Real-World Efficacy of Beclomethasone Dipropionate/Formoterol Fumarate/Glycopyrronium on Diaphragmatic Workload Assessed by Ultrasound and Lung Function in Patients with Uncontrolled Asthma
Abstract
Highlights
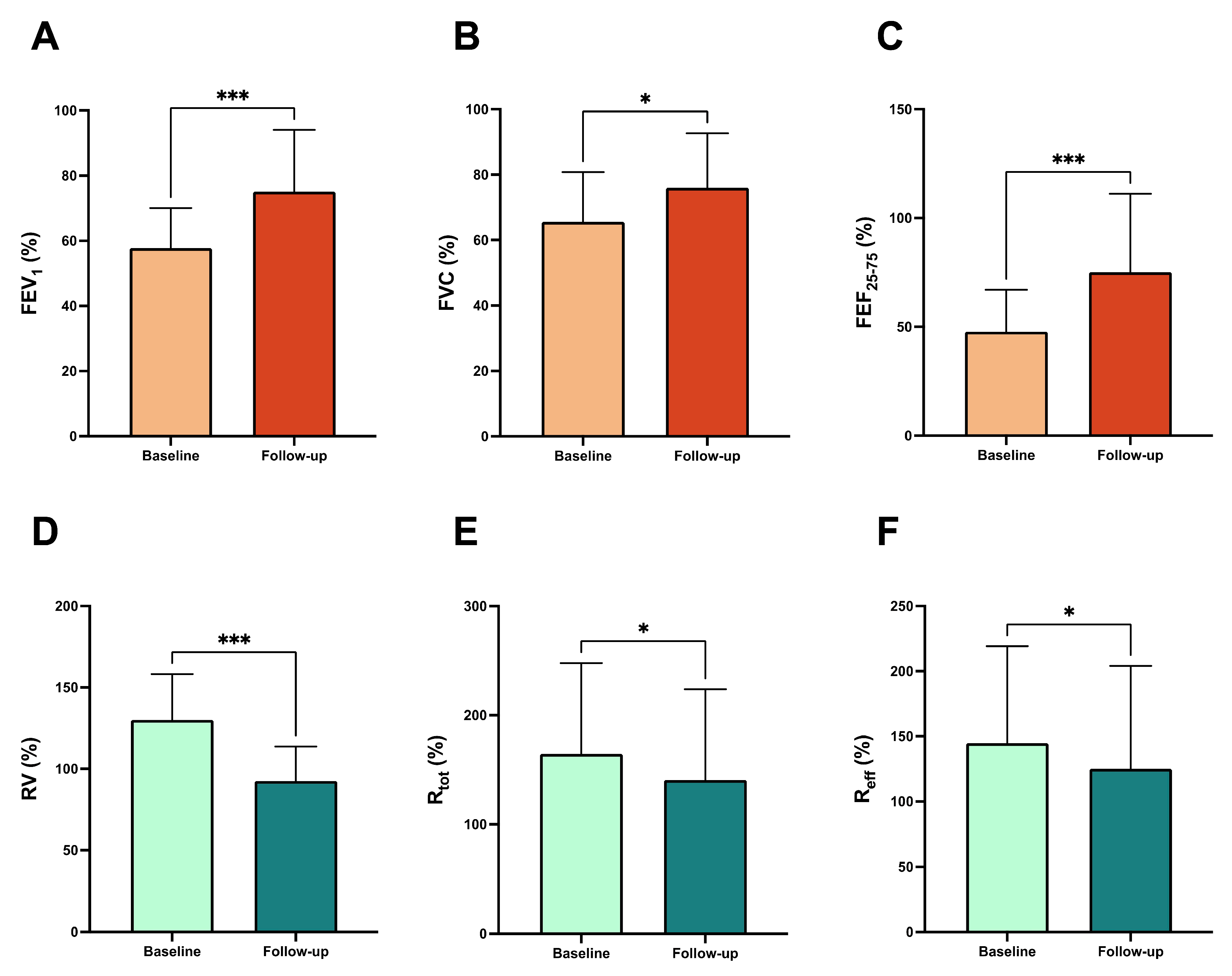
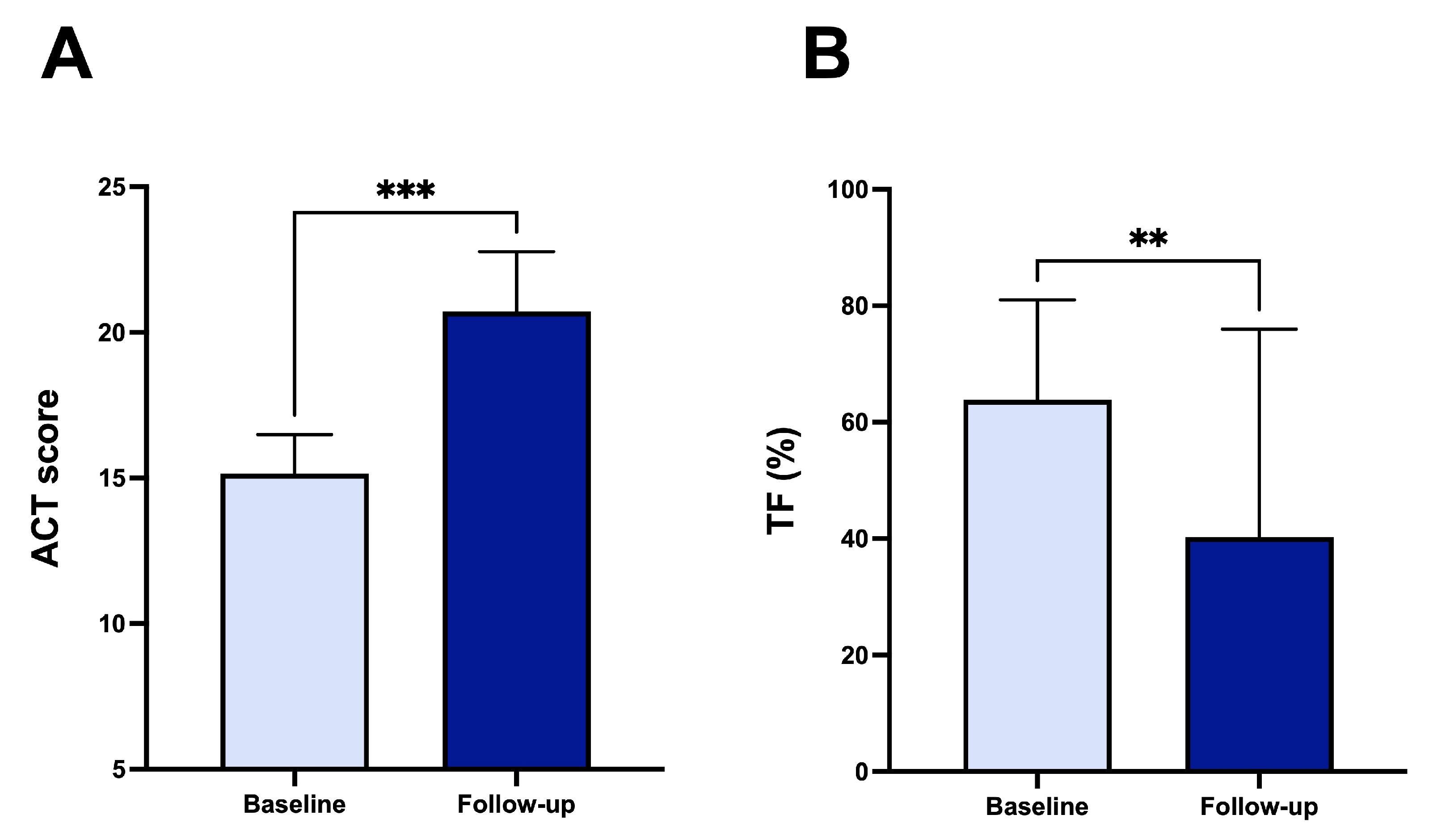
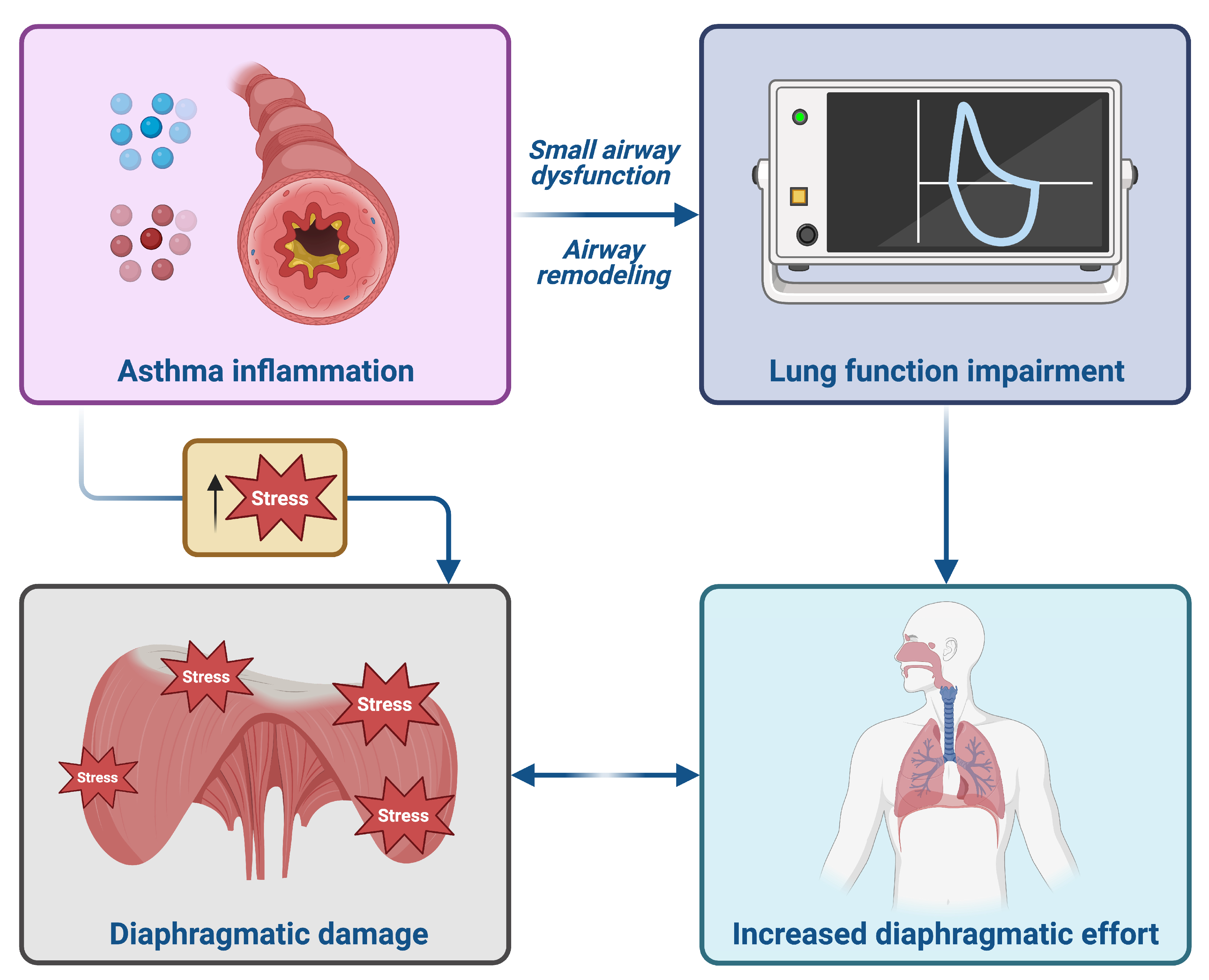
- Triple inhaled therapy with beclomethasone dipropionate/formoterol fumarate/glycopyrronium (BDP/FF/G) significantly improved airflow, reduced residual volume, and enhanced symptom control in patients with uncontrolled asthma.
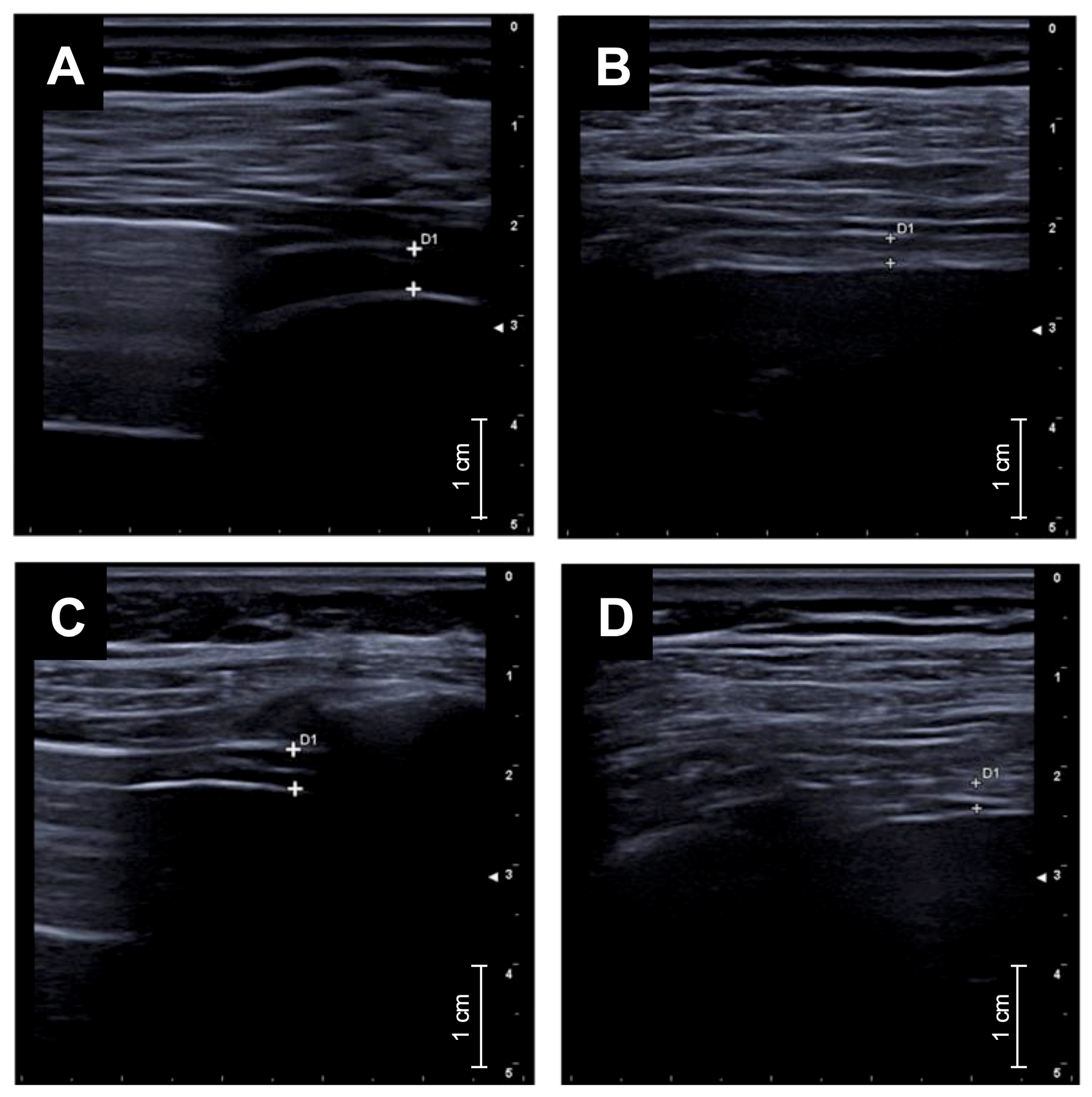
- Diaphragmatic ultrasound showed a marked reduction in diaphragmatic thickening fraction, indicating decreased respiratory workload.
- These results suggest that triple inhaled therapy may not only improve lung function and symptoms but also reduce respiratory muscle strain in real-world settings.
- Diaphragmatic ultrasound could represent a novel, non-invasive biomarker to monitor treatment response in uncontrolled asthma.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Lung Function Tests
2.2. Diaphragmatic Ultrasound Protocol
2.3. Statistical Analysis
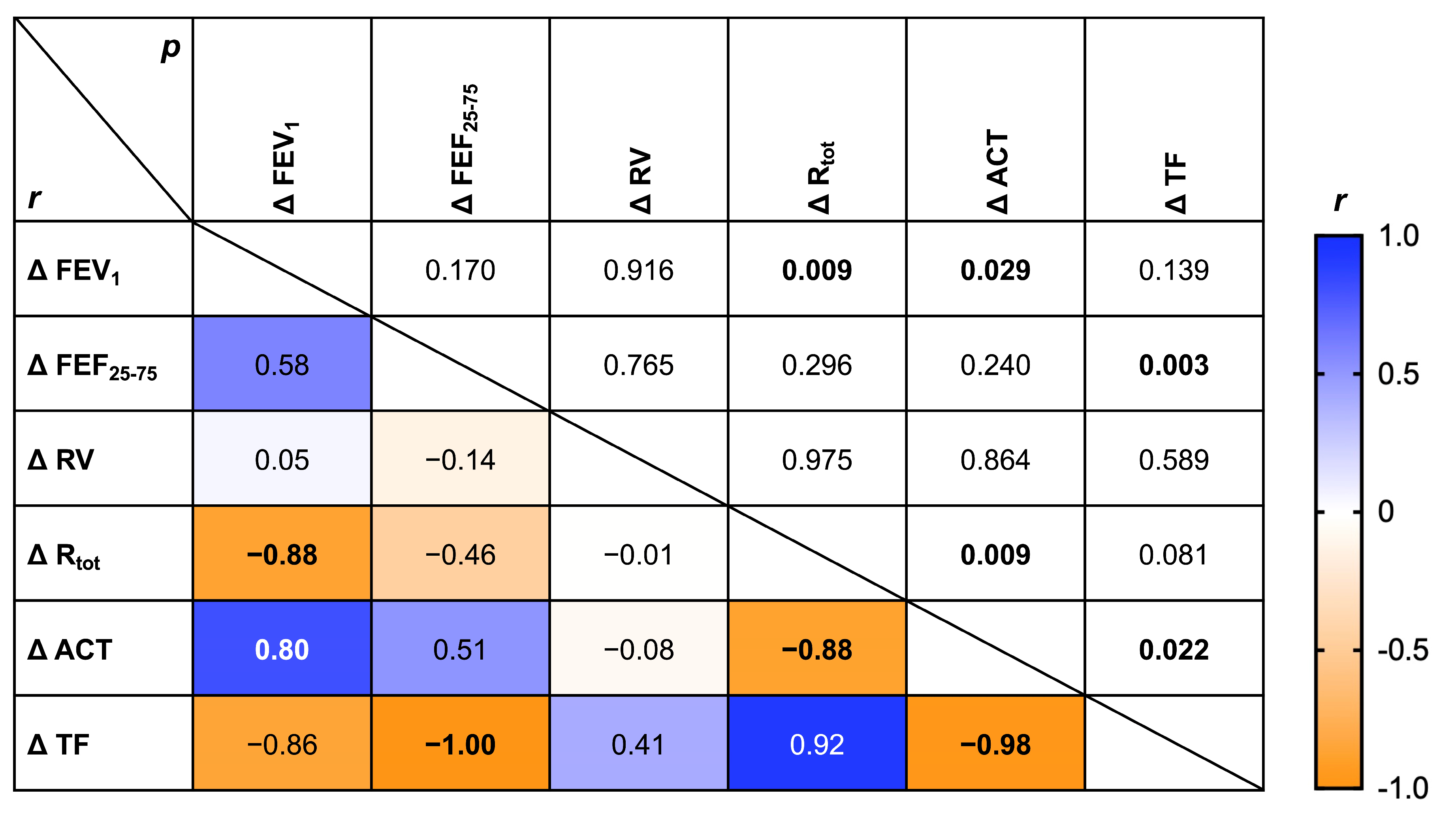
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Crimi, C.; Vatrella, A.; Tinello, C.; Terracciano, R.; Pelaia, G. Molecular Targets for Biological Therapies of Severe Asthma. Front. Immunol. 2020, 11, 603312. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef]
- Global Initiative for Asthma, Global Strategy for Asthma Management and Prevention. 2025. Available online: www.ginasthma.org (accessed on 26 July 2025).
- Virchow, J.C.; Kuna, P.; Paggiaro, P.; Papi, A.; Singh, D.; Corre, S.; Zuccaro, F.; Vele, A.; Kots, M.; Georges, G.; et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): Two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet 2019, 394, 1737–1749. [Google Scholar] [CrossRef]
- Lipworth, B.; Manoharan, A.; Anderson, W. Unlocking the quiet zone: The small airway asthma phenotype. Lancet Respir. Med. 2014, 2, 497–506. [Google Scholar] [CrossRef]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef]
- Postma, D.S.; Brightling, C.; Baldi, S.; Berge, M.V.D.; Fabbri, L.M.; Gagnatelli, A.; Papi, A.; Van der Molen, T.; Rabe, K.F.; Siddiqui, S.; et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): Baseline data from a prospective cohort study. Lancet Respir. Med. 2019, 7, 402–416. [Google Scholar] [CrossRef]
- Pelaia, C.; Armentaro, G.; Lupia, C.; Maiorano, A.; Montenegro, N.; Miceli, S.; Condoleo, V.; Cassano, V.; Bruni, A.; Garofalo, E.; et al. Effects of High-Flow Nasal Cannula on Right Heart Dysfunction in Patients with Acute-on-Chronic Respiratory Failure and Pulmonary Hypertension. J. Clin. Med. 2023, 12, 5472. [Google Scholar] [CrossRef]
- Abenavoli, L.; Spagnuolo, R.; Scarlata, G.G.M.; Scarpellini, E.; Boccuto, L.; Luzza, F. Ultrasound Prevalence and Clinical Features of Nonalcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Diseases: A Real-Life Cross-Sectional Study. Medicina 2023, 59, 1935. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarlata, G.G.; Spagnuolo, R.; Luzza, F. Can dysmetabolic comorbidities carry hepatic fat accumulation in patients with inflammatory bowel diseases? Minerva Gastroenterol. 2025, 71, 74–76. [Google Scholar] [CrossRef]
- Sorino, C.; Mondoni, M.; Lococo, F.; Marchetti, G.; Feller-Kopman, D. Optimizing the management of complicated pleural effusion: From intrapleural agents to surgery. Respir. Med. 2022, 191, 106706. [Google Scholar] [CrossRef]
- Zanforlin, A.; Smargiassi, A.; Inchingolo, R.; di Marco Berardino, A.; Valente, S.; Ramazzina, E. Ultrasound analysis of diaphragm kinetics and the diagnosis of airway obstruction: The role of the M-mode index of obstruction. Ultrasound Med. Biol. 2014, 40, 1065–1071. [Google Scholar] [CrossRef]
- Usmani, O.S.; Scichilone, N.; Mignot, B.; Belmans, D.; Van Holsbeke, C.; De Backer, J.; De Maria, R.; Cuoghi, E.; Topole, E.; Georges, G. Airway Deposition of Extrafine Inhaled Triple Therapy in Patients with COPD: A Model Approach Based on Functional Respiratory Imaging Computer Simulations. Int. J. Chron Obs. Pharmacol. Dis. 2020, 15, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, A.; Lupia, C.; Montenegro, N.; Neri, G.; Bruni, A.; Garofalo, E.; Longhini, F.; Crimi, C.; Maglio, A.; Vatrella, A.; et al. Effects of inhaled beclomethasone dipropionate/formoterol fumarate/glycopyrronium on diaphragmatic workload and lung function in uncontrolled asthma: A case report. Front. Med. 2024, 11, 1357362. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, N.R.; McGowan, A.; Ramsey, K.A.; Borg, B.; Kivastik, J.; Knight, S.L.; Sylvester, K.; Burgos, F.; Swenson, E.R.; McCarthy, K.; et al. European Respiratory Society/American Thoracic Society technical statement: Standardisation of the measurement of lung volumes, 2023 update. Eur. Respir. J. 2023, 62, 2201519. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- Boussuges, A.; Rives, S.; Finance, J.; Chaumet, G.; Vallée, N.; Risso, J.-J.; Brégeon, F. Ultrasound Assessment of Diaphragm Thickness and Thickening: Reference Values and Limits of Normality When in a Seated Position. Front. Med. 2021, 8, 742703. [Google Scholar] [CrossRef]
- Boussuges, A.; Finance, J.; Chaumet, G.; Brégeon, F. Diaphragmatic motion recorded by M-mode ultrasonography: Limits of normality. ERJ Open Res. 2021, 7, 00714-2020. [Google Scholar] [CrossRef]
- Zambon, M.; Greco, M.; Bocchino, S.; Cabrini, L.; Beccaria, P.F.; Zangrillo, A. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: A systematic review. Intensive Care Med. 2017, 43, 29–38. [Google Scholar] [CrossRef]
- Liu, G.; Philp, A.M.; Corte, T.; Travis, M.A.; Schilter, H.; Hansbro, N.G.; Burns, C.J.; Eapen, M.S.; Sohal, S.S.; Burgess, J.K.; et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol. Ther. 2021, 225, 107839. [Google Scholar] [CrossRef]
- Pelaia, C.; Pelaia, G.; Crimi, C.; Maglio, A.; Stanziola, A.A.; Calabrese, C.; Terracciano, R.; Longhini, F.; Vatrella, A. Novel Biological Therapies for Severe Asthma Endotypes. Biomedicines 2022, 10, 1064. [Google Scholar] [CrossRef]
- Bagnasco, D.; Ansotegui, I.; Baiardini, I.; Benfante, A.; Bernstein, J.; Bikov, A.; Bondi, B.; Boulet, L.; Panaitescu, C.; Canonica, G.; et al. Triple inhaled therapy in asthma: Beliefs, behaviours and doubts. Pulm. Pharmacol. Ther. 2024, 87, 102333. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Freitas, P.D.; Silva, A.G.; Porras, D.C.; Stelmach, R.; Cukier, A.; Fernandes, F.L.A.; Martins, M.A.; Carvalho, C.R.F. Dynamic hyperinflation and exercise limitations in obese asthmatic women. J. Appl. Physiol. 2017, 123, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Levi, M. Structure and function of the respiratory muscles in patients with COPD: Impairment or adaptation? Eur. Respir. J. 2003, 22 (Suppl. S46), 41s–51s. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.V.; Cardenas, L.Z.; Albuquerque, A.L.P.; Carvalho, C.R.R.; Caruso, P. Diaphragmatic ultrasound: A review of its methodological aspects and clinical uses. J. Bras. Pneumol. 2020, 46, e20200064. [Google Scholar] [CrossRef] [PubMed]
- Portacci, A.; Iorillo, I.; Quaranta, V.N.; Amendolara, M.; Sana, F.; Pezzuto, V.; Ferrulli, S.; Dragonieri, S.; Carpagnano, G.E. Diaphragm function in patients with asthma and healthy controls: A cross-sectional study. Respir. Med. 2025, 239, 108008. [Google Scholar] [CrossRef]
- Usmani, O.; Li, G.; De Backer, J.; Sadafi, H.; Wu, L.; Marshall, J. Modeled small airways lung deposition of two fixed-dose triple therapy combinations assessed with in silico functional respiratory imaging. Respir. Res. 2023, 24, 226. [Google Scholar] [CrossRef]
- Siafakas, N.; Trachalaki, A. By deflating the lungs pulmonologists help the cardiologists. A literature review. Pulmonology 2023, 29 (Suppl. S4), S86–S91. [Google Scholar] [CrossRef]
- Eysa, A.; Hafez, M.; Moazen, E. Ultrasonographic evaluation of diaphragm function in patients with chronic obstructive pulmonary disease and patients with bronchial asthma: Comparative study. J. Recent Adv. Med. 2023, 4, 144–154. [Google Scholar] [CrossRef]
| Characteristic | N = 21 |
|---|---|
| Age, mean (±SD), years | 66.10 (±9.66) |
| Male gender, N (%) | 8 (38.10) |
| Female gender, N (%) | 13 (61.90) |
| Weight, mean (±SD), kg | 77.60 (±13.70) |
| Height, mean (±SD), cm | 169.00 (±6.52) |
| BMI, median (IQR), kg/m2 | 27.50 (21.00–30.50) |
| Smokers and ex-smokers, N (%) | 7 (33.33) |
| CRSwNP, N (%) | 6 (28.57) |
| GERD, N (%) | 4 (19.05) |
| Atopy, N (%) | 10 (47.62) |
| On treatment with high-dose ICS/LABA, N (%) | 21 (100.00) |
| T2-high endotype, N (%) | 11 (52.38) |
| T2-low endotype, N (%) | 10 (47.62) |
| GINA treatment step 4, N (%) | 6 (28.57) |
| GINA treatment step 5, N (%) | 15 (71.43) |
| Exacerbations in the last year, mean (±SD) | 3.12 (2.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiorano, A.; Ferrante Bannera, A.; Lupia, C.; Pastore, D.; Chiarella, E.; Piazzetta, G.L.; Maglio, A.; Vatrella, A.; Pelaia, G.; Pelaia, C. Real-World Efficacy of Beclomethasone Dipropionate/Formoterol Fumarate/Glycopyrronium on Diaphragmatic Workload Assessed by Ultrasound and Lung Function in Patients with Uncontrolled Asthma. Adv. Respir. Med. 2025, 93, 40. https://doi.org/10.3390/arm93050040
Maiorano A, Ferrante Bannera A, Lupia C, Pastore D, Chiarella E, Piazzetta GL, Maglio A, Vatrella A, Pelaia G, Pelaia C. Real-World Efficacy of Beclomethasone Dipropionate/Formoterol Fumarate/Glycopyrronium on Diaphragmatic Workload Assessed by Ultrasound and Lung Function in Patients with Uncontrolled Asthma. Advances in Respiratory Medicine. 2025; 93(5):40. https://doi.org/10.3390/arm93050040
Chicago/Turabian StyleMaiorano, Antonio, Anna Ferrante Bannera, Chiara Lupia, Daniela Pastore, Emanuela Chiarella, Giovanna Lucia Piazzetta, Angelantonio Maglio, Alessandro Vatrella, Girolamo Pelaia, and Corrado Pelaia. 2025. "Real-World Efficacy of Beclomethasone Dipropionate/Formoterol Fumarate/Glycopyrronium on Diaphragmatic Workload Assessed by Ultrasound and Lung Function in Patients with Uncontrolled Asthma" Advances in Respiratory Medicine 93, no. 5: 40. https://doi.org/10.3390/arm93050040
APA StyleMaiorano, A., Ferrante Bannera, A., Lupia, C., Pastore, D., Chiarella, E., Piazzetta, G. L., Maglio, A., Vatrella, A., Pelaia, G., & Pelaia, C. (2025). Real-World Efficacy of Beclomethasone Dipropionate/Formoterol Fumarate/Glycopyrronium on Diaphragmatic Workload Assessed by Ultrasound and Lung Function in Patients with Uncontrolled Asthma. Advances in Respiratory Medicine, 93(5), 40. https://doi.org/10.3390/arm93050040









