Pharmacological Immunomodulation via Collagen–Polyvinylpyrrolidone or Pirfenidone Plays a Role in the Recovery of Patients with Severe COVID-19 Through Similar Mechanisms of Action Involving the JAK/STAT Signalling Pathway: A Pilot Study
Abstract
Highlights
- Collagen–polyvinylpyrrolidone and pirfenidone have been shown to be therapeutically useful in patients with severe COVID-19.
- Collagen–polyvinylpyrrolidone or pirfenidone could control the inflammation associated with COVID-19, perhaps by regulating the JAK/STAT pathway.
- Collagen–polyvinylpyrrolidone and pirfenidone can be considered as adjuvant therapies for the treatment of infectious diseases involving exacerbated inflammation.
Abstract
1. Introduction
2. Materials and Methods
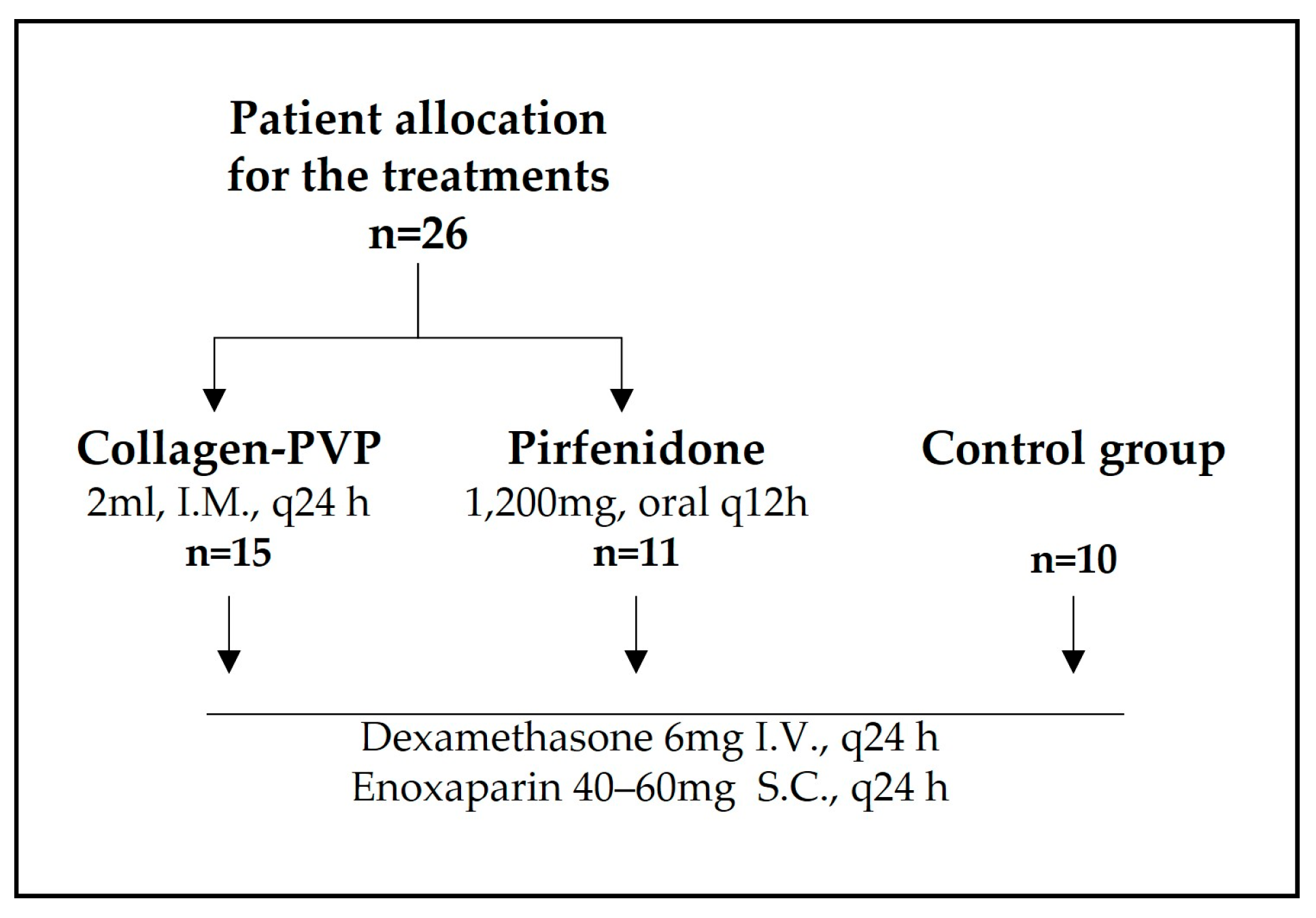
2.1. Clinical Follow-Up
2.2. Ethics
2.3. Human Genome Microarray Assay for Fibroblasts and Monocytes Treated In Vitro with Collagen-PVP
2.4. Statistical Analysis
3. Results
3.1. Treatment of Patients with COVID-19 with Collagen-PVP or Pirfenidone Improves Some Disease Indicators
3.2. Collagen-PVP Modulates Different Signalling Pathways According to Cell Lineage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Collagen-PVP | Collagen–polyvinylpyrrolidone |
| ICU | Intensive care unit |
| qCSI | quick COVID-19 severity index |
| JAK | Janus kinase |
| STAT | signal transducers and activators of transcription |
| SD | Standard deviation |
| ILK | Integrin-Linked Kinase |
| mTOR | Mechanistic Target of Rapamycin Kinase |
| Gαq | Gq protein alpha subunit |
| Rho | Ras homology of the Guanosine Triphosphate family proteins |
| ERK | Extracellular Signal-Regulated Kinases |
| MAPK | Mitogen-Activated Protein Kinases |
Appendix A
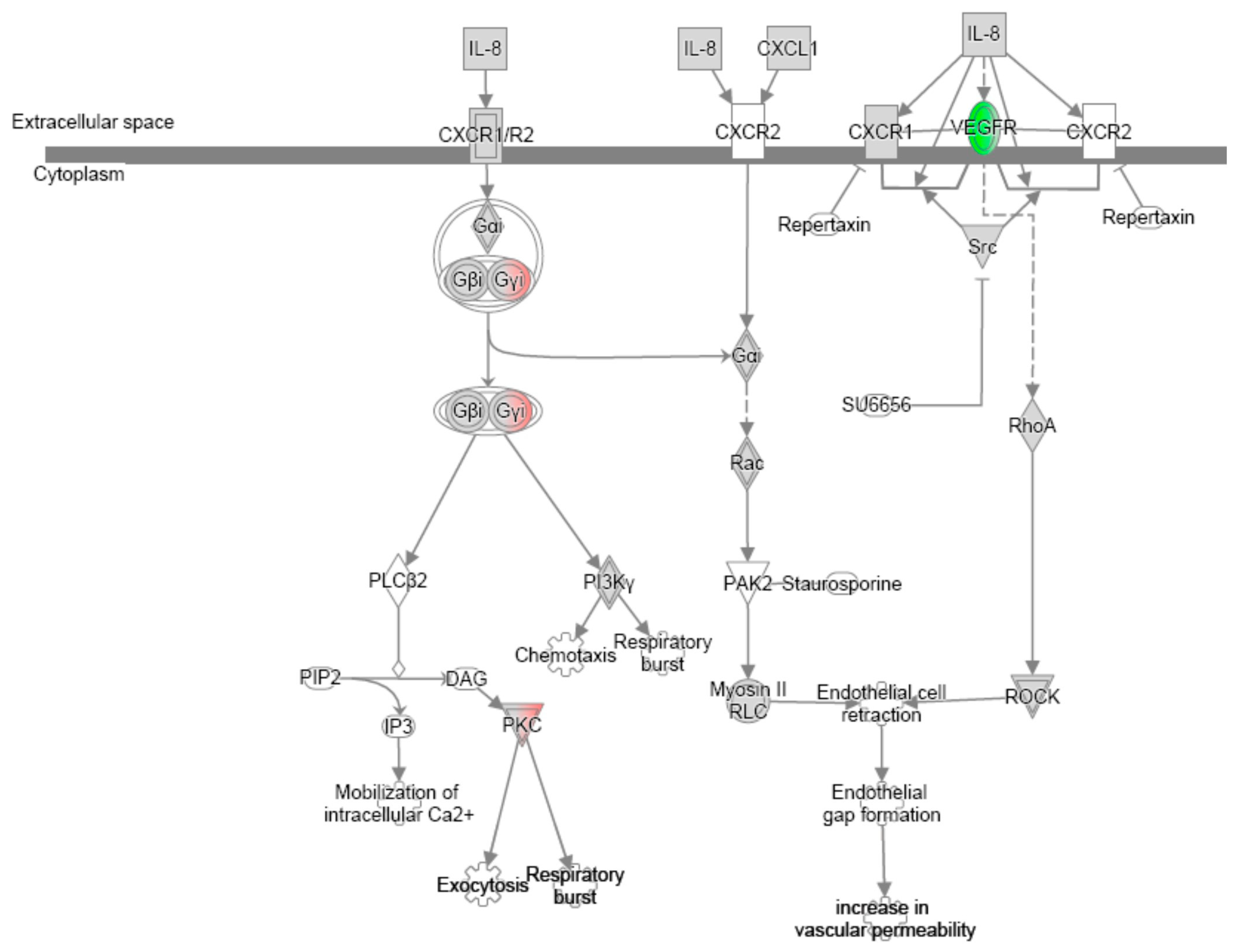
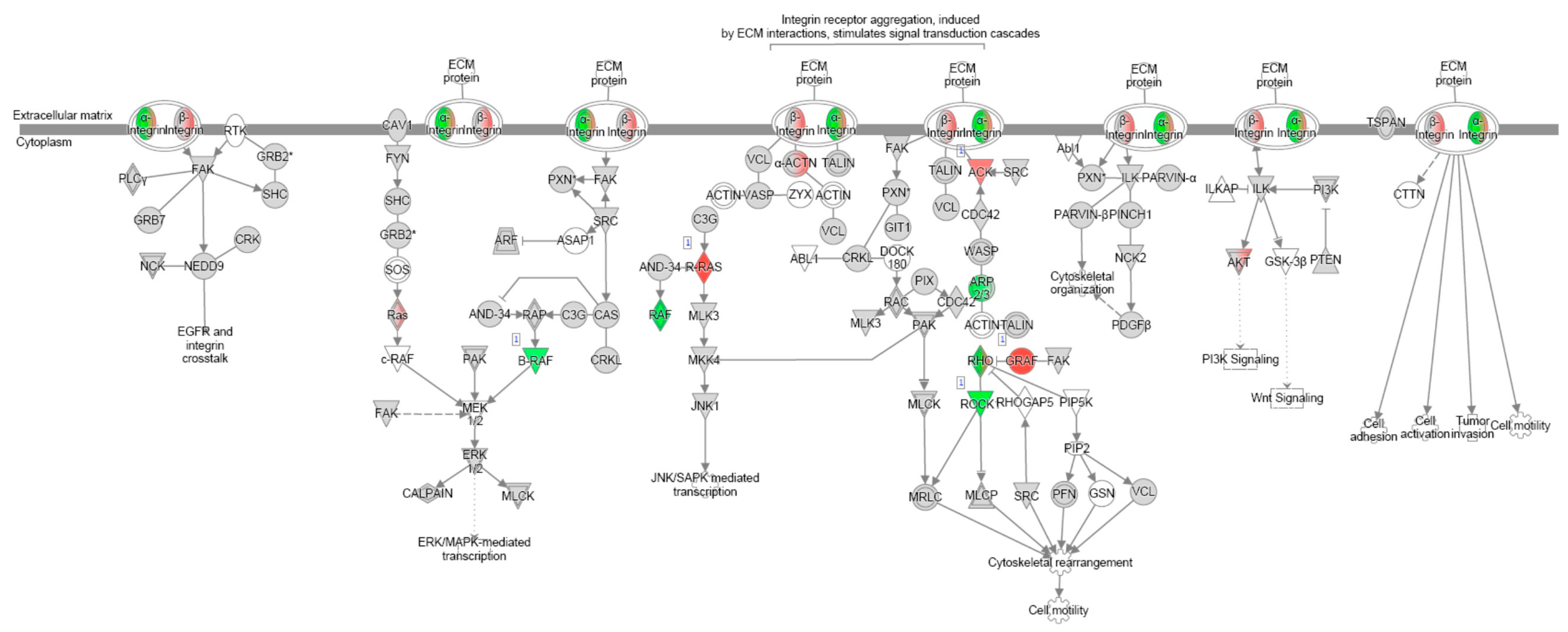
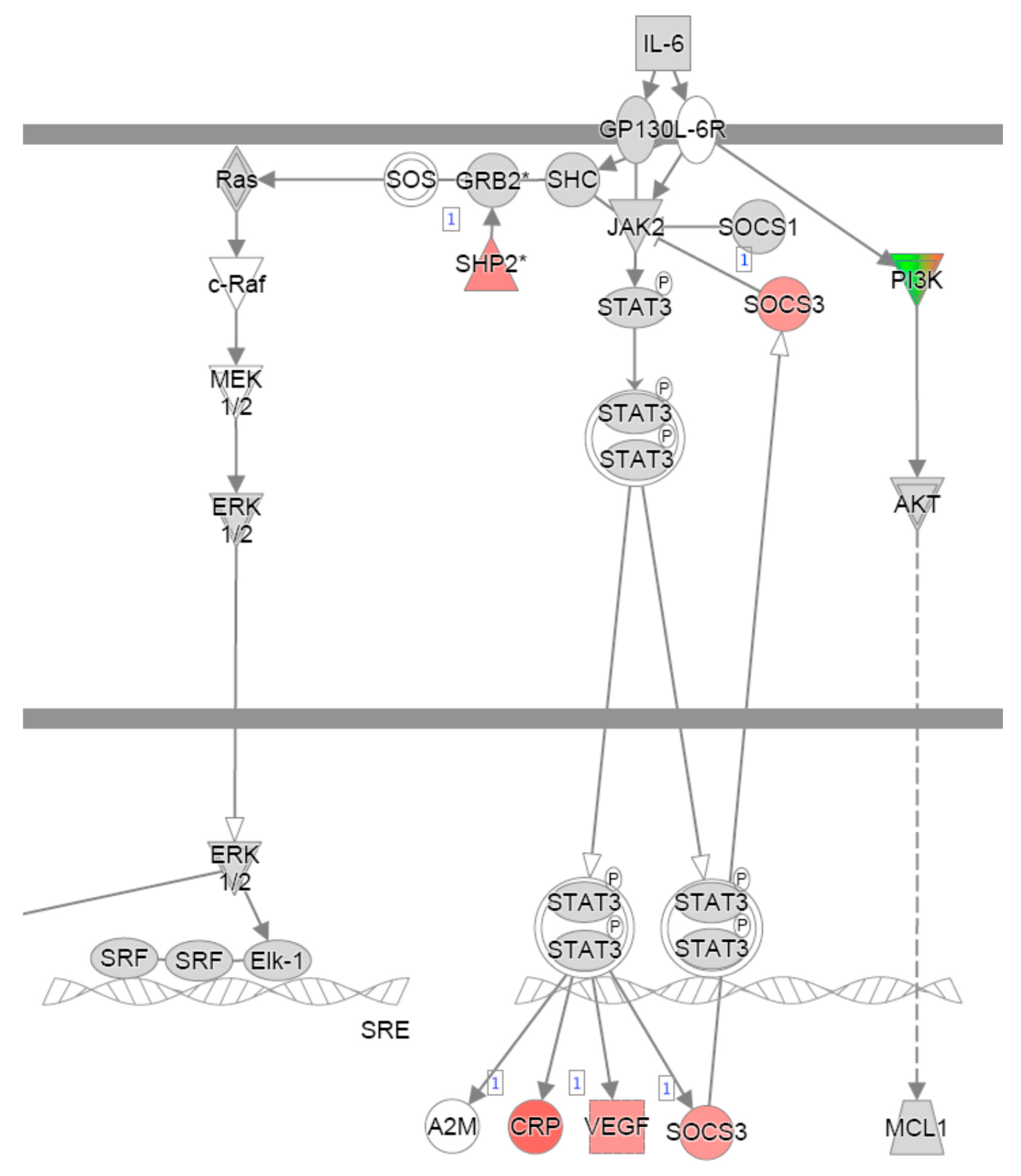
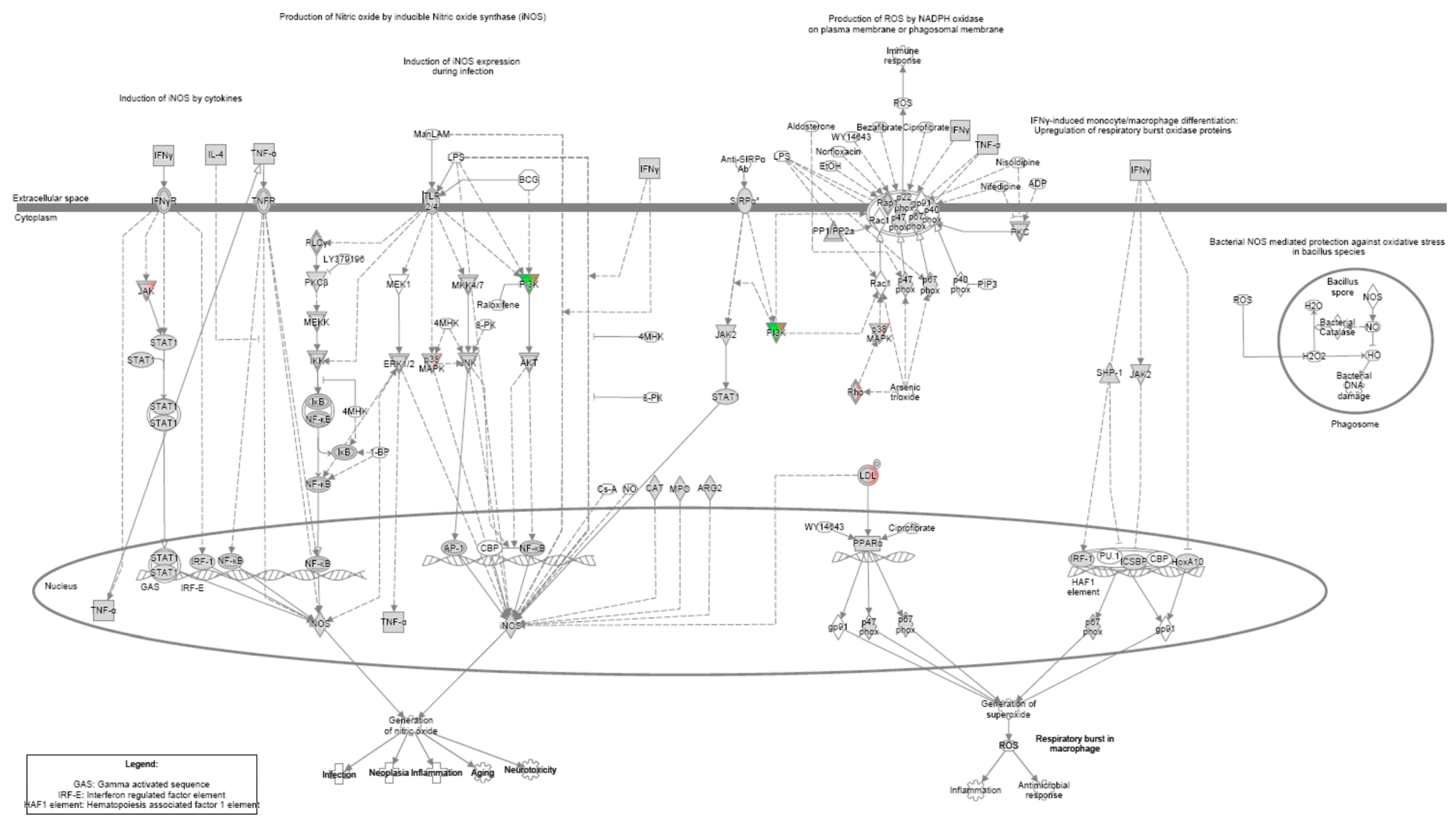
References
- Kinross, P.; Suetens, C.; Dias, J.G.; Alexakis, L.; Wijermans, A.; Colzani, E.; Monnet, D.L.; Prevention, E.C.F.D. Control (ECDC) Public Health Emergency Team Rapidly increasing cumulative incidence of coronavirus disease (COVID-19) in the European Union/European Economic Area and the United Kingdom, 1 January to 15 March 2020. Eurosurveillance 2020, 25, 2000285. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Giacobbe, D.R. The novel Chinese coronavirus (2019nCoV) infections: Challenges for fighting the storm. Eur. J. Clin. Investig. 2020, 50, e13209. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of the coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wang, M.X.; Ang, I.Y.H.; Tan, S.H.X.; Lewis, R.F.; Chen, J.I.-P.; Gutierrez, R.A.; Gwee, S.X.W.; Chua, P.E.Y.; Yang, Q.; et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J. Clin. Med. 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.; Li, Z.; Chua, Y.X.; Chaw, W.L.; Zhao, Z.; Er, B.; Pung, R.; Chiew, C.J.; Lye, D.C.; Heng, D.; et al. Evaluation of the Effectiveness of Surveillance and Containment Measures for the First 100 Patients with COVID-19 in Singapore—January 2–February 29, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Applegate, W.B.; Ouslander, J.G. COVID-19 Presents High Risk to Older Persons. J. Am. Geriatr. Soc. 2020, 68, 681. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Acharya, G. Novel corona virus disease (COVID-19) in pregnancy: What clinical recommendations to follow? Acta Obstet. Gynecol. Scand. 2020, 99, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Sevillano Pires, L.; Andrino, B.; Llaneras, K.; Grasso, D. El Mapa del Coronavirus: Así Crecen los Casos Día a Día y País Por País. Available online: https://elpais.com/sociedad/2020/03/16/actualidad/1584360628_538486.html (accessed on 19 March 2020).
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Flaumenhaft, R.; Enjyoji, K.; Schmaier, A.A. Vasculopathy in COVID-19. Blood 2022, 140, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, A.D.; Yalcin, A.N. Future perspective: Biologic agents in patients with severe COVID-19. Immunopharmacol. Immunotoxicol. 2021, 43, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tale, S.; Ghosh, S.; Meitei, S.P.; Kolli, M.; Garbhapu, A.K.; Pudi, S. Post-COVID-19 pneumonia pulmonary fibrosis. QJM Int. J. Med. 2020, 113, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Vasarmidi, E.; Tsitoura, E.; Spandidos, D.A.; Tzanakis, N.; Antoniou, K.M. Pulmonary fibrosis in the aftermath of the COVID-19 era (Review). Exp. Ther. Med. 2020, 20, 2557–2560. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934. [Google Scholar] [CrossRef] [PubMed]
- Fadel, R.; Morrison, A.R.; Vahia, A.; Smith, Z.R.; Chaudhry, Z.; Bhargava, P.; Miller, J.; Kenney, R.M.; Alangaden, G.; Ramesh, M.S.; et al. Early Short-Course Corticosteroids in Hospitalized Patients With COVID-19. Clin. Infect. Dis. 2020, 71, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Cron, R.Q.; Hartwell, J.; Manson, J.J.; Tattersall, R.S. Silencing the cytokine storm: The use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020, 2, e358–e367. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wang, F.; Huang, Z.; Chen, X.; Wang, Y. Perspectives on anti-IL-1 inhibitors as potential therapeutic interventions for severe COVID-19. Cytokine 2021, 143, 155544. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef] [PubMed]
- On behalf of the Gemelli-ICU Study Group; Montini, L.; DE Pascale, G.; Bello, G.; Grieco, D.L.; Grasselli, G.; Pesenti, A.; Ranieri, V.M.; Cecconi, M.; D’arcangelo, E.; et al. Compassionate use of anti-IL6 receptor antibodies in critically ill patients with acute respiratory distress syndrome due to SARS-CoV-2. Minerva Anestesiol. 2021, 87, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Pettilä, V.; Karvonen, M.K.; Jalkanen, J.; Nightingale, P.; Brealey, D.; Mancebo, J.; Ferrer, R.; Mercat, A.; Patroniti, N.; et al. Effect of Intravenous Interferon β-1a on Death and Days Free From Mechanical Ventilation Among Patients With Moderate to Severe Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2020, 323, 725. [Google Scholar] [CrossRef] [PubMed]
- Callejas Rubio, J.L.; Aomar Millán, I.; Moreno Higueras, M.; Muñoz Medina, L.; López López, M.; Ceballos Torres, Á. Tratamiento y evolución del síndrome de tormenta de citoquinas asociados a infección por SARS-CoV-2 en pacientes octogenarios. Rev. Esp. Geriatría Gerontol. 2020, 55, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gómez, G.; Lima, E.; Krötzsch, G.; Pacheco-Marín, R.; Rodríguez-Fuentes, N.; Quintanar-Guerrero, D.; Krötzsch, E. Physicochemical and functional characterization of the collagen–polyvinylpyrrolidone copolymer. J. Phys. Chem. B 2014, 118, 9272–9283. [Google Scholar] [CrossRef] [PubMed]
- Krötzsch-Gómez, F.E.; Furuzawa-Carballeda, J.; Reyes-Márquez, R.; Quiróz-Hernández, E.; Díaz de León, L. Cytokine expression is downregulated by collagen–polyvinylpyrrolidone in hypertrophic scars. J Invest Dermatol. 1998, 111, 828–834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Furuzawa-Carballeda, J.; Krotzsch, E.; Barile-Fabris, L.; Alcala, M.; Espinosa-Morales, R. Subcutaneous administration of collagen-polyvinylpyrrolidone down regulates IL-1beta, TNF-alpha, TGF-beta1, ELAM-1 and VCAM-1 expression in scleroderma skin lesions. Clin. Exp. Dermatol. 2005, 30, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pozos, K.; Lee-Montiel, F.; Perez-Villalva, R.; Uribe, N.; Gamba, G.; Bazan-Perkins, B.; Bobadilla, N.A. Polymerized type I collagen reduces chronic cyclosporine nephrotoxicity. Nephrol. Dial. Transplant. 2010, 25, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Sánchez, C.R.; Olaya, E.; Testas, M.; Garcia-López, N.; Coste, G.; Arrellin, G.; Luna, A.; Krötzsch, F.E. Collagen-PVP, a modulator of collagen synthesis, decreases intraperitoneal adhesions. J. Surg. Res. 2003, 110, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, H.; Oku, H.; Yamane, S.; Tsuruta, Y.; Suzuki, R. A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-alpha at the translational level. Eur. J. Pharmacol. 2002, 446, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Ebina, M.; Kondoh, Y.; Ogura, T.; Azuma, A.; Suga, M.; Taguchi, Y.; Takahashi, H.; Nakata, K.; Sato, A.; et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010, 35, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Armendariz-Borunda, J.; Islas-Carbajal, M.C.; Meza-Garcia, E.; Rincon, A.R.; Lucano, S.; Sandoval, A.S.; Salazar, A.; Berumen, J.; Alvarez, A.; Covarrubias, A.; et al. A pilot study in patients with established advanced liver fibrosis using pirfenidone. Gut 2006, 55, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Flores, S.; Priego-Ranero, Á.; Azamar-Llamas, D.; Olvera-Prado, H.; Rivas-Redonda, K.I.; Ochoa-Hein, E.; Perez-Ortiz, A.; Rendón-Macías, M.E.; Rojas-Castañeda, E.; Urbina-Terán, S.; et al. Effect of polymerised type I collagen on hyperinflammation of adult outpatients with symptomatic COVID-19. Clin. Transl. Med. 2022, 12, e763. [Google Scholar] [CrossRef] [PubMed]
- Seifirad, S. Pirfenidone: A novel hypothetical treatment for COVID-19. Med. Hypotheses 2020, 144, 110005. [Google Scholar] [CrossRef] [PubMed]
- Furuzawa-Carballeda, J.; Rodríquez-Calderón, R.; de León, L.D.; Alcocer-Varela, J. Mediators of inflammation are down-regulated while apoptosis is up-regulated in rheumatoid arthritis synovial tissue by polymerized collagen. Clin. Exp. Immunol. 2002, 130, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Batiha, G.E.-S.; Faidah, H.; Al-Gareeb, A.I.; Saad, H.M.; Simal-Gandara, J. Pirfenidone and post-COVID-19 pulmonary fibrosis: Invoked again for realistic goals. Inflammopharmacology 2022, 30, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Bagca, B.G.; Avci, C.B. The potential inhibition of the JAK/STAT pathway by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor. Rev. 2020, 54, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Zamora, J.A.; Quiroz, T.; Vega, A.D. Successful treatment with Remdesivir and corticosteroids in a patient with COVID-19-associated pneumonia: A case report. Tratamiento exitoso con Remdesivir y corticoides en un paciente con neumonía asociada a COVID-19: Reporte de un caso. Medwave 2020, 20, e7998. [Google Scholar] [CrossRef] [PubMed]
- Aygün, I.; Kaya, M.; Alhajj, R. Identifying side effects of commonly used drugs in the treatment of COVID 19. Sci. Rep. 2020, 10, 21508. [Google Scholar] [CrossRef] [PubMed]
- Del Carpio-Orantes, L.; García-Méndez, S.; Sánchez-Díaz, J.S.; Aguilar-Silva, A.; Contreras-Sánchez, E.R.; Hernández, S.N.H. Use of Fibroquel® (Polymerized type I collagen) in patients with hypoxemic inflammatory pneumonia secondary to COVID-19 in Veracruz, Mexico. J. Anesthesia Crit. Care Open Access 2021, 13, 69–73. [Google Scholar] [CrossRef]
- Acat, M.; Gulhan, P.Y.; Oner, S.; Turan, M.K. Comparison of pirfenidone and corticosteroid treatments at the COVID-19 pneumonia with the guide of artificial intelligence supported thoracic computed tomography. Int. J. Clin. Pract. 2021, 75, e14961. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.A.; Kathuria, A.; Al Mahmeed, W.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; Cosentino, F.; et al. Post-COVID syndrome, inflammation, and diabetes. J. Diabetes Complicat. 2022, 6, 108336. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Liu, C.; Su, L.; Zhang, D.; Fan, J.; Yang, Y.; Xiao, M.; Xie, J.; Xu, Y.; et al. IP-10 and MCP-1 as biomarkers associated with the severity of the disease of COVID-19. Mol. Med. 2020, 26, 97. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2021, 137, 155323. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-J.; Liu, Y.; Zhang, Z.; Yang, P.-R.; Li, Z.-G.; Song, M.-Y.; Qi, X.-M.; Han, Z.-F.; Pang, J.-L.; Li, B.-C.; et al. Pirfenidone ameliorates silica-induced lung inflammation and fibrosis in mice by inhibiting the secretion of interleukin-17A. Acta Pharmacol. Sin. 2022, 43, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, J.P.; Ponnuswamy, A.; Dutta, P.; Abdelaziz, A.; Kamil, H. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, H.; Huang, X.; Wang, S.; Ouyang, X.; Wang, Y.; Cao, Q.; Yang, L.; Tao, Y.; Lai, H. Pirfenidone attenuates cardiac hypertrophy against isoproterenol by inhibiting activation of the janus tyrosine kinase-2/signal transducer and activator of transcription 3 (JAK-2/STAT3) signaling pathway. Bioengineered 2022, 13, 12772–12782. [Google Scholar] [CrossRef] [PubMed]
- Borja-Flores, A.; Macías-Hernández, S.I.; Hernández-Molina, G.; Perez-Ortiz, A.; Reyes-Martínez, E.; de la Torre, J.B.-C.; Ávila-Jiménez, L.; Vázquez-Bello, M.C.; León-Mazón, M.A.; Furuzawa-Carballeda, J.; et al. Long-Term Effectiveness of Polymerized-Type I Collagen Intra-Articular Injections in Patients with Symptomatic Knee Osteoarthritis: Clinical and Radiographic Evaluation in a Cohort Study. Adv. Orthop. 2020, 2020, 9398274. [Google Scholar] [CrossRef] [PubMed]
- Furuzawa-Carballeda, J.; Cabral, A.R.; Zapata-Zuñiga, M.; Alcocer-Varela, J. Subcutaneous administration of polymerized-type I collagen for the treatment of patients with rheumatoid arthritis. An open-label pilot trial. J. Rheumatol. 2003, 30, 256–259. [Google Scholar] [PubMed]
- Olivares-Martínez, E.; Hernández-Ramírez, D.F.; Núñez-Álvarez, C.A.; Meza-Sánchez, D.E.; Chapa, M.; Méndez-Flores, S.; Priego-Ranero, Á.; Azamar-Llamas, D.; Olvera-Prado, H.; Rivas-Redonda, K.I.; et al. Polymerized Type I Collagen Downregulates STAT-1 Phosphorylation Through Engagement with LAIR-1 in Circulating Monocytes, Avoiding Long COVID. Int. J. Mol. Sci. 2025, 26, 1018. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7, 10489. [Google Scholar] [CrossRef] [PubMed]
- Taraballi, F.; Corradetti, B.; Minardi, S.; Powel, S.; Cabrera, F.; Van Eps, J.L.; Weiner, B.K.; Tasciotti, E. Biomimetic collagenous scaffold to tune inflammation by targeting macrophages. J. Tissue Eng. 2016, 7, 2041731415624667. [Google Scholar] [CrossRef] [PubMed]
- Sarode, A.Y.; Jha, M.K.; Zutshi, S.; Ghosh, S.K.; Mahor, H.; Sarma, U.; Saha, B. Residue-Specific Message Encoding in CD40-Ligand. iScience 2020, 23, 101441. [Google Scholar] [CrossRef] [PubMed]
- Furuzawa-Carballeda, J.; Rojas, E.; Valverde, M.; Castillo, I.; de León, L.D.; Krötzsch, E. Cellular and humoral responses to collagen–polyvinylpyrrolidone administered during short and long periods in humans. Can. J. Physiol. Pharmacol. 2003, 81, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
| Variable | n (%) or Mean (±SD) |
|---|---|
| Group | |
| A (Collagen-PVP) | 15 (57.7) |
| B (Pirfenidone) | 11 (42.3) |
| C (Control) | 10 (27.8) |
| Sex | |
| Male | 17 (47.2) |
| Female | 19 (52.8) |
| Age | 50.8 (19–84) |
| Discharge reason | |
| Patient improvement | 21 (58.3) |
| Death | 15 (41.7) |
| Variable | Group n (%), Mean (±SD) or Median | |||
|---|---|---|---|---|
| Collagen-PVP n = 15 | Pirfenidone n = 11 | Control n = 10 | p Value | |
| Sex | 0.799 | |||
| Male | 8 (53.3) | 5 (45.5) | 4 (40.0) | |
| Female | 7 (46.7) | 6 (54.5) | 6 (60.0) | |
| Age | 47.20 (±13.7) | 56.45 (±13.0) | 50.10 (±19.2) | 0.406 |
| Discharge reason | 0.297 | |||
| Patient improvement | 11 (73.3) | 5 (45.5) | 5 (50.0) | |
| Death | 4 (26.7) | 6 (54.6) | 5 (50.0) | |
| Follow-up days (hospital stay) | 12.67 (±6.8) | 13.18 (±8.1) | 24.10 (±14.2) | 0.015 |
| Weight | 71.47 (±13.3) | 73.18 (±7.7) | 68.60 (±18.1) | 0.738 |
| Size | 1.62 (±0.1) | 1.60 (±0.1) | 1.60 (±0.1) | 0.677 |
| BMI | 27.22 (±4.7) | 28.90 (±4.3) | 26.54 (±5.9) | 0.552 |
| Comorbidities | 4 (80.0) | 5 (100.0) | - | 0.297 |
| SBP-admission | 120.60 (±14.4) | 119.54 (±26.9) | 119.80 (±15.3) | 0.989 |
| DBP-admission | 71.40 (±11.9) | 74.45 (±12.4) | 74.40 (±17.4) | 0.810 |
| MBP-admission | 87.80 (±11.7) | 89.48 (±16.9) | 89.53 (±16.2) | 0.943 |
| SBP-discharge | 108.70 (±8.6) | 104.10 (±18.4) | 104.50 (±9.7) | 0.717 |
| DBP-discharge | 67.00 (±7.3) | 61.5 (±9.6) | 67.83 (±6.4) | 0.224 |
| MBP-discharge | 62.23 (±35.9) | 75.70 (±12.3) | 53.37 (±40.3) | 0.322 |
| HR-admission | 90.13 (±15.7) | 84.82 (±22.5) | 84.90 (±24.6) | 0.751 |
| HR-discharge | 74.30 (±16.6) | 81.70 (±21.8) | 88.50 (±15.0) | 0.393 |
| BF-admission | 24.5 | 23.0 | 22.0 | 0.303 |
| BF-discharge | 21.0 | 23.0 | 18.0 | 0.172 |
| Body temperature-admission | 36.33 (±0.6) | 36.23 (±0.8) | 36.16 (±0.8) | 0.846 |
| Body temperature-discharge | 36.18 (±0.4) | 36.48 (±0.7) | 36.67 (±0.3) | 0.164 |
| Shock index-admission | 0.8 | 0.7 | 0.6 | 0.457 |
| Shock index-discharge | 0.7 | 0.7 | 0.9 | 0.390 |
| SpO2-admission | 80.0 | 93.0 | 93.5 | 0.059 |
| SpO2-discharge | 91.5 | 91.0 | 93.0 | 0.390 |
| Use of supplemental oxygen | 4 (50.0) | 7 (100.0) | 1 (100.0) | 0.069 |
| FiO2-admission | 0.9 | 0.9 | 0.7 | 0.240 |
| FiO2-discharge | 0.2 | 0.9 | 0.3 | 0.196 |
| Kirby-admission | 96.7 | 90.9 | 130.8 | 0.100 |
| Kirby-discharge | 135.1 | 133.3 | 103.3 | 0.397 |
| qCSI-admission | 8.0 | 6.0 | 5.0 | 0.363 |
| qCSI-discharge | 2.0 | 4.5 | 6.0 | 0.019 |
| Glucose-admission | 108.5 | 151.5 | 146.0 | 0.397 |
| Glucose-discharge | 90.0 | 119.5 | 179.0 | 0.018 |
| BUN-admission | 14.5 | 25.5 | 23.0 | 0.568 |
| BUN-discharge | 18.0 | 16.0 | 12.0 | 0.673 |
| Creatinine-admission | 0.7 | 0.9 | 0.9 | 0.265 |
| Creatinine-discharge | 0.6 | 0.7 | 0.4 | 0.608 |
| Leukocytes-admission | 10.9 | 11.1 | 11.4 | 0.951 |
| Leukocytes-discharge | 8.1 | 10.6 | 9.3 | 0.110 |
| Blood gas pH-admission | 7.5 | 7.5 | 7.4 | 0.807 |
| Blood gas pH-discharge | 7.5 | 7.3 | 7.4 | 0.185 |
| PCO2-admission | 31.0 | 29.0 | 30.0 | 0.462 |
| PCO2-discharge | 33.0 | 60.5 | 37.0 | 0.219 |
| PO2-admission | 64.5 | 69.0 | 65.5 | 0.690 |
| PO2-discharge | 57.0 | 55.5 | 55.0 | 0.908 |
| HCO3-admission | 21.85 (±5.4) | 19.04 (±3.7) | 20.73 (±6.2) | 0.462 |
| HCO3-discharge | 23.72 (±3.8) | 29.38 (±8.6) | 27.08 (±5.4) | 0.378 |
| Surgical interventions | 2 (20.0) | 5 (45.5) | - | 0.217 |
| Admission to the ICU | 2 (16.7) | 5 (45.5) | 8 (80.0) | 0.012 |
| Fibroblasts | Monocytes | ||
|---|---|---|---|
| Up Regulated | Down Regulated | Up Regulated | Down Regulated |
| 618 | 620 | 328 | 140 |
| Fibroblasts | Monocytes | ||
|---|---|---|---|
| Upregulated | Downregulated | Upregulated | Downregulated |
| Cell cycle | IL-2 signalling | IL-6 signalling | |
| Integrin signalling | Th2 signalling | ||
| IL-8 signalling | Protein kinase A signalling | ||
| NFAT signalling on inflammation | IL-3 signalling | ||
| Integrin signalling | |||
| Upregulated | Downregulated |
|---|---|
| Nitric oxide production | Apoptosis |
| Phospholipase C signalling | |
| ILK-mediated signalling | |
| mTOR signalling | |
| Gαq signalling | |
| Rho family GTPase- mediated signaling | |
| ERK/MAPK signalling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendieta-Zerón, H.; Cruz-Arenas, E.; Díaz-Meza, S.; Cabrera-Wrooman, A.; Mandujano-Tinoco, E.A.; Salgado, R.M.; Tovar, H.; Muñiz-García, D.; Orozco-Castañeda, L.J.; Hernández-Enríquez, S.; et al. Pharmacological Immunomodulation via Collagen–Polyvinylpyrrolidone or Pirfenidone Plays a Role in the Recovery of Patients with Severe COVID-19 Through Similar Mechanisms of Action Involving the JAK/STAT Signalling Pathway: A Pilot Study. Adv. Respir. Med. 2025, 93, 24. https://doi.org/10.3390/arm93040024
Mendieta-Zerón H, Cruz-Arenas E, Díaz-Meza S, Cabrera-Wrooman A, Mandujano-Tinoco EA, Salgado RM, Tovar H, Muñiz-García D, Orozco-Castañeda LJ, Hernández-Enríquez S, et al. Pharmacological Immunomodulation via Collagen–Polyvinylpyrrolidone or Pirfenidone Plays a Role in the Recovery of Patients with Severe COVID-19 Through Similar Mechanisms of Action Involving the JAK/STAT Signalling Pathway: A Pilot Study. Advances in Respiratory Medicine. 2025; 93(4):24. https://doi.org/10.3390/arm93040024
Chicago/Turabian StyleMendieta-Zerón, Hugo, Esteban Cruz-Arenas, Salvador Díaz-Meza, Alejandro Cabrera-Wrooman, Edna Ayerim Mandujano-Tinoco, Rosa M. Salgado, Hugo Tovar, Daniel Muñiz-García, Laura Julieta Orozco-Castañeda, Sonia Hernández-Enríquez, and et al. 2025. "Pharmacological Immunomodulation via Collagen–Polyvinylpyrrolidone or Pirfenidone Plays a Role in the Recovery of Patients with Severe COVID-19 Through Similar Mechanisms of Action Involving the JAK/STAT Signalling Pathway: A Pilot Study" Advances in Respiratory Medicine 93, no. 4: 24. https://doi.org/10.3390/arm93040024
APA StyleMendieta-Zerón, H., Cruz-Arenas, E., Díaz-Meza, S., Cabrera-Wrooman, A., Mandujano-Tinoco, E. A., Salgado, R. M., Tovar, H., Muñiz-García, D., Orozco-Castañeda, L. J., Hernández-Enríquez, S., Rodríguez-Piña, M. D., Mulia-Soto, A. S., Meneses-Calderón, J., Mondragón-Terán, P., & Krötzsch, E. (2025). Pharmacological Immunomodulation via Collagen–Polyvinylpyrrolidone or Pirfenidone Plays a Role in the Recovery of Patients with Severe COVID-19 Through Similar Mechanisms of Action Involving the JAK/STAT Signalling Pathway: A Pilot Study. Advances in Respiratory Medicine, 93(4), 24. https://doi.org/10.3390/arm93040024







