Beyond the Apnea–Hypopnea Index: Exploring Time-Dependent Hazard Ratios of Respiratory Events in Obstructive Sleep Apnea
Abstract
Highlights
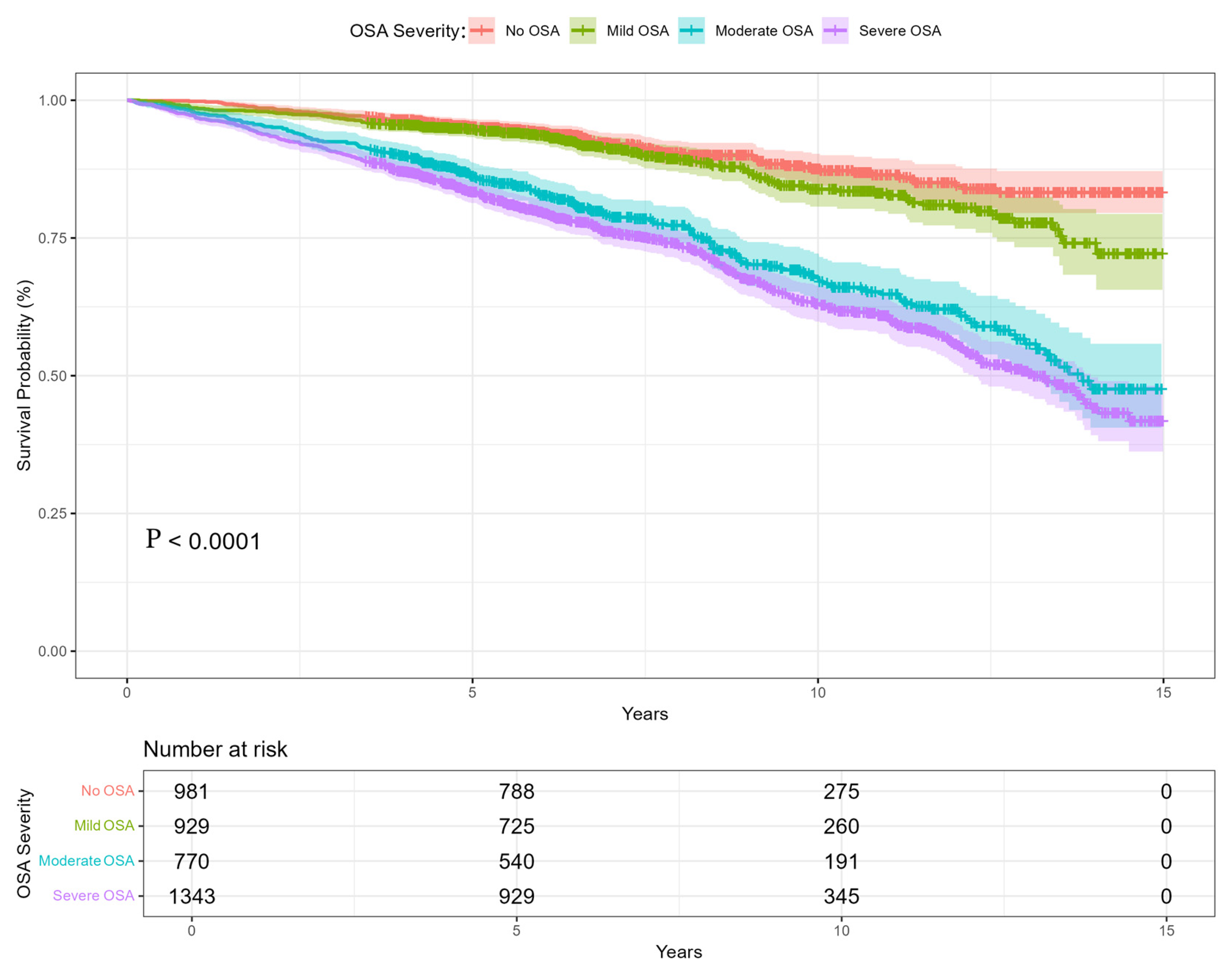
- The highest long-term mortality risk was associated with severe OSA, particularly events occurring during REM sleep and significant oxygen desaturation. Dynamic modeling revealed temporal patterns of risk progression, providing insights beyond those captured by static, point-in-time analyses.
- REM-related apneas and oxygen desaturation measures emerge as stronger predictors of long-term outcomes than conventional AHI-based metrics.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcome Variables
2.3. Polysomnography
2.4. Statistics
3. Results
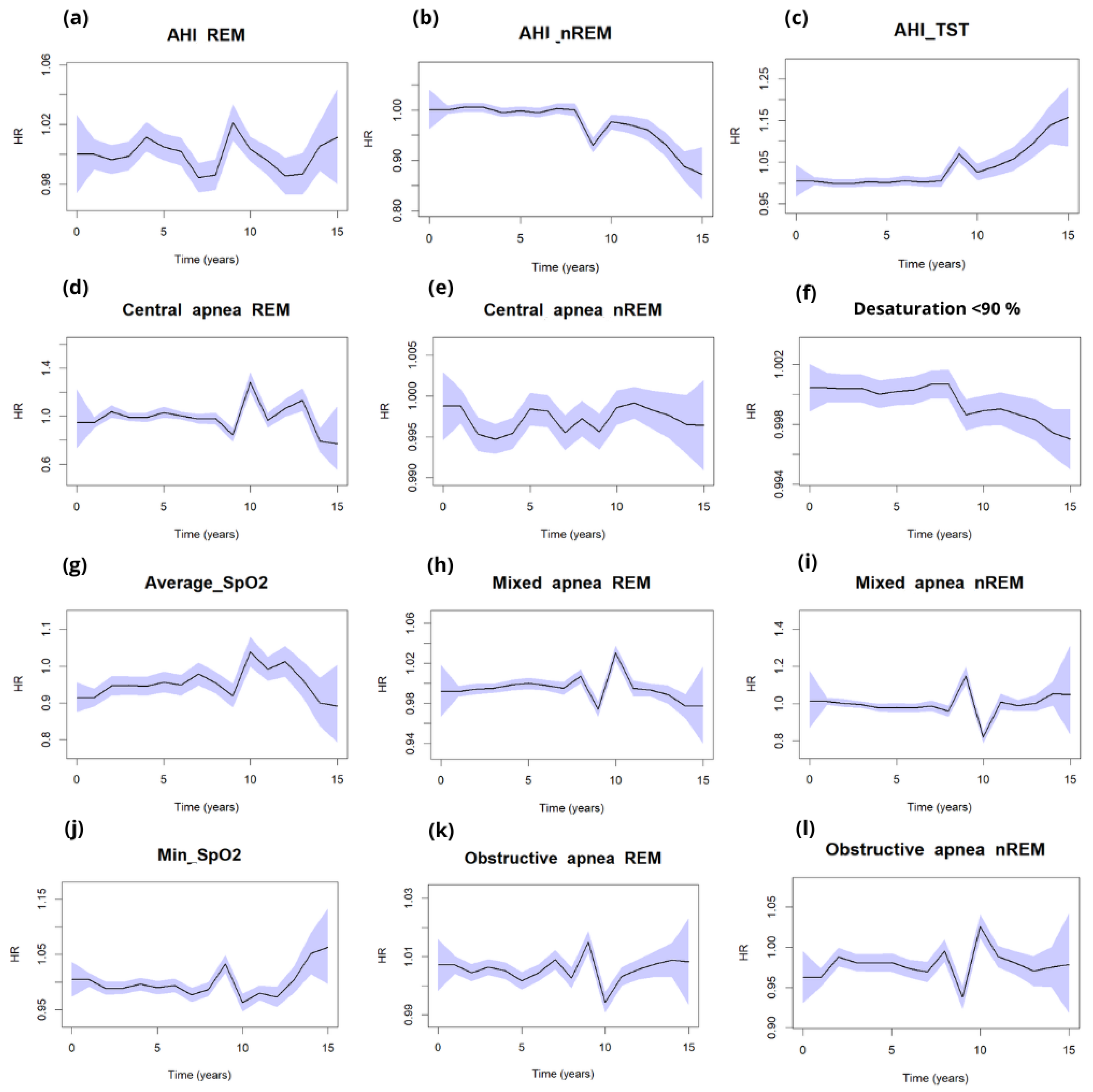
4. Discussion
Polysomnographic Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHI | apnea–hypopnea Index |
| ASA | acetylsalicylic acid |
| BMI | body mass index |
| ESS | Epworth Sleepiness Scale |
| OSA | obstructive sleep apnea |
| PSG | polysomnography |
| TST | total sleep time |
| T90 | time with desaturation below 90% |
| SpO2 | oxygen saturation |
| NREM | non-rapid eye movement sleep |
| REM | rapid eye movement sleep |
| AHITST | apnea–hypopnea index during total sleep time |
| AHIREM | apnea–hypopnea index during REM sleep |
| AHINREM | apnea–hypopnea index during NREM sleep |
Appendix A
| Variables | 0–5 Years HR (Univariable) | 0–10 Years HR (Univariable) | 0–15 Years HR (Univariable) |
|---|---|---|---|
| Age | 2.16 (1.99–2.46, p < 0.001) | 2.26 (2.09–2.45, p < 0.001) | 2.34 (2.18–2.54, p < 0.001) |
| Body mass index (BMI) | 1.22 (1.11–1.34, p < 0.001) | 1.27 (1.19–1.36, p < 0.001) | 1.31 (1.24–1.40, p < 0.001) |
| Epworth Sleepiness Scale | 1.29 (1.17–1.41, p < 0.001) | 1.19 (1.11–1.28, p < 0.001) | 1.22 (1.14–1.30, p < 0.001) |
| Neck circumference | 1.30 (1.18–1.43, p < 0.001) | 1.25 (1.16–1.34, p < 0.001) | 1.30 (1.21–1.38, p < 0.001) |
| Systolic blood pressure | 1.01 (1.01–1.02, p < 0.001) | 1.01 (1.01–1.01, p < 0.001) | 1.01 (1.00–1.01, p < 0.001) |
| Diastolic blood pressure | 1.00 (0.99–1.01, p = 0.556) | 0.99 (0.98–1.00, p = 0.009) * | 0.99 (0.98–0.99, p < 0.001) |
| Morning fatigue | 0.85 (0.69–1.05, p = 0.139) | 0.76 (0.65–0.89, p = 0.001) | 0.91 (0.78–1.05, p = 0.199) |
| Sleepiness | 0.99 (0.73–1.35, p = 0.965) | 0.98 (0.77–1.25, p = 0.883) | 0.97 (0.78–1.21, p = 0.772) |
| Snoring | 0.97 (0.71–1.34, p = 0.864) | 0.82 (0.65–1.03, p = 0.090) | 1.05 (0.84–1.31, p = 0.693) |
| Morning headaches | 0.92 (0.76–1.12, p = 0.424) | 0.96 (0.83–1.11, p = 0.590) | 0.93 (0.81–1.07, p = 0.316) |
| Hypertension | 1.92 (1.53–2.40, p < 0.001) | 2.10 (1.77–2.50, p < 0.001) | 2.24 (1.91–2.63, p < 0.001) |
| Diabetes | 1.64 (1.32–2.03, p < 0.001) | 2.03 (1.73–2.39, p < 0.001) | 2.36 (2.03–2.75, p < 0.001) |
| Atrial fibrillation | 1.72 (1.24–2.38, p = 0.001) | 1.67 (1.30–2.14, p < 0.001) | 2.18 (1.71–2.78, p < 0.001) |
| Dyslipidemia | 1.32 (1.07–1.62, p = 0.008) * | 1.36 (1.16–1.59, p < 0.001) | 1.53 (1.32–1.77, p < 0.001) |
| Depression | 0.86 (0.48–1.52, p = 0.601) | 0.56 (0.37–0.84, p = 0.005) | 0.66 (0.45–0.96, p = 0.032) * |
| Stroke | 2.89 (2.03–4.11, p < 0.001) | 2.05 (1.59–2.64, p < 0.001) | 2.47 (1.94–3.15, p < 0.001) |
| Myocardial Infarction | 0.28 (0.13–0.60, p = 0.001) | 0.40 (0.23–0.69, p = 0.001) | 0.49 (0.29–0.83, p = 0.008) * |
| Hypothyroidism | 1.28 (0.76–2.14, p = 0.353) | 1.91 (1.22–2.97, p = 0.004) * | 1.66 (1.11–2.49, p = 0.014) * |
| Post thyroidectomy | 2.26 (1.01–5.07, p = 0.048) * | 1.86 (0.96–3.59, p = 0.064) | 1.21 (0.65–2.26, p = 0.544) |
| Hyperthyroidism | 1.54 (1.22–1.94, p < 0.001) | 2.02 (1.70–2.40, p < 0.001) | 2.47 (2.10–2.91, p < 0.001) |
| Oral hypoglycemic agents | 2.56 (1.80–3.64, p < 0.001) | 2.74 (2.09–3.59, p < 0.001) | 3.19 (2.47–4.11, p < 0.001) |
| Insulin | 2.90 (1.36–6.18, p = 0.006) | 2.90 (1.50–5.60, p = 0.002) | 2.46 (1.27–4.74, p = 0.007) * |
| Vitamin K antagonist | 1.67 (0.99–2.80, p = 0.052) | 1.98 (1.32–2.98, p = 0.001) | 2.55 (1.71–3.80, p < 0.001) |
| New oral anticoagulants | 2.95 (2.03–4.29, p < 0.001) | 2.39 (1.74–3.28, p < 0.001) | 2.59 (1.91–3.51, p < 0.001) |
| Aspirin (ASA) | 1.85 (1.49–2.29, p < 0.001) | 1.97 (1.68–2.30, p < 0.001) | 2.04 (1.76–2.36, p < 0.001) |
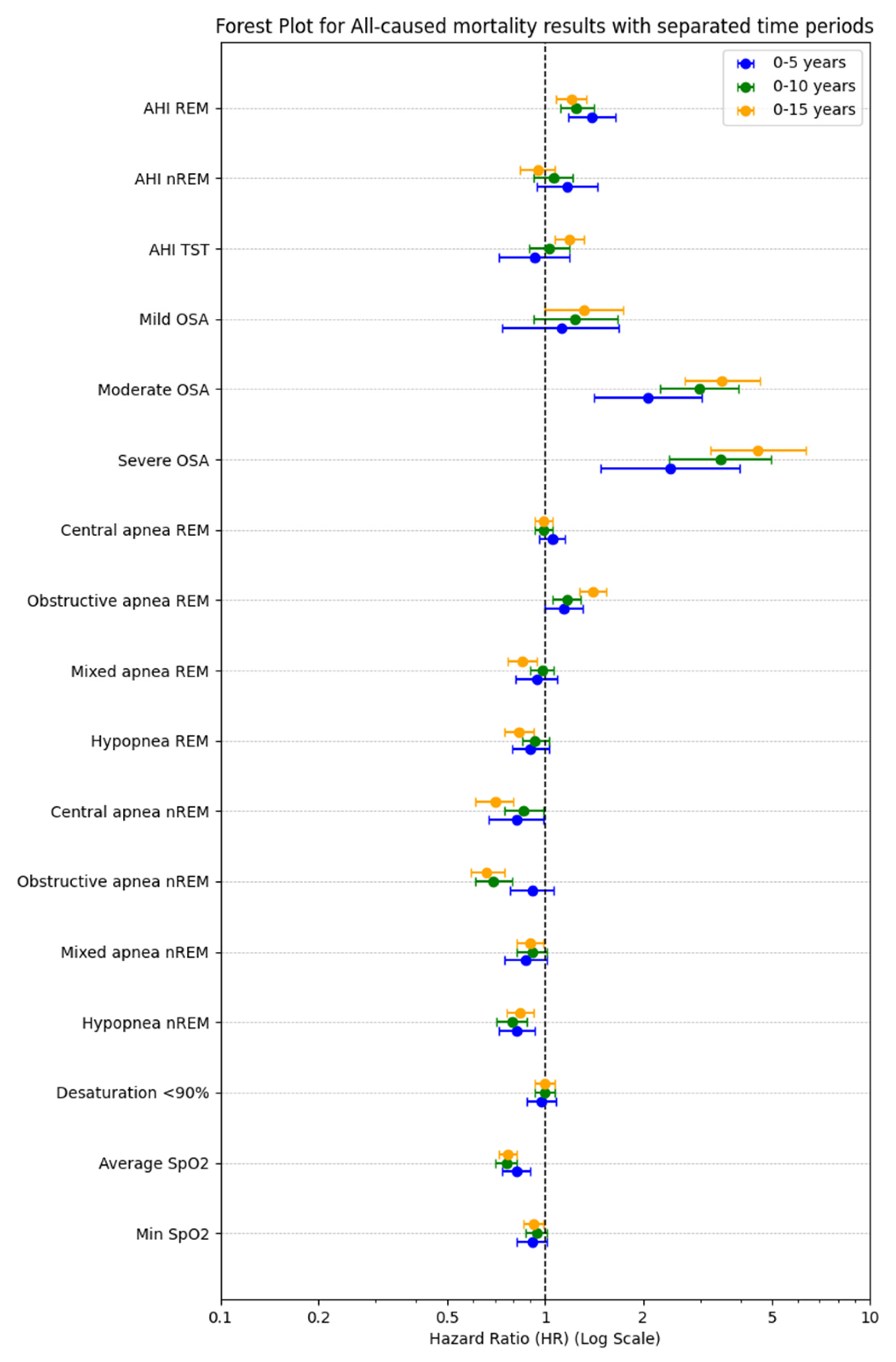
| AHI REM | 1.65 (1.52–1.78, p < 0.001) | 1.48 (1.40–1.57, p < 0.001) | 1.37 (1.30–1.45, p < 0.001) |
| AHI NREM | 1.62 (1.51–1.75, p < 0.001) | 1.50 (1.42–1.59, p < 0.001) | 1.42 (1.35–1.50, p < 0.001) |
| AHI TST | 1.49 (1.37–1.62, p < 0.001) | 1.42 (1.35–1.49, p < 0.001) | 1.46 (1.39–1.53, p < 0.001) |
| Mild OSA | 1.09 (0.73–1.64, p = 0.666) | 1.32 (0.98–1.76, p = 0.063) | 1.31 (1.00–1.72, p = 0.051) |
| Moderate OSA | 2.14 (1.51–3.03, p < 0.001) | 2.89 (2.24–3.74, p < 0.001) | 3.13 (2.46–3.99, p < 0.001) |
| Severe OSA | 2.80 (2.04–3.86, p < 0.001) | 3.66 (2.89–4.64, p < 0.001) | 3.70 (2.96–4.62, p < 0.001) |
| Central apnea REM | 1.03 (0.93–1.13, p = 0.610) | 1.03 (0.98–1.09, p = 0.265) | 1.06 (1.01–1.11, p = 0.011) * |
| Obstructive apnea REM | 1.11 (1.03–1.20, p = 0.007) * | 1.08 (1.03–1.15, p = 0.004) * | 1.08 (1.03–1.14, p = 0.003) |
| Mixed apnea REM | 1.04 (0.96–1.13, p = 0.354) | 1.08 (1.02–1.13, p = 0.004) * | 1.01 (0.96–1.07, p = 0.626) |
| Hypopnea REM | 1.01 (0.91–1.11, p = 0.857) | 1.01 (0.94–1.08, p = 0.768) | 0.97 (0.91–1.04, p = 0.381) |
| Central apnea NREM | 1.04 (0.94–1.14, p = 0.488) | 1.09 (1.02–1.16, p = 0.009) * | 1.05 (0.99–1.12, p = 0.087) |
| Obstructive apnea NREM | 1.04 (0.94–1.15, p = 0.413) | 1.05 (0.98–1.12, p = 0.193) | 1.03 (0.96–1.09, p = 0.438) |
| Mixed apnea NREM | 1.03 (0.94–1.14, p = 0.527) | 1.12 (1.06–1.19, p < 0.001) | 1.07 (1.01–1.13, p = 0.026) * |
| Hypopnea NREM | 0.97 (0.88–1.07, p = 0.520) | 1.00 (0.93–1.08, p = 0.931) | 1.05 (0.98–1.13, p = 0.138) |
| Desaturation < 90%ent | 1.31 (1.22–1.40, p < 0.001) | 1.31 (1.24–1.37, p < 0.001) | 1.29 (1.23–1.36, p < 0.001) |
| Average SpO2 | 0.75 (0.70–0.80, p < 0.001) | 0.70 (0.67–0.74, p < 0.001) | 0.74 (0.72–0.77, p < 0.001) |
| Min SpO2 | 0.78 (0.72–0.84, p < 0.001) | 0.74 (0.70–0.78, p < 0.001) | 0.73 (0.69–0.76, p < 0.001) |
References
- Kuczyński, W.; Kudrycka, A.; Małolepsza, A.; Karwowska, U.; Białasiewicz, P.; Białas, A. The Epidemiology of Obstructive Sleep Apnea in Poland-Polysomnography and Positive Airway Pressure Therapy. Int. J. Environ. Res. Public Health 2021, 18, 2109. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Jennum, P.; Tønnesen, P.; Ibsen, R.; Kjellberg, J. All-cause mortality from obstructive sleep apnea in male and female patients with and without continuous positive airway pressure treatment: A registry study with 10 years of follow-up. Nat. Sci. Sleep 2015, 7, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bouloukaki, I.; Grote, L.; McNicholas, W.T.; Hedner, J.; Verbraecken, J.; Parati, G.; Lombardi, C.; Basoglu, O.K.; Pataka, A.; Marrone, O.; et al. Mild obstructive sleep apnea increases hypertension risk, challenging traditional severity classification. J. Clin. Sleep Med. 2020, 16, 889–898. [Google Scholar] [CrossRef]
- Ruehland, W.R.; Rochford, P.D.; O’dOnoghue, F.J.; Pierce, R.J.; Singh, P.; Thornton, A.T. The New AASM Criteria for Scoring Hypopneas: Impact on the Apnea Hypopnea Index. Sleep 2009, 32, 150. [Google Scholar] [CrossRef]
- Kuczyński, W.; Kudrycka, A.; Pierzchala, K.; Grabska-Kobyłecka, I.; Pencina, M.; Sakowski, S.; Białasiewicz, P. Overall Mortality and Comorbidities in Obstructive Sleep Apnea in Poland. Med. Sci. Monit. 2025, 31, e950826. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Varol, Y.; Uçar, Z.Z.; Arslan, B.O.; Karasu, I. Apnea-hypopnea index and the polysomnographic risk factors for predicting 5- to 8-year mortality in patients with OSA. Sleep Breath. 2024, 28, 103–112. [Google Scholar] [CrossRef]
- Kundel, V.; Ahn, A.; Arzt, M.; Asin, J.; Azarbarzin, A.; Collop, N.; Das, A.; Fang, J.C.; Khayat, R.; Penzel, T.; et al. Insights, recommendations, and research priorities for central sleep apnea: Report from an expert panel. J. Clin. Sleep Med. 2025, 21, 405–416. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Pepin, J.L.; Cibella, F.; Barbera, C.D.; Marrone, O.; Verbraecken, J.; Saaresranta, T.; Basoglu, O.K.; Trakada, G.; Bouloukaki, I.; et al. Excessive Daytime Sleepiness in Obstructive Sleep Apnea Patients Treated with Continuous Positive Airway Pressure: Data from the European Sleep Apnea Database. Front. Neurol. 2021, 12, 690008. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Finn, L.A.; Hagen, E.W.; Young, T.; Hla, K.M.; Van Cauter, E.; Peppard, P.E. Obstructive sleep apnea during REM sleep and hypertension: Results of the Wisconsin sleep cohort. Am. J. Respir. Crit. Care Med. 2014, 190, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhu, R.; Tian, Y.; Wang, K. Association of obstructive sleep apnoea with the risk of vascular outcomes and all-cause mortality: A meta-analysis. BMJ Open 2017, 7, e013983. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Li, Y.; He, F.; Fernandez-Mendoza, J.; Gaines, J.; Liao, D.; Basta, M.; Bixler, E.O. Mild-to-moderate sleep apnea is associated with incident hypertension: Age effect. Sleep 2019, 42, zsy265. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Karagkouni, E.; He, F.; Li, Y.; Karataraki, M.; Fernandez-Mendoza, J.; Bixler, E.O. Mild-to-moderate obstructive sleep apnea and mortality risk in a general population sample: The modifying effect of age and cardiovascular/cerebrovascular comorbidity. J. Sleep Res. 2023, 33, e13944. [Google Scholar] [CrossRef]
- Azarian, M.; Ramezani, A.; Sharafkhaneh, A.; Maghsoudi, A.; Kryger, M.; Thomas, R.J.; Westover, M.B.; Razjouyan, J. The Association between All-cause Mortality and Obstructive Sleep Apnea in Adults: A U-Shaped Curve. Ann. Am. Thorac. Soc. 2025, 22, 581–590. [Google Scholar] [CrossRef]
- Kim, J.-W.; Won, T.-B.; Rhee, C.-S.; Park, Y.M.; Yoon, I.-Y.; Cho, S.-W. Polysomnographic phenotyping of obstructive sleep apnea and its implications in mortality in Korea. Sci. Rep. 2020, 10, 13207. [Google Scholar] [CrossRef]
- Milicic Ivanovski, D.; Milicic Stanic, B.; Kopitovic, I. Comorbidity Profile and Predictors of Obstructive Sleep Apnea Severity and Mortality in Non-Obese Obstructive Sleep Apnea Patients. Medicina 2023, 59, 873. [Google Scholar] [CrossRef]
- Altman, D.G.; Royston, P. The cost of dichotomising continuous variables. Br. Med. J. 2006, 332, 1080. [Google Scholar] [CrossRef]
- Zhang, Z.; Reinikainen, J.; Adeleke, K.A.; Pieterse, M.E.; Groothuis-Oudshoorn, C.G. Big-data Clinical Trial Column Time-varying covariates and coefficients in Cox regression models. Ann. Transl. Med. 2018, 6, 121. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Mazzuca, E.; Baiamonte, P.; Bouckaert, B.; Verbeke, W.; Pevernagie, D.A. REM sleep obstructive sleep apnoea. Eur. Respir. Rev. 2024, 33, 230166. [Google Scholar] [CrossRef]
- Labarca, G.; Vena, D.; Hu, W.-H.; Esmaeili, N.; Gell, L.; Yang, H.C.; Wang, T.-Y.; Messineo, L.; Taranto-Montemurro, L.; Sofer, T.; et al. Sleep Apnea Physiological Burdens and Cardiovascular Morbidity and Mortality. Am. J. Respir. Crit. Care Med. 2023, 208, 802–813. [Google Scholar] [CrossRef]

| n = 4023 | |||||
|---|---|---|---|---|---|
| Variables | n | Mean | SD | Min | Max |
| Age | 4023 | 55.88 | 12.88 | 19 | 93 |
| BMI ‡ | 4023 | 31.69 | 6.01 | 18.08 | 59.97 |
| Neck circumference | 4023 | 42.61 | 3.55 | 30 | 60 |
| TST | 4023 | 336.06 | 70.19 | 152 | 529.3 |
| Wake | 4023 | 54.52 | 49.58 | 0 | 330 |
| REM | 4023 | 60.76 | 30.79 | 0 | 278 |
| NREM | 4023 | 278.47 | 60.06 | 0 | 481 |
| AHIREM ¶ | 4023 | 18.49 | 25.38 | 0 | 143.3 |
| AHINREM ¶ | 4023 | 18.39 | 26.77 | 0 | 158.5 |
| AHITST ¶ | 4023 | 26.68 | 27.58 | 0 | 294 |
| Average saturation | 4023 | 91.55 | 3.78 | 49 | 98 |
| Time < 90% saturation | 4023 | 76.94 | 14.44 | 0.7 | 98 |
| BP systolic ~ | 4023 | 135.9 | 18.2 | 90 | 250 |
| BP diastolic ~ | 4023 | 86.09 | 11.56 | 50 | 140 |
| Epworth SS ‖ | 4023 | 8.35 | 4.69 | 0 | 24 |
| Morning fatigue † | 2986 (74.22%) | ||||
| Snoring † | 3631 (90.26%) | ||||
| Morning headache † | 1736 (43.15%) | ||||
| Hypertension † | 2459 (61.12%) | ||||
| Atrial fibrillation † | 202 (5.02%) | ||||
| Dyslipidemia † | 953 (23.69%) | ||||
| Stroke † | 163 (4.05%) | ||||
| Myocardial Infraction † | 202 (5.02%) | ||||
| Depression † | 186 (4.62%) | ||||
| Diabetes † | 654 (16.26%) | ||||
| Hypothyroidism † | 161 (4.00%) | ||||
| Post thyroidectomy † | 75 (1.86%) | ||||
| Hyperthyroidism † | 38 (0.94%) | ||||
| Oral hypoglycemic agents † | 496 (12.33%) | ||||
| Insulin † | 126 (3.13%) | ||||
| ASA † | 722 (17.95%) | ||||
| Vitamin K antagonist | 100 (2.49%) | ||||
| New oral anticoagulants | 75 (1.86%) | ||||
| No OSA (AHI < 5) ¶ | 981 (24.38%) | ||||
| Mild OSA (AHI ≥ 5. AHI < 15) ¶ | 929 (23.09%) | ||||
| moderate OSA (AHI ≥ 15. AHI < 30) ¶ | 770 (19.14%) | ||||
| Severe OSA (AHI ≥ 30) ¶ | 1343 (33.38%) | ||||
| Specific Cause of Death | n (%) | Gender M/F | OS TIME YEARS Mean (SD) | AHIREM Mean (SD) | AHINREM Mean (SD) | AHITST Mean (SD) | |
|---|---|---|---|---|---|---|---|
| Cardiovascular | Chronic heart failure | 97 (11.37%) | 82/15 | 5.33 (3.14) | 31.05 (28.67) | 31.70 (28.70) | 39.57 (30.27) |
| Myocardial infarction | 72 (8.44%) | 56/16 | 4.67 (3.39) | 31.93 (32.58) | 36.04 (36.71) | 44.91 (45.26) | |
| Stroke, intracerebral hemorrhage | 62 (7.27%) | 43/19 | 5.45 (3.54) | 31.99 (29.86) | 29.92 (32.49) | 38.85 (30.40) | |
| Arteriosclerosis | 17 (1.99%) | 14/3 | 6.48 (4.22) | 31.77 (27.14) | 32.06 (24.26) | 38.10 (28.36) | |
| Cardiomyopathy | 11 (1.29%) | 9/2 | 5.42 (3.89) | 38.55 (37.99) | 36.90 (33.75) | 43.19 (29.61) | |
| Aneurysm | 11 (1.29%) | 10/1 | 5.98 (3.63) | 32.61 (27.94) | 37.00 (32.36) | 42.71 (26.08) | |
| Cardiac arrest | 6 (0.70%) | 5/1 | 4.63 (3.81) | 18.87 (18.07) | 28.08 (44.86) | 33.95 (42.04) | |
| Endocarditis | 6 (0.70%) | 1/1 | 4.03 (2.62) | 23.82 (26.09) | 23.12 (36.78) | 30.70 (28.87) | |
| Arterial hypertension | 2 (0.23%) | 42/10 | 2.82 (2.70) | 49.60 (42.99) | 59.60 (54.02) | 57.90 (52.61) | |
| Pulmonary | Chronic obstructive pulmonary disease | 52 (6.10%) | 25/9 | 4.81 (3.13) | 35.38 (30.12) | 37.70 (37.27) | 49.63 (34.80) |
| Pneumonia | 34 (3.99%) | 9/3 | 7.89 (4.27) | 31.86 (28.01) | 34.37 (33.10) | 36.24 (30.64) | |
| Pulmonary embolism | 12 (1.41%) | 2/1 | 5.42 (3.74) | 30.72 (35.37) | 37.89 (38.69) | 56.08 (33.63) | |
| Interstitial pulmonary disease | 3 (0.35%) | 1/0 | 4.54 (5.36) | 31.00 (53.69) | 51.03 (74.08) | 55.53 (64.07) | |
| Respiratory failure | 1 (0.12%) | 1/0 | 3.55 (nan) | 72.90 (nan) | 20.20 (nan) | 27.40 (nan) | |
| COVID-19 | 25 (2.93%) | 22/3 | 6.89 (3.29) | 27.47 (25.05) | 30.32 (26.36) | 37.10 (22.80) | |
| Diabetes | 18 (2.11%) | 13/5 | 5.81 (3.69) | 25.55 (31.36) | 35.11 (34.82) | 47.81 (25.06) | |
| Suicide | 18 (2.11%) | 14/4 | 3.99 (2.62) | 19.73 (25.55) | 16.80 (24.45) | 17.54 (24.15) | |
| Car accidents | 8 (0.94%) | 7/1 | 4.31 (3.68) | 14.41 (16.00) | 17.84 (22.57) | 19.58 (19.10) | |
| Obesity | 5 (0.59%) | 4/1 | 4.34 (3.77) | 61.98 (20.19) | 59.22 (33.46) | 60.02 (30.83) | |
| Cancer | Lung cancer | 69 (8.09%) | 52/17 | 5.12 (3.43) | 30.83 (28.65) | 31.82 (33.59) | 38.69 (31.24) |
| Gastrointestinal cancer | 61 (7.15%) | 43/18 | 5.85 (3.64) | 31.70 (27.35) | 29.25 (28.54) | 35.41 (25.97) | |
| Genitourinary and reproductive system cancer | 22 (2.58%) | 16/6 | 7.03 (3.34) | 32.63 (36.48) | 39.21 (37.26) | 44.50 (34.04) | |
| Hematopoietic and lymphatic system neoplasms | 20 (2.34%) | 11/9 | 6.67 (3.13) | 34.80 (30.17) | 32.60 (32.21) | 33.59 (30.55) | |
| Skin cancer | 16 (1.88%) | 11/5 | 6.38 (3.61) | 11.99 (14.76) | 11.06 (16.27) | 23.16 (27.39) | |
| Unspecified or metastatic neoplasms | 13 (1.52%) | 12/1 | 6.58 (3.57) | 38.94 (36.48) | 33.44 (22.39) | 34.97 (23.33) | |
| Nervous system neoplasms | 11 (1.29%) | 6/5 | 5.89 (2.80) | 33.73 (28.11) | 35.66 (33.87) | 40.67 (28.90) | |
| Breast cancer | 6 (0.70%) | 0/6 | 6.85 (3.45) | 25.13 (21.31) | 24.72 (28.55) | 26.18 (26.87) | |
| Head and neck cancer | 6 (0.70%) | 6/0 | 7.53 (3.33) | 30.40 (26.51) | 19.70 (34.57) | 22.83 (32.34) | |
| Endocrine neoplasms | 2 (0.23%) | 1/1 | 7.25 (3.30) | 25.10 (35.50) | 20.40 (28.85) | 26.45 (21.85) | |
| Other | 91 (10.67%) | 78/13 | 5.71 (4.09) | 32.25 (30.69) | 38.57 (37.38) | 46.28 (34.89) | |
| Digestive system diseases | 37 (4.34%) | 27/10 | 6.53 (3.79) | 25.94 (27.27) | 26.96 (27.04) | 37.60 (28.31) | |
| Kidney and urinary system diseases | 11 (1.29%) | 6/3 | 5.78 (2.96) | 21.10 (28.97) | 27.04 (33.18) | 34.06 (28.94) | |
| Neurological diseases | 9 (1.06%) | 6/2 | 4.72 (2.69) | 29.78 (25.47) | 26.54 (26.04) | 26.98 (22.95) | |
| External causes of morbidity | 8 (0.94%) | 5/2 | 7.23 (4.16) | 25.85 (28.48) | 30.65 (25.19) | 30.41 (25.15) | |
| Mental and behavioral disorders | 7 (0.82%) | 0/6 | 6.44 (3.87) | 29.27 (25.62) | 30.20 (29.29) | 35.53 (25.10) | |
| Injuries and trauma | 4 (0.47%) | 3/1 | 4.66 (3.49) | 40.77 (32.35) | 57.00 (44.60) | 56.30 (37.86) | |
| Variables | 0–5 Years HR (Multivariable) | 0–10 Years HR (Multivariable) | 0–15 Years HR (Multivariable) |
|---|---|---|---|
| Age | 2.16 (1.99–2.46, p < 0.001) | 2.26 (2.09–2.45, p < 0.001) | 2.34 (2.18–2.54, p < 0.001) |
| Body mass index (BMI) | 1.04 (0.92–1.18, p = 0.507) | 1.18 (1.08–1.29, p < 0.001) | 1.20 (1.10–1.30, p < 0.001) |
| Epworth Sleepiness Scale | 1.28 (1.16–1.42, p < 0.001) | 1.20 (1.11–1.30, p < 0.001) | 1.17 (1.09–1.26, p < 0.001) |
| Neck circumference | 1.21 (1.07–1.37, p = 0.002) | 1.12 (1.02–1.22, p = 0.013) * | 1.15 (1.06–1.24, p = 0.001) |
| Systolic blood pressure | 1.02 (1.01–1.02, p < 0.001) | 1.02 (1.02–1.02, p < 0.001) | 1.02 (1.02–1.02, p < 0.001) |
| Diastolic blood pressure | 0.98 (0.97–1.00, p = 0.004) | 0.97 (0.96–0.98, p < 0.001) | 0.96 (0.96–0.97, p < 0.001) |
| Morning fatigue | 0.78 (0.62–0.98, p = 0.031) * | 0.71 (0.60–0.84, p < 0.001) | 0.90 (0.77–1.05, p = 0.185) |
| Sleepiness | 0.88 (0.63–1.22, p = 0.436) | 0.92 (0.71–1.19, p = 0.518) | 0.81 (0.64–1.03, p = 0.081) |
| Snoring | 0.96 (0.70–1.33, p = 0.823) | 0.76 (0.60–0.96, p = 0.023) * | 0.95 (0.75–1.19, p = 0.639) |
| Morning headaches | 0.96 (0.79–1.18, p = 0.709) | 1.01 (0.87–1.18, p = 0.886) | 0.94 (0.82–1.08, p = 0.362) |
| Hypertension | 1.57 (1.23–2.00, p < 0.001) | 1.66 (1.39–1.99, p < 0.001) | 1.72 (1.45–2.03, p < 0.001) |
| Diabetes | 1.40 (0.95–2.07, p = 0.092) | 1.30 (0.99–1.71, p = 0.056) | 1.38 (1.07–1.78, p = 0.013) * |
| Atrial fibrillation | 1.18 (0.79–1.77, p = 0.425) | 1.05 (0.78–1.42, p = 0.752) | 1.33 (1.00–1.77, p = 0.053) |
| Dyslipidemia | 0.91 (0.72–1.14, p = 0.411) | 0.86 (0.72–1.02, p = 0.086) | 0.96 (0.82–1.13, p = 0.639) |
| Depression | 0.65 (0.35–1.18, p = 0.157) | 0.62 (0.41–0.94, p = 0.023) | 0.66 (0.45–0.96, p = 0.031) * |
| Stroke | 2.41 (1.66–3.49, p < 0.001) | 1.53 (1.17–1.99, p = 0.002) | 1.77 (1.38–2.28, p < 0.001) |
| Myocardial Infarction | 0.29 (0.14–0.62, p = 0.001) | 0.39 (0.22–0.68, p = 0.001) | 0.40 (0.23–0.68, p = 0.001) |
| Hypothyroidism | 1.34 (0.78–2.29, p = 0.291) | 2.37 (1.50–3.75, p < 0.001) | 1.81 (1.18–2.76, p = 0.006) |
| Post thyroidectomy | 2.00 (0.88–4.52, p = 0.096) | 1.78 (0.92–3.45, p = 0.087) | 1.34 (0.72–2.51, p = 0.353) |
| Hyperthyroidism | 0.79 (0.52–1.18, p = 0.251) | 1.17 (0.88–1.54, p = 0.278) | 1.26 (0.97–1.65, p = 0.084) |
| Oral hypoglycemic agents | 1.71 (1.14–2.58, p = 0.010) * | 1.42 (1.04–1.95, p = 0.028) | 1.64 (1.23–2.19, p = 0.001) |
| Insulin | 2.29 (1.06–4.93, p = 0.034) * | 1.90 (0.97–3.73, p = 0.063) | 1.44 (0.74–2.80, p = 0.288) |
| Vitamin K antagonist | 1.40 (0.77–2.54, p = 0.269) | 1.78 (1.14–2.78, p = 0.011) | 1.80 (1.17–2.76, p = 0.007) |
| New oral anticoagulants | 2.56 (1.67–3.94, p < 0.001) | 2.07 (1.44–2.98, p < 0.001) | 1.71 (1.21–2.43, p = 0.002) |
| Aspirin (ASA) | 1.56 (1.24–1.96, p < 0.001) | 1.58 (1.32–1.88, p < 0.001) | 1.46 (1.24–1.72, p < 0.001) |
| AHIREM | 1.38 (1.18–1.62, p < 0.001) | 1.26 (1.12–1.41, p < 0.001) | 1.23 (1.11–1.37, p < 0.001) |
| AHINREM | 1.14 (0.92–1.41, p = 0.222) | 1.10 (0.96–1.26, p = 0.165) | 1.02 (0.91–1.15, p = 0.684) |
| AHITST | 1.09 (0.85–1.38, p = 0.502) | 1.12 (1.00–1.25, p = 0.047) * | 1.27 (1.17–1.37, p < 0.001) |
| Mild OSA | 1.14 (0.75–1.72, p = 0.538) | 1.34 (1.00–1.80, p = 0.049) * | 1.39 (1.06–1.84, p = 0.018) * |
| Moderate OSA | 2.10 (1.43–3.08, p < 0.001) | 3.10 (2.35–4.09, p < 0.001) | 3.65 (2.80–4.74, p < 0.001) |
| Severe OSA | 2.34 (1.43–3.82, p = 0.001) | 3.74 (2.64–5.28, p < 0.001) | 4.72 (3.40–6.56, p < 0.001) |
| Central apnea REM | 1.03 (0.94–1.13, p = 0.511) | 0.97 (0.91–1.04, p = 0.360) | 0.97 (0.91–1.03, p = 0.286) |
| Obstructive apnea REM | 1.14 (1.00–1.31, p = 0.049) * | 1.18 (1.06–1.31, p = 0.002) | 1.38 (1.26–1.51, p < 0.001) |
| Mixed apnea REM | 0.96 (0.83–1.10, p = 0.549) | 0.96 (0.88–1.05, p = 0.408) | 0.87 (0.79–0.96, p = 0.006) * |
| Hypopnea REM | 0.92 (0.81–1.05, p = 0.219) | 0.91 (0.82–1.00, p = 0.049) * | 0.81 (0.73–0.89, p < 0.001) |
| Central apnea NREM | 0.82 (0.67–1.00, p = 0.045) * | 0.86 (0.75–1.00, p = 0.045) * | 0.70 (0.61–0.79, p < 0.001) |
| Obstructive apnea NREM | 0.90 (0.77–1.06, p = 0.201) | 0.70 (0.62–0.80, p < 0.001) | 0.67 (0.60–0.75, p < 0.001) |
| Mixed apnea NREM | 0.86 (0.74–1.00, p = 0.046) * | 0.93 (0.84–1.02, p = 0.133) | 0.91 (0.83–1.01, p = 0.066) |
| Hypopnea NREM | 0.80 (0.71–0.91, p = 0.001) | 0.83 (0.75–0.93, p = 0.001) | 0.88 (0.80–0.97, p = 0.008) * |
| Desaturation < 90%ent | 1.15 (1.05–1.25, p = 0.002) | 1.06 (1.00–1.13, p = 0.064) | 1.06 (1.00–1.13, p = 0.044) |
| Average SpO2 | 0.84 (0.76–0.93, p < 0.001) | 0.78 (0.73–0.83, p < 0.001) | 0.82 (0.78–0.87, p < 0.001) |
| Min SpO2 | 0.86 (0.79–0.94, p = 0.001) | 0.85 (0.79–0.91, p < 0.001) | 0.82 (0.78–0.87, p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuczyński, W.; Kudrycka, A.; Pierzchała, K.; Grabska-Kobyłecka, I.; Pencina, M.; Sakowski, S.; Białasiewicz, P. Beyond the Apnea–Hypopnea Index: Exploring Time-Dependent Hazard Ratios of Respiratory Events in Obstructive Sleep Apnea. Adv. Respir. Med. 2025, 93, 46. https://doi.org/10.3390/arm93050046
Kuczyński W, Kudrycka A, Pierzchała K, Grabska-Kobyłecka I, Pencina M, Sakowski S, Białasiewicz P. Beyond the Apnea–Hypopnea Index: Exploring Time-Dependent Hazard Ratios of Respiratory Events in Obstructive Sleep Apnea. Advances in Respiratory Medicine. 2025; 93(5):46. https://doi.org/10.3390/arm93050046
Chicago/Turabian StyleKuczyński, Wojciech, Aleksandra Kudrycka, Karol Pierzchała, Izabela Grabska-Kobyłecka, Michael Pencina, Sebastian Sakowski, and Piotr Białasiewicz. 2025. "Beyond the Apnea–Hypopnea Index: Exploring Time-Dependent Hazard Ratios of Respiratory Events in Obstructive Sleep Apnea" Advances in Respiratory Medicine 93, no. 5: 46. https://doi.org/10.3390/arm93050046
APA StyleKuczyński, W., Kudrycka, A., Pierzchała, K., Grabska-Kobyłecka, I., Pencina, M., Sakowski, S., & Białasiewicz, P. (2025). Beyond the Apnea–Hypopnea Index: Exploring Time-Dependent Hazard Ratios of Respiratory Events in Obstructive Sleep Apnea. Advances in Respiratory Medicine, 93(5), 46. https://doi.org/10.3390/arm93050046






