Detecting Airway Involvement in Non-Asthmatic Eosinophilic Disorders: Diagnostic Utility of Fractional Exhaled Nitric Oxide (FeNO)
Abstract
Highlights
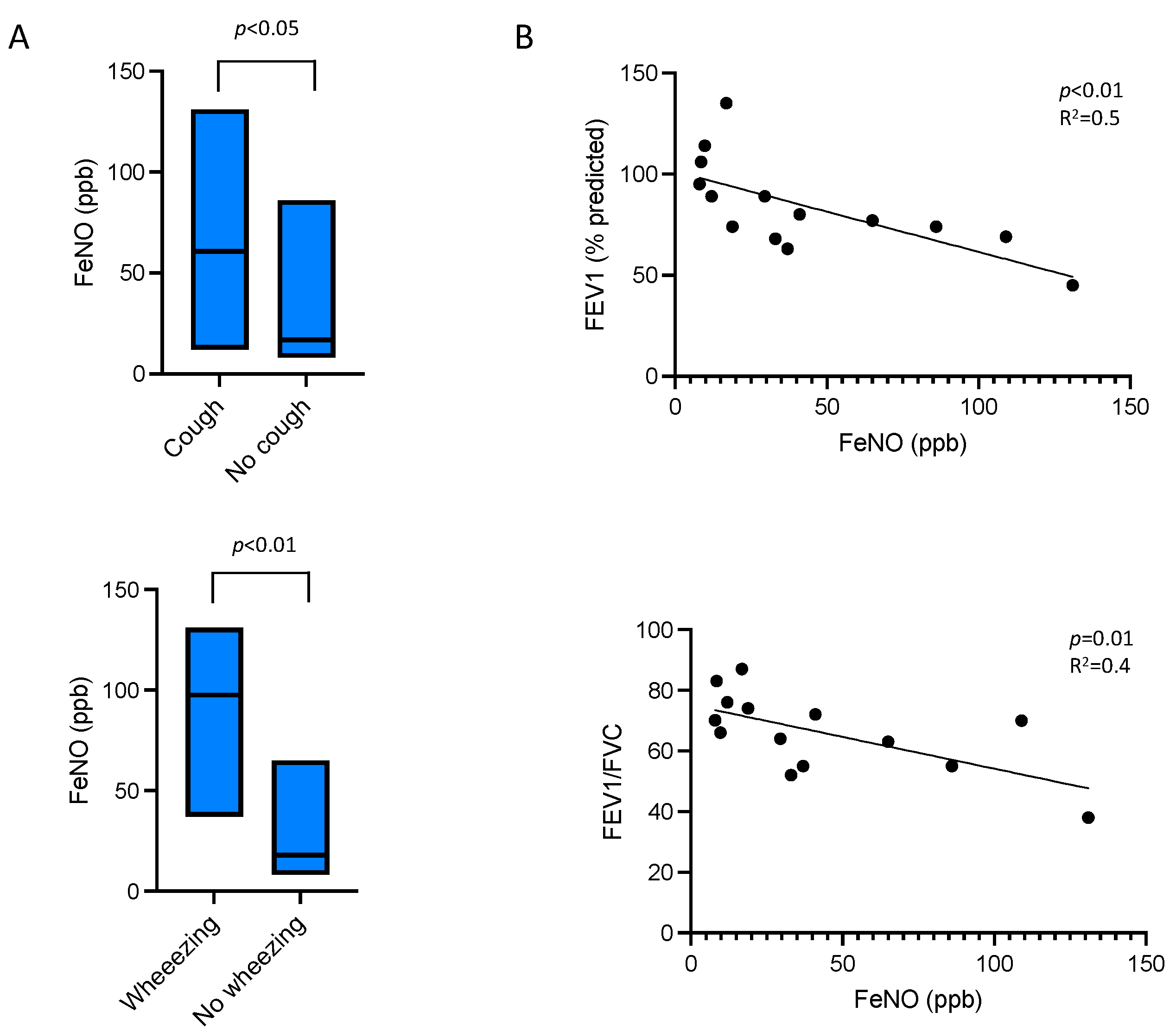
- In patients with persistent eosinophilia from disorders other than asthma, elevated FeNO levels were associated with airflow obstruction and respiratory symptoms such as cough and wheezing.
- Unlike in asthma, blood eosinophil counts showed no correlation with FeNO levels.
- FeNO measurement can help detect bronchial involvement in non-asthmatic eosinophilic disorders where respiratory symptoms may be less apparent or masked by extra-respiratory manifestations.
- Combining FeNO assessment with clinical evaluation could facilitate earlier and adapted treatment for patients with eosinophilic bronchial involvement who might otherwise go unrecognized.
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FeNO | Fractional Exhaled Nitric Oxide |
| FEV1 | Forced Expiratory Volume in 1 Second |
| FVC | Forced Vital Capacity |
| HES | Hypereosinophilic Syndrome |
| mMRC | Modified Medical Research Council |
References
- Wechsler, M.E.; Munitz, A.; Ackerman, S.J.; Drake, M.G.; Jackson, D.J.; Wardlaw, A.J.; Dougan, S.K.; Berdnikovs, S.; Schleich, F.; Matucci, A.; et al. Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clin. Proc. 2021, 96, 2694–2707. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.J.; Bochner, B.S. Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol. Allergy Clin. N. Am. 2007, 27, 357–375. [Google Scholar] [CrossRef]
- McBrien, C.N.; Menzies-Gow, A. The Biology of Eosinophils and Their Role in Asthma. Front. Med. 2017, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.; Rohmer, J.; Etienne, N.; Abou Chahla, W.; Baudet, A.; Chan Hew Wai, A.; Chenivesse, C.; Rusek, I.C.; Cottin, V.; Decamp, M.; et al. French guidelines for the etiological workup of eosinophilia and the management of hypereosinophilic syndromes. Orphanet J. Rare Dis. 2023, 18, 100. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Khatri, S.B.; Iaccarino, J.M.; Barochia, A.; Soghier, I.; Akuthota, P.; Brady, A.; Soghier, I.; Akuthota, P.; Brady, A.; Covar, R.A.; et al. Use of Fractional Exhaled Nitric Oxide to Guide the Treatment of Asthma: An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2021, 204, e97–e109. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Shomali, W.; Gotlib, J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2019, 94, 1149–1167. [Google Scholar] [CrossRef]
- Spry, C.J.; Davies, J.; Tai, P.C.; Olsen, E.G.; Oakley, C.M.; Goodwin, J.F. Clinical features of fifteen patients with the hypereosinophilic syndrome. Q. J. Med. 1983, 52, 1–22. [Google Scholar] [PubMed]
- Xie, J.; Zhang, J.; Zhang, X.; Zhang, Q.; Chung, K.F.; Wang, C.; Lai, K. Cough in hypereosinophilic syndrome: Case report and literature review. BMC Pulm. Med. 2020, 20, 90. [Google Scholar] [CrossRef]
- Kuek, S.L.; Pettman, C.; Neeland, M.R.; Harrison, J.; Mehr, S.; Shanthikumar, S.; Beggs, S. Eosinophilia and wheeze: Thinking beyond asthma. Breathe 2024, 20, 230126. [Google Scholar] [CrossRef]
- Karrasch, S.; Linde, K.; Rücker, G.; Sommer, H.; Karsch-Völk, M.; Kleijnen, J.; Jörres, R.A.; Schneider, A. Accuracy of FENO for diagnosing asthma: A systematic review. Thorax 2017, 72, 109–116. [Google Scholar] [CrossRef]
- Song, W.J.; Kim, H.J.; Shim, J.-S.; Won, H.-K.; Kang, S.-Y.; Sohn, K.-H.; Kim, B.-K.; Jo, E.-J.; Kim, M.-H.; Kim, S.-H.; et al. Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variant asthma and eosinophilic bronchitis in adults with chronic cough: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2017, 140, 701–709. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Li, M.; Xu, X. Diagnostic value of fractional exhaled nitric oxide in cough-variant asthma: An updated meta-analysis. J. Asthma 2020, 57, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.H.; Wright, C.E.; Hart, S.; Crooks, M.; Morice, A. Phenotyping patients with chronic cough: Evaluating the ability to predict the response to anti-inflammatory therapy. Ann. Allergy Asthma Immunol. 2018, 120, 285–291. [Google Scholar] [CrossRef]
- Alcazar-Navarrete, B.; Rodríguez, O.R.; Baena, P.C.; Palacios, P.J.R.; Agusti, A. Persistently elevated exhaled nitric oxide fraction is associated with increased risk of exacerbation in COPD. Eur. Respir. J. 2018, 51, 1701457. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, W.; Wang, L.; Xu, N.; Ding, Q.; Cao, C. Exhaled nitric oxide in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2695–2705. [Google Scholar] [CrossRef]
- Kunisaki, K.M.; Rice, K.L.; Janoff, E.N.; Rector, T.S.; Dennis, E. Niewoehner Exhaled nitric oxide, systemic inflammation, and the spirometric response to inhaled fluticasone propionate in severe chronic obstructive pulmonary disease: A prospective study. Ther. Adv. Respir. Dis. 2008, 2, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Barta, I.; Horvath, I.; Csiszer, E. Relationship between exhaled nitric oxide and treatment response in COPD patients with exacerbations. Respirology 2010, 15, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Purohit, A.; Barnig, C.; Casset, A.; de Blay, F. Niox and Niox Mino: Comparison of exhaled NO in grass pollen allergic adult volunteers. Allergy 2007, 62, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Mih, J.D.; George, S.C. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2007, 37, 97–104. [Google Scholar] [CrossRef] [PubMed]
| n = 14 | |
|---|---|
| Age (years) | 65.7 ± 15.5 |
| Gender (M/F) | 7/7 |
| BMI (kg/m2) | 24.7 ± 4.0 |
| Blood eosinophil count (×109/L) | 1.45 (0.9–3.1) |
| Causes of hypereosinophilia | |
| Idiopathic HES | 3 (21.4%) |
| Ig4-related disease | 1 (7.1%) |
| Eosinophilic fasciitis | 1 (7.1%) |
| Solid malignant tumor | 1 (7.1%) |
| Idiopathic hypereosinophilia | 8 (57.1%) |
| Respiratory symptoms | |
| Cough | 6 (42.9%) |
| Dyspnea (≥1 mMRC) | 8 (57.1%) |
| Wheezing | 4 (28.6%) |
| FeNO (ppb) | 31.25 (11.45–70.25) |
| Pulmonary function tests | |
| FEV1 (L) | 2.07 ± 0.75 |
| FEV1 (% predicted) | 84.1 ± 23.0 |
| FVC (L) | 3.10 ± 0.99 |
| FVC (% predicted) | 100.1 ± 16.8 |
| FEV1/FVC (%) | 66.1 ± 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raoul, N.; Laurent, L.; Ritter, O.; Roux-Claudé, P.; Al Freijat, F.; Magy-Bertrand, N.; Westeel, V.; Barnig, C. Detecting Airway Involvement in Non-Asthmatic Eosinophilic Disorders: Diagnostic Utility of Fractional Exhaled Nitric Oxide (FeNO). Adv. Respir. Med. 2025, 93, 36. https://doi.org/10.3390/arm93050036
Raoul N, Laurent L, Ritter O, Roux-Claudé P, Al Freijat F, Magy-Bertrand N, Westeel V, Barnig C. Detecting Airway Involvement in Non-Asthmatic Eosinophilic Disorders: Diagnostic Utility of Fractional Exhaled Nitric Oxide (FeNO). Advances in Respiratory Medicine. 2025; 93(5):36. https://doi.org/10.3390/arm93050036
Chicago/Turabian StyleRaoul, Nicolas, Lucie Laurent, Ophélie Ritter, Pauline Roux-Claudé, Faraj Al Freijat, Nadine Magy-Bertrand, Virginie Westeel, and Cindy Barnig. 2025. "Detecting Airway Involvement in Non-Asthmatic Eosinophilic Disorders: Diagnostic Utility of Fractional Exhaled Nitric Oxide (FeNO)" Advances in Respiratory Medicine 93, no. 5: 36. https://doi.org/10.3390/arm93050036
APA StyleRaoul, N., Laurent, L., Ritter, O., Roux-Claudé, P., Al Freijat, F., Magy-Bertrand, N., Westeel, V., & Barnig, C. (2025). Detecting Airway Involvement in Non-Asthmatic Eosinophilic Disorders: Diagnostic Utility of Fractional Exhaled Nitric Oxide (FeNO). Advances in Respiratory Medicine, 93(5), 36. https://doi.org/10.3390/arm93050036






