787), with no effect of PBD supplementation observed (p = 0.1369).
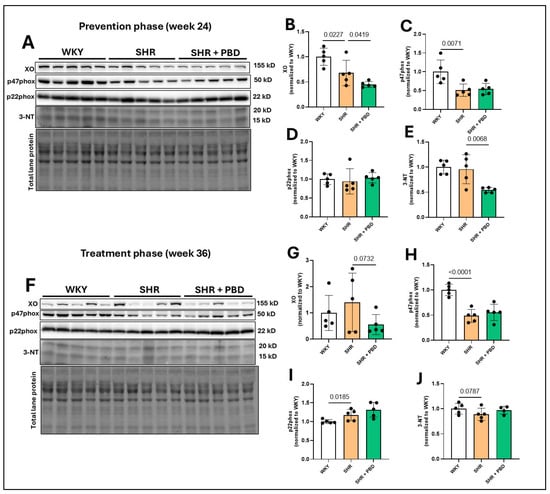
Figure 6.
Expression of pro-oxidative enzymes. At 4 weeks of age, spontaneously hypertensive rats (SHRs) consumed either a control purified diet or a plant-based diet (PBD) for 24 weeks. Wistar Kyoto (WKY) rats also consumed the control diet for 24 weeks. Animals were then euthanized (prevention phase). For the treatment phase, a subgroup of SHRs were switched from the control diet to a PBD after 24 weeks, while remaining WKY and SHRs continued the control diet for 12 additional weeks. Lung protein was isolated and protein expression of (A,B,F,G) XO, (A,C,F,H) p47phox, (A,D,F,I) p22phox and (A,E,F,J) 3-NT, at (A–E) week 24 or (F–J) week 36 via Western blot. Statistical comparisons were made with Student’s t-test between WKY vs. SHR or SHR vs. SHR + PBD (n = 5/group). Lane 13 was excluded (F) because of a large, vertical, nonspecific line running through the bands of interest. The raw blot can be found in the Supplementary Materials. Data are expressed as mean ± SD. Abbreviations: NT, nitrotyrosine; PBD, plant-based diet; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto and XO, xanthine oxidase.
The expression of the antioxidant enzyme, superoxide dismutase (SOD)1, which neutralizes superoxide in the cytosol, was not significantly different between groups at week 24 (Figure 7A,B) or week 36 (Figure 7G,H). However, SOD2, a mitochondrial superoxide-neutralizing protein, was significantly reduced at week 24 (Figure 7A,C) in SHRs consuming the control diet vs. WKYs (0.75 ± 0.18 vs. 1.00 ± 0.13, p = 0.0007), which reduced further at week 36 (Figure 7G,I) in SHR, relative to WKY (0.67 ± 0.08 vs. 1.00 ± 0.06, p < 0.0001). PBD did not significantly impact the expression of SOD2 at any time point. The antioxidant enzyme catalase (CAT), a protein that converts hydrogen peroxide to water, was significantly reduced in SHRs consuming the control diet vs. WKYs (0.64 ± 0.14 vs. 1.00 ± 0.0.25, p = 0.0138) at week 24 (Figure 7A,D), for which PBD supplementation attenuated (0.78 ± 0.11, p = 0.0672). Differences in CAT were no longer statistically significant between groups at week 36 (Figure 7G,J). Glutathione peroxidase (GPx)1, an enzyme that also neutralizes hydrogen peroxide, was significantly reduced at week 24 (Figure 7A,E) in SHRs consuming the control diet vs. WKYs (0.52 ± 0.13 vs. 2.00 ± 0.08, p < 0.0001). PBD supplementation did not significantly change GPx1 expression relative to SHRs alone (p = 0.2045). No significant differences in GPx1 expression were observed between groups at week 36 (Figure 7G,K). The expression of nuclear factor erythroid 2-related factor 2 (NRF2), a transcription factor and regulator of glutathiones, was reduced (non-significantly) at week 24 (Figure 7A,F) in SHRs consuming the control diet vs. WKYs (0.67 ± 0.19 vs. 1.00 ± 0.35, p = 0.0519). PBD supplementation did not significantly impact the expression of NRF2 relative to HSR (p = 0.1314). At week 36, however, there were no significant differences in the expression of NRF2 between groups (Figure 7 G,L).
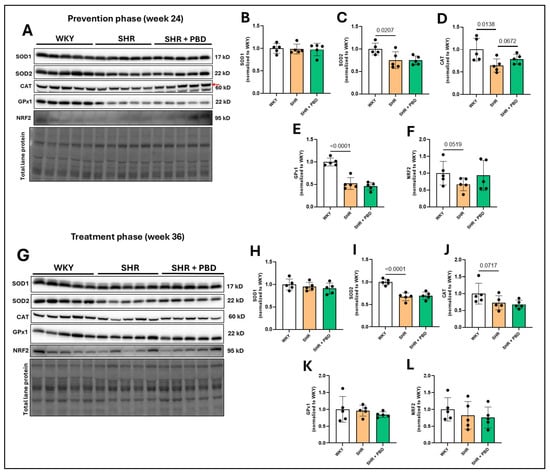
Figure 7.
Expression of antioxidant proteins. At 4 weeks of age, spontaneously hypertensive rats (SHRs) consumed either a control purified diet or a plant-based diet (PBD) for 24 weeks. Wistar Kyoto (WKY) rats also consumed the control diet for 24 weeks. Animals were then euthanized (prevention phase). For the treatment phase, a subgroup of SHRs were switched from the control diet to a PBD after 24 weeks, while the remaining WKYs and SHRs continued the control diet for 12 additional weeks. Lung protein was isolated and protein expression of (A,B,G,H) SOD1, (A,C,G,I) SOD2, (A,D,G,J) CAT, (A,E,G,K) GPx1 and (A,F,G,L) NRF2, at (A–F) week 24 or (G–L) week 36, via Western blot. The red arrow in the CAT blot for panel A points to the band of interest. Statistical comparisons were made with Student’s t-test between WKY vs. SHR or SHR vs. SHR + PBD (n = 5/group). Data are expressed as mean ± SD. Abbreviations: CAT, catalase; GPx, glutathione peroxidase; NRF2, nuclear factor erythroid 2-related factor 2; PBD, plant-based diet; SHR, spontaneously hypertensive rat; SOD, superoxide dismutase and WKY, Wistar Kyoto.
3.5. Impacts of PBD on Lung Architecture and Fibrosis in Female SHRs
No pathological alterations in lung architecture or blood vessels were observed between WKY and SHR, with similar observations found in SHR + PBD (Figure 8A). Similarly, no significant differences in collagen were observed via Sirius red staining (Figure 8B).
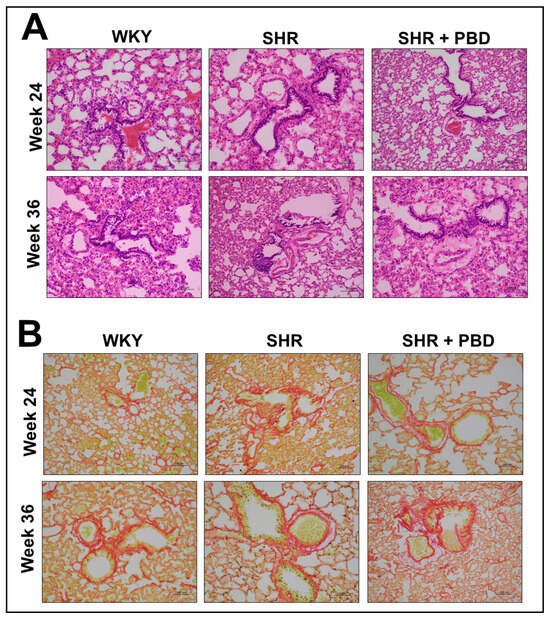
Figure 8.
Histological staining of lung tissue sections. At 4 weeks of age, spontaneously hypertensive rats (SHRs) consumed either a control purified diet or a plant-based diet (PBD) for 24 weeks. Wistar Kyoto (WKY) rats also consumed the control diet for 24 weeks. Animals were then euthanized (prevention phase). For the treatment phase, a subgroup of SHRs were switched from the control diet to a PBD after 24 weeks, while the remaining WKYs and SHRs continued the control diet for 12 additional weeks. Lungs were excised and stained for either (A) H&E or (B) Sirius red. Red staining in panel B indicates fibrosis. Total magnification is 200×. Abbreviations: PBD, plant-based diet; SHR, spontaneously hypertensive rat and WKY, Wistar Kyoto.
4. Discussion
A plant-based diet both prevented and treated pathological molecular changes in proteins involved in endothelial function, epithelial junction and fibrosis in the lung (Figure 9). However, despite these changes, no changes in lung architecture or differences in collagen deposition were noted. This is in contrast to previous findings, in which 14–18 week-old male SHRs had significant pathological alteration in lung tissue [19]. We used female rats that were 28 or 40 weeks old. However, female rats have rarely been utilized in studies of PH; thus, we reveal a key difference in phenotype between sexes. It is possible, however, that with aging, more pronounced pathological changes in lung architecture would be observed. For example, male SHRs tend to develop heart failure 6–12 months sooner than female SHRs [33,34]. Nonetheless, human PH is more dominant in women; thus, female SHRs are more translational than male SHRs.
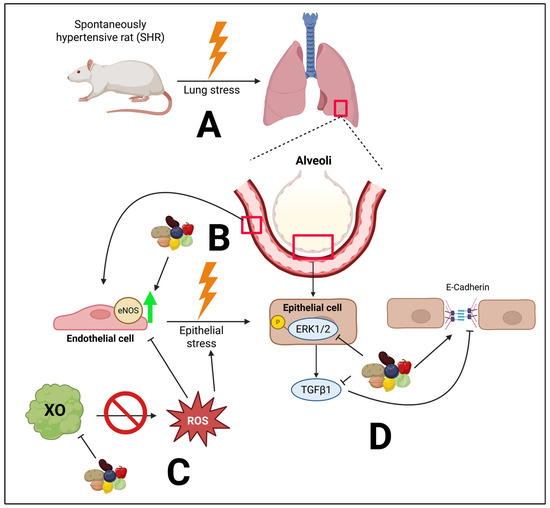
Figure 9.
Overall hypothesized mechanisms by which a PBD favorably impacts the lungs in essential hypertension. A major cause of this stress is microvascular endothelial dysfunction. (A) Essential hypertension can lead to pulmonary hypertension and lung stress. (B) Compromised eNOS can drive epithelial stress. A PBD preserves the expression of eNOS, thus potentially improving microvascular endothelial dysfunction. (C) A PBD inhibits XO expression, likely reducing the release of superoxide, a form of ROS, which can compromise endothelial function and drive epithelial stress. (D) The phosphorylation of ERK1/2 can increase TGFβ1 activity, resulting in reduced E-cadherin, compromising barrier function. However, a PBD attenuated these molecular abnormalities. Created in BioRender. Najjar, R. (2025) https://BioRender.com/f926qw3. Biorender publication agreement number: MX28YBH0G6.
Changes in inflammatory signaling and redox proteins were less clear and more ambiguous (Figure 5, Figure 6 and Figure 7), and conclusions are difficult to draw from this data as a result. While a clear reduction in XO in SHRs consuming PBD versus SHRs consuming the control diet was observed, XO was lower in SHRs at week 24 vs. WKYs, but not at week 36, while 3-NT was lower in PBD vs. SHR at week 24, but not week 36. Despite these discrepancies, a clear finding we observed was complete prevention and reversal of diminished eNOS protein expression in lung tissue with PBD supplementation (Figure 2). This suggests that a PBD could improve lung microvascular endothelial function independently of hypertension. This is important, as microvascular dysfunction of lungs leads to poor gas exchange, potential hypoxia and lung injury, which drives the development of PH [6]. As part of these pathological changes in SHRs, E-cadherin was downregulated, which a PBD attenuated (Figure 3), and activated TGF-β1 was increased, which a PBD also attenuated during both the prevention and treatment phase (Figure 4). Considering these molecular changes in the lungs of SHRs and the protective action of PBD, it is likely that a PBD could prevent and/or treat PH, although additional studies are needed. Because this study is a secondary analysis, pulmonary hypertension was not directly assessed: nor was lung function.
In humans, polyphenol-rich fruit intake is associated with improved lung function [35,36]. Additionally, healthy PBDs, characterized by the intake of whole fruits, vegetables, whole grains and legumes, were associated with a 46% reduced risk of chronic obstructive pulmonary disease (COPD), while an unhealthy PBD, characterized by refined grains and processed foods, was associated with a 39% higher risk [37]. Even in the presence of air pollution, a healthy PBD can counteract the increased risk of COPD, while an unhealthy PBD exacerbates it [38]. This is likely due to the polyphenol and phytochemical content of healthy PBDs, as whole plant foods are rich sources of polyphenols compared to their refined counterparts. Indeed, polyphenol-rich foods and other isolated phytochemicals have been found to be efficacious in animal models of PH [39]. In a model of hypoxia-induced PH, for example, apple polyphenols improved both endothelial-dependent and vascular smooth muscle cell-dependent function in isolated pulmonary arteries [40]. The expression of eNOS also increased. In the lungs specifically, blueberry extract was found to improve lung function and reduce lung oxidative stress in an animal model of monocrotaline-induced PH, using male rats [41]. In this model, however, NRF2 and SODs were upregulated with the blueberry extract, which was not observed in the present study (Figure 7), despite blueberries being a part of the PBD. The mix of plant foods in the PBD used in this study was used in an effort to partially recapitulate a human PBD; thus, our findings are more translational than food extracts or single phytochemical interventions.
Despite the protective role of estrogen in cardiovascular diseases [42], females, including those that are premenopausal, have higher rates of PH than males. Oddly, estrogen is protective in PH in animal models [43,44], representing a paradox. Thus, it is not clear why females have a higher incidence of PH. Future studies should evaluate sex differences in SHRs and also evaluate young versus aged animals to elucidate these mechanisms.
Several limitations exist in the present study. Firstly, we did not use a traditional model of PH [45]. Instead, we used an essential hypertension model in the SHR. However, the existing literature illustrates that SHRs develop PH [19,46,47,48,49]. Thus, we have confidence that the model is appropriate. Secondly, we did not directly measure PH in these animals, which may have been attenuated by the intervention. However, because systemic hypertension was not impacted by PBD supplementation relative to SHRs consuming the control diet, it is likely that PH was unchanged in SHRs between diets; however, future studies should validate this. In addition, we cannot be certain whether the majority of eNOS is from endothelial cells or from other cell types in the lungs which also express eNOS. Prior studies have found that eNOS activity from lung endothelial cells in PH is downregulated—thus, we believe that our data reflects the microvascular endothelium, at least partially [50]. Lung epithelial cells also express eNOS, but this expression is half of that of lung endothelial cells [51]. We also are not certain whether our junction markers (Figure 3) reflect purely epithelial cells. However, we aimed to specifically collect lung tissue for protein analysis at the terminal end of the tissue, rather than towards the large bronchi; as such, we believe we have captured protein from the end of the bronchial tree which comprises the small airway and contains substantial alveoli. In addition, E-cadherin is observed primarily in epithelial cells of the small airways [52]. As such, we believe that our data primarily reflects epithelial junction proteins, rather than junction proteins of other cell types. Lastly, while we did not observe histopathological changes in the lung, the known pathological effects of the changes in the expression of proteins involved in endothelial function, epithelial junctions and fibrosis were attenuated, suggesting that female SHRs of an increased age would likely be needed to show histological abnormalities.
5. Conclusions
In conclusion, a PBD favorably improved proteins associated with PH in the lung, using both prevention and treatment models in female SHRs. This reveals a potential novel therapeutic strategy in the treatment of lung abnormalities associated with PH. Future studies should make direct comparisons between sexes and assess whether a PBD improves PH. Pilot clinical studies utilizing a PBD to treat human PH may be warranted; however, confirmation of the ability of a PBD to target PH itself requires investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/arm93060049/s1.
Author Contributions
R.S.N.: Writing—review and editing, Writing—original draft, Visualization, Methodology, Investigation, Formal analysis, Conceptualization, Funding acquisition, Project administration. J.J.: Writing—review and editing, Methodology, Investigation, Resources. A.T.G.: Writing—review and editing, Funding acquisition, Supervision, Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agriculture and Food Research Initiative grant no. 2023-67012-39756/project accession no. 1030574 from the USDA National Institute of Food and Agriculture, as well as the National Institutes of Health, grant no. 5R01DK083890-13.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Georgia State University (protocol: A23025; approval date: 20 March 2023).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Chang, K.Y.; Duval, S.; Badesch, D.B.; Bull, T.M.; Chakinala, M.M.; De Marco, T.; Frantz, R.P.; Hemnes, A.; Mathai, S.C.; Rosenzweig, E.B.; et al. Mortality in Pulmonary Arterial Hypertension in the Modern Era: Early Insights From the Pulmonary Hypertension Association Registry. J. Am. Heart Assoc. 2022, 11, e024969. [Google Scholar] [CrossRef]
- Itelman, E.; Segel, M.J.; Kuperstein, R.; Feinberg, M.; Segev, A.; Segal, G.; Maor, E.; Grossman, E. Pulmonary Hypertension Is Associated With Systemic Arterial Hypertension Among Patients With Normal Left Ventricular Diastolic Function. J. Am. Heart Assoc. 2021, 10, e023603. [Google Scholar] [CrossRef]
- Memon, H.A.; Park, M.H. Pulmonary Arterial Hypertension in Women. Methodist Debakey Cardiovasc. J. 2017, 13, 224–237. [Google Scholar] [CrossRef]
- Lau, E.M.T.; Giannoulatou, E.; Celermajer, D.S.; Humbert, M. Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2017, 14, 603–614. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Richards, E.M.; Raizada, M.K. Pulmonary hypertension: Pathophysiology beyond the lung. Pharmacol. Res. 2020, 151, 104518. [Google Scholar] [CrossRef] [PubMed]
- Marinho, Y.; Villarreal, E.S.; Loya, O.; Oliveira, S.D. Mechanisms of lung endothelial cell injury and survival in pulmonary arterial hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2024, 327, L972–L983. [Google Scholar] [CrossRef] [PubMed]
- Adu-Amankwaah, J.; Shi, Y.; Song, H.; Ma, Y.; Liu, J.; Wang, H.; Yuan, J.; Sun, K.; Hu, Q.; Tan, R. Signaling pathways and targeted therapy for pulmonary hypertension. Signal Transduct. Target. Ther. 2025, 10, 207. [Google Scholar] [CrossRef]
- Wang, A.; Pan, Q.; Zhang, J.; Gong, S.; Zhang, F.; Liang, N.; Yang, Y.; Jiang, Z. Role of inflammation in endothelial responses in Pulmonary Hypertension. Biomed. Pharmacother. 2025, 188, 118206. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Callejo, M.; Barberá, J.A.; Duarte, J.; Perez-Vizcaino, F. Impact of Nutrition on Pulmonary Arterial Hypertension. Nutrients 2020, 12, 169. [Google Scholar] [CrossRef]
- Wharton, R.C.; Wang, J.G.; Choi, Y.; Eisenberg, E.; Jackson, M.K.; Hanson, C.; Liu, B.; Washko, G.R.; Kalhan, R.; Jacobs, D.R.; et al. Associations of a plant-centered diet and lung function across early to mid-adulthood: The CARDIA Lung Study. Respir. Res. 2024, 25, 122. [Google Scholar] [CrossRef]
- Lin, P.H.; Leslie, D.; Levine, M.; Davis, G.; Esselstyn, C. Plant-Based Diet Reverses Vascular Endothelial Dysfunction in Patients with Peripheral Arterial Disease. Int. J. Dis. Reversal Prev. 2020, 2, 15. [Google Scholar] [CrossRef]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. A defined, plant-based diet utilized in an outpatient cardiovascular clinic effectively treats hypercholesterolemia and hypertension and reduces medications. Clin. Cardiol. 2018, 41, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S.; Turner, C.G.; Wong, B.J.; Feresin, R.G. Berry-Derived Polyphenols in Cardiovascular Pathologies: Mechanisms of Disease and the Role of Diet and Sex. Nutrients 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Stocker, R.; Croft, K.D. Specific Dietary Polyphenols Attenuate Atherosclerosis in Apolipoprotein E–Knockout Mice by Alleviating Inflammation and Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 2443–2458. [Google Scholar] [CrossRef]
- Najjar, R.S.; Hekmatyar, N.; Wang, Y.; Ngo, V.; Lail, H.L.; Tejada, J.P.; Danh, J.P.; Wanders, D.; Feresin, R.G.; Mehta, P.K.; et al. Prevention and reversal of hypertension-induced coronary microvascular dysfunction by a plant-based diet. J. Am. Heart Assoc. 2025, in press. [Google Scholar] [CrossRef]
- Aharinejad, S.; Schraufnagel, D.E.; Böck, P.; MacKay, C.A.; Larson, E.K.; Miksovsky, A.; Marks, S.C., Jr. Spontaneously hypertensive rats develop pulmonary hypertension and hypertrophy of pulmonary venous sphincters. Am. J. Pathol. 1996, 148, 281–290. [Google Scholar]
- Claassen, V. 10—Housing Conditions. In Techniques in the Behavioral and Neural Sciences; Elsevier: Amsterdam, The Netherlands, 1994; Volume 12, pp. 225–250. [Google Scholar]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic Analysis of the Content of 502 Polyphenols in 452 Foods and Beverages: An Application of the Phenol-Explorer Database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Guenther, P.M.; Subar, A.F.; Kirkpatrick, S.I.; Dodd, K.W. Americans Do Not Meet Federal Dietary Recommendations1. J. Nutr. 2010, 140, 1832–1838. [Google Scholar] [CrossRef]
- Kawashima, S.; Yokoyama, M. Dysfunction of Endothelial Nitric Oxide Synthase and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 998–1005. [Google Scholar] [CrossRef]
- Heiss, C.; Rodriguez-Mateos, A.; Kelm, M. Central role of eNOS in the maintenance of endothelial homeostasis. Antioxid. Redox Signal. 2015, 22, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.Y.; Choi, S.J.; Lee, J.S.; Jo, J.; Lee, J.; Kim, J.; Cha, H.J. Loss of E-cadherin activates EGFR-MEK/ERK signaling, which promotes invasion via the ZEB1/MMP2 axis in non-small cell lung cancer. Oncotarget 2013, 4, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Chelladurai, P.; Seeger, W.; Pullamsetti, S.S. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur. Respir. J. 2012, 40, 766–782. [Google Scholar] [CrossRef]
- Church, A.C.; Martin, D.H.; Wadsworth, R.; Bryson, G.; Fisher, A.J.; Welsh, D.J.; Peacock, A.J. The reversal of pulmonary vascular remodeling through inhibition of p38 MAPK-alpha: A potential novel anti-inflammatory strategy in pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L333–L347. [Google Scholar] [CrossRef]
- Das, M.; Zawada, W.M.; West, J.; Stenmark, K.R. JNK2 regulates vascular remodeling in pulmonary hypertension. Pulm. Circ. 2018, 8, 2045894018778156. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Minczuk, K.; Huang, Q.; Kemp, B.A.; Howell, N.L.; Chordia, M.D.; Roy, R.J.; Patrie, J.T.; Qureshi, Z.; Kramer, C.M.; et al. Progressive Cardiac Metabolic Defects Accompany Diastolic and Severe Systolic Dysfunction in Spontaneously Hypertensive Rat Hearts. J. Am. Heart Assoc. 2023, 12, e026950. [Google Scholar] [CrossRef]
- Mirsky, I.; Pfeffer, J.M.; Pfeffer, M.A.; Braunwald, E. The contractile state as the major determinant in the evolution of left ventricular dysfunction in the spontaneously hypertensive rat. Circ. Res. 1983, 53, 767–778. [Google Scholar] [CrossRef]
- Morton, L.; Braakhuis, A.J. The Effects of Fruit-Derived Polyphenols on Cognition and Lung Function in Healthy Adults: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4273. [Google Scholar] [CrossRef]
- Pounis, G.; Arcari, A.; Costanzo, S.; Di Castelnuovo, A.; Bonaccio, M.; Persichillo, M.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Favorable association of polyphenol-rich diets with lung function: Cross-sectional findings from the Moli-sani study. Respir. Med. 2018, 136, 48–57. [Google Scholar] [CrossRef]
- Varraso, R.; Dumas, O.; Tabung, F.K.; Boggs, K.M.; Fung, T.T.; Hu, F.; Giovannucci, E.; Speizer, F.E.; Willett, W.C.; Camargo, C.A., Jr. Healthful and Unhealthful Plant-Based Diets and Chronic Obstructive Pulmonary Disease in U.S. Adults: Prospective Study. Nutrients 2023, 15, 765. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, C.; Fang, X.; Zhao, J.; Chao, W.; Bo, Y.; Zhou, L. Healthful Plant-Based Dietary Patterns Associated with Reduced Adverse Effects of Air Pollution on COPD: Findings from a Large Cohort Study. Nutrients 2025, 17, 1055. [Google Scholar] [CrossRef] [PubMed]
- Jasemi, S.V.; Khazaei, H.; Aneva, I.Y.; Farzaei, M.H.; Echeverria, J. Medicinal Plants and Phytochemicals for the Treatment of Pulmonary Hypertension. Front. Pharmacol. 2020, 11, 145. [Google Scholar] [CrossRef]
- Hua, C.; Zhao, J.; Wang, H.; Chen, F.; Meng, H.; Chen, L.; Zhang, Q.; Yan, J.; Yuan, L. Apple polyphenol relieves hypoxia-induced pulmonary arterial hypertension via pulmonary endothelium protection and smooth muscle relaxation: In vivo and in vitro studies. Biomed. Pharmacother. 2018, 107, 937–944. [Google Scholar] [CrossRef]
- Türck, P.; Fraga, S.; Salvador, I.; Campos-Carraro, C.; Lacerda, D.; Bahr, A.; Ortiz, V.; Hickmann, A.; Koetz, M.; Belló-Klein, A.; et al. Blueberry extract decreases oxidative stress and improves functional parameters in lungs from rats with pulmonary arterial hypertension. Nutrition 2020, 70, 110579. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Farhat, M.Y.; Chen, M.F.; Bhatti, T.; Iqbal, A.; Cathapermal, S.; Ramwell, P.W. Protection by oestradiol against the development of cardiovascular changes associated with monocrotaline pulmonary hypertension in rats. Br. J. Pharmacol. 1993, 110, 719–723. [Google Scholar] [CrossRef]
- Umar, S.; Iorga, A.; Matori, H.; Nadadur, R.D.; Li, J.; Maltese, F.; van der Laarse, A.; Eghbali, M. Estrogen rescues preexisting severe pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2011, 184, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Agrawal, V.; Lawrie, A.; Bonnet, S. The Latest in Animal Models of Pulmonary Hypertension and Right Ventricular Failure. Circ. Res. 2022, 130, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Gomart, S.; Damoiseaux, C.; Jespers, P.; Makanga, M.; Labranche, N.; Pochet, S.; Michaux, C.; Berkenboom, G.; Naeije, R.; McEntee, K.; et al. Pulmonary vasoreactivity in spontaneously hypertensive rats—Effects of endothelin-1 and leptin. Respir. Res. 2014, 15, 12. [Google Scholar] [CrossRef]
- Roldán Gómez, F.J.; Aranda Fraustro, A.; Gómez Soto, L.; Pulido Zamudio, T.; Sánchez Mendoza, A.; Barbosa Carreño, J.A. Heritable pulmonary arterial hypertension in rats with spontaneous systemic hypertension. Rev. Española Cardiol. (Engl. Ed.) 2024, 77, 510–600. [Google Scholar] [CrossRef]
- Langer, A.; Schreckenberg, R.; Schlüter, K.-D. Right Ventricular Hypertrophy in Spontaneously Hypertensive Rats (SHR/NHsd) Is Associated with Inter-Individual Variations of the Pulmonary Endothelin System. Biology 2024, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, U.P.; Schladweiler, M.C.; Ledbetter, A.D.; Watkinson, W.P.; Campen, M.J.; Winsett, D.W.; Richards, J.R.; Crissman, K.M.; Hatch, G.E.; Costa, D.L. The Spontaneously Hypertensive Rat as a Model of Human Cardiovascular Disease: Evidence of Exacerbated Cardiopulmonary Injury and Oxidative Stress from Inhaled Emission Particulate Matter. Toxicol. Appl. Pharmacol. 2000, 164, 250–263. [Google Scholar] [CrossRef]
- Ghosh, S.; Gupta, M.; Xu, W.; Mavrakis, D.A.; Janocha, A.J.; Comhair, S.A.; Haque, M.M.; Stuehr, D.J.; Yu, J.; Polgar, P.; et al. Phosphorylation inactivation of endothelial nitric oxide synthesis in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L1199–L1205. [Google Scholar] [CrossRef]
- German, Z.; Chambliss, K.L.; Pace, M.C.; Arnet, U.A.; Lowenstein, C.J.; Shaul, P.W. Molecular basis of cell-specific endothelial nitric-oxide synthase expression in airway epithelium. J. Biol. Chem. 2000, 275, 8183–8189. [Google Scholar] [CrossRef]
- Sohal, S.S.; Walters, E.H. Epithelial mesenchymal transition (EMT) in small airways of COPD patients. Thorax 2013, 68, 783–784. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).