Impact of Invasive Mechanical Ventilation on the Lung Microbiome
Abstract
Highlights
- Prolonged invasive mechanical ventilation disrupts lung microbiota, reducing diversity and promoting colonization by gram-negative bacteria and fungi.
- These alterations are closely linked to the development of ventilator-associated pneumonia.
- Microbiota disruption increases the risk of infection, inflammation, and ICU mortality.
- Microbiota profiling may enable early detection of dysbiosis and improve infection control.
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.1.1. Search Sources
2.1.2. Research Question
- -
- Population: Humans with intra- or extrapulmonary pathologies
- -
- Intervention: Invasive mechanical ventilation
- -
- Comparison: Not applicable
- -
- Outcome: Pulmonary microbiota
2.1.3. Search Terms
2.1.4. Eligibility Criteria
2.1.5. Exclusion Criteria
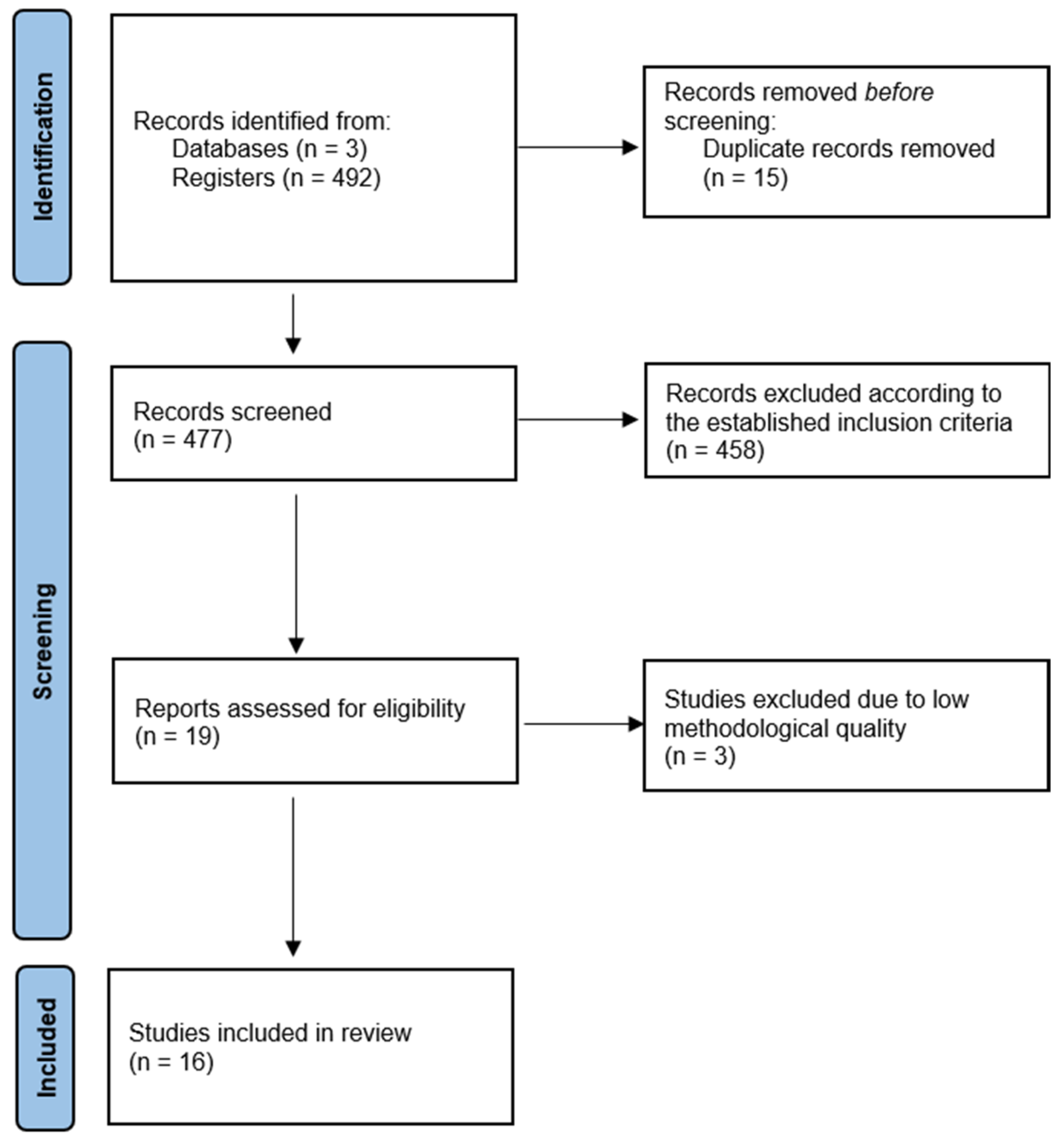
2.2. Study Selection
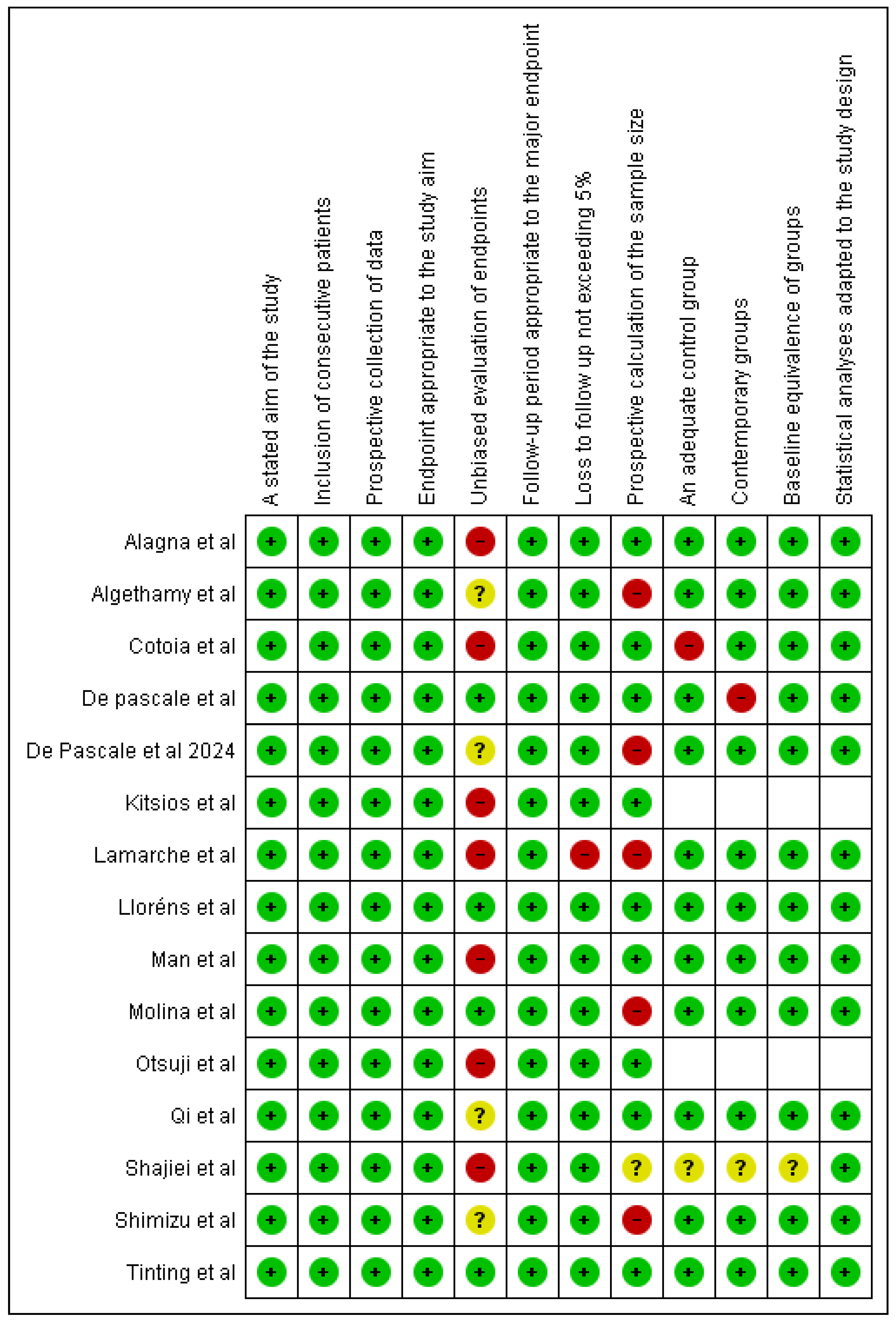
2.3. Quality Assessment
2.4. Data Extraction and Synthesis
3. Results
3.1. Characteristics of the Included Studies
3.2. Methodological Quality
3.3. Demographics of the Included Studies
3.4. Comparison with No Intervention
3.5. Prevalence of Bacterial Families in ICU Patients
3.6. Dynamics of Pulmonary Microbiome Research
3.7. Antibiotics Used
3.8. Microorganisms Detected
3.9. Bacterial Distribution According to Gram Staining
3.9.1. Gram-Positive Bacteria
3.9.2. Gram-Negative Bacteria
3.10. Viruses
3.11. Fungi
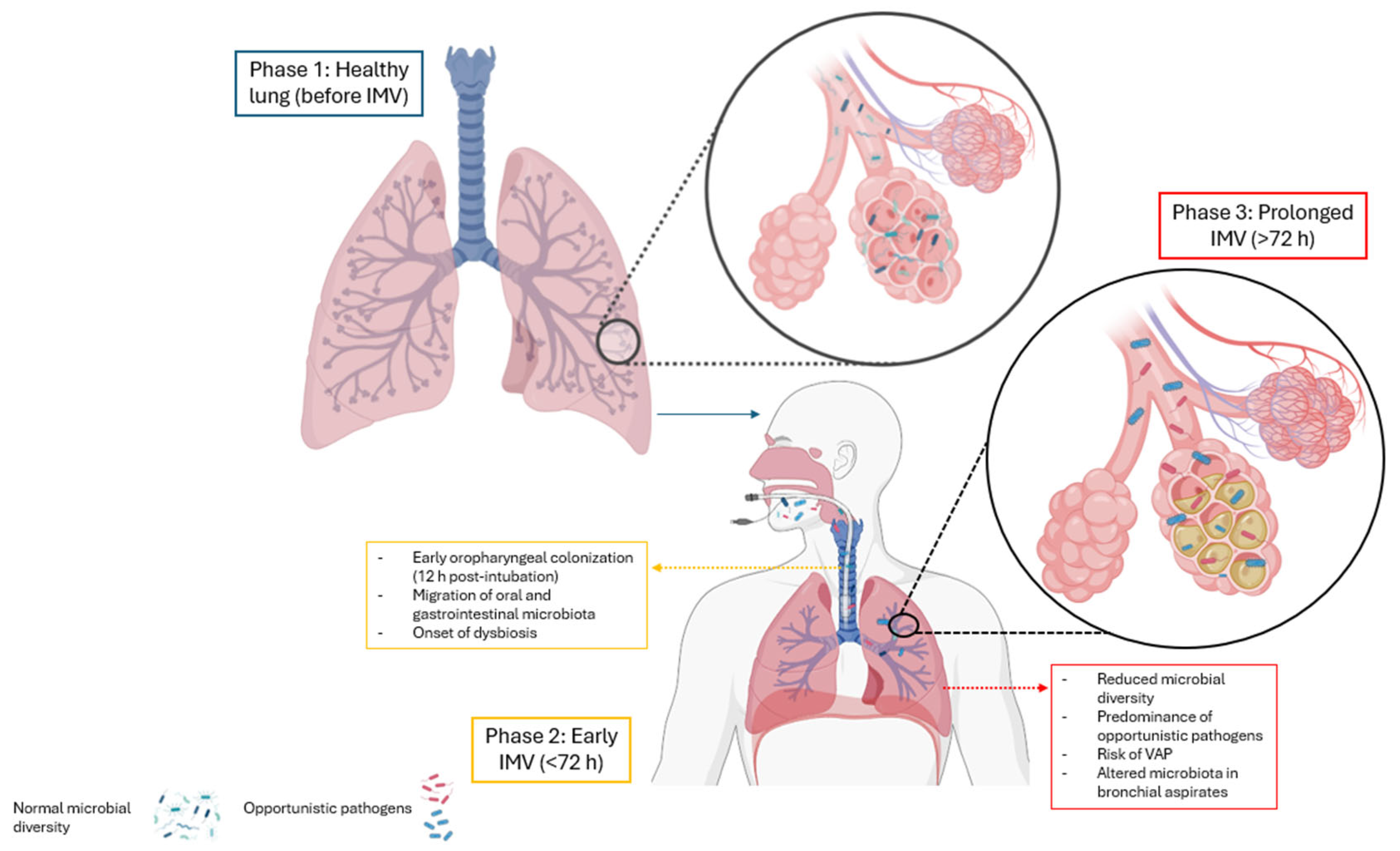
3.12. Impact on the Lung Microbiota
3.13. Effects of Mechanical Ventilation on the Lung Microbiota
4. Discussion
Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verhoeff, K.; Mitchell, J.R. Cardiopulmonary physiology: Why the heart and lungs are inextricably linked. Adv. Physiol. Educ. 2017, 41, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Shah, Z.; Baloch, Z.; Cui, X. The role of microbiota in respiratory health and diseases, particularly in tuberculosis. Biomed. Pharmacother. 2021, 143, 112108. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Tian, Z. Role of microbiota on lung homeostasis and diseases. Sci. China Life Sci. 2017, 60, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Shukla, S.D.; Rehman, S.F.; Bowerman, K.L.; Keely, S.; Hugenholtz, P.; Armstrong-James, D.P.H.; Adcock, I.M.; Chotirmall, S.H.; Chung, K.F.; et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir. Med. 2019, 7, 907–920. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jang, Y.-S. Recent Insights into Cellular Crosstalk in Respiratory and Gastrointestinal Mucosal Immune Systems. Immune Netw. 2020, 20, e44. [Google Scholar] [CrossRef]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Wiley, N.C.; Dinan, T.G.; Ross, R.P.; Stanton, C.; Clarke, G.; Cryan, J.F. The microbiota-gut-brain axis as a key regulator of neural function and the stress response: Implications for human and animal health. J. Anim. Sci. 2017, 95, 3225–3246. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, F.; Wang, Y.; Ji, J.; Xu, Y.; Huang, Y.; Zhang, M.; Li, M.; Xia, J.; Wang, B. Methodological comparison of bronchoalveolar lavage fluid-based detection of respiratory pathogens in diagnosis of bacterium/fungus-associated pneumonia in critically ill patients. Front. Public Health 2023, 11, 1168812. [Google Scholar] [CrossRef]
- Montassier, E.; Kitsios, G.D.; Radder, J.E.; Le Bastard, Q.; Kelly, B.J.; Panzer, A.; Lynch, S.V.; Calfee, C.S.; Dickson, R.P.; Roquilly, A. Robust airway microbiome signatures in acute respiratory failure and hospital-acquired pneumonia. Nat. Med. 2023, 29, 2793–2804. [Google Scholar] [CrossRef]
- Liu, C.; Wu, K.; Sun, T.; Chen, B.; Yi, Y.; Ren, R.; Xie, L.; Xiao, K. Effect of invasive mechanical ventilation on the diversity of the pulmonary microbiota. Crit. Care 2022, 26, 252. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Brodie, E.L.; Weng, L.; Lynch, S.V.; Garcia, O.; Brown, R.; Hugenholtz, P.; DeSantis, T.Z.; Andersen, G.L.; Wiener-Kronish, J.P.; et al. Loss of bacterial diversity during antibiotic treatment of intubated patients colonized with Pseudomonas aeruginosa. J. Clin. Microbiol. 2007, 45, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, M.; Ricard, J.-D.; Roux, D. Respiratory microbiome in mechanically ventilated patients: A narrative review. Intensive Care Med. 2021, 47, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, M.; Ricard, J.-D.; Roux, D. Lung Microbiome in Critically Ill Patients. Life 2021, 12, 7. [Google Scholar] [CrossRef]
- Emonet, S.; Lazarevic, V.; Refondini, C.L.; Gaïa, N.; Leo, S.; Girard, M.; Boyer, V.N.; Wozniak, H.; Després, L.; Renzi, G.; et al. Identification of respiratory microbiota markers in ventilator-associated pneumonia. Intensive Care Med. 2019, 45, 1082–1092. [Google Scholar] [CrossRef]
- Zakharkina, T.; Martin-Loeches, I.; Matamoros, S.; Povoa, P.; Torres, A.; Kastelijn, J.B.; Hofstra, J.J.; de Wever, B.; de Jong, M.; Schultz, M.J.; et al. The dynamics of the pulmonary microbiome during mechanical ventilation in the intensive care unit and the association with occurrence of pneumonia. Thorax 2017, 72, 803–810. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Lamarche, D.; Johnstone, J.; Zytaruk, N.; Clarke, F.; Hand, L.; Loukov, D.; Szamosi, J.C.; Rossi, L.; Schenck, L.P.; Verschoor, C.P.; et al. Microbial dysbiosis and mortality during mechanical ventilation: A prospective observational study. Respir. Res. 2018, 19, 245. [Google Scholar] [CrossRef]
- Man, W.H.; A van Houten, M.; E Mérelle, M.; Vlieger, A.M.; Chu, M.L.J.N.; Jansen, N.J.G.; Sanders, E.A.M.; Bogaert, D. Bacterial and viral respiratory tract microbiota and host characteristics in children with lower respiratory tract infections: A matched case-control study. Lancet Respir. Med. 2019, 7, 417–426. [Google Scholar] [CrossRef]
- Alagna, L.; Mancabelli, L.; Magni, F.; Chatenoud, L.; Bassi, G.; Del Bianco, S.; Fumagalli, R.; Turroni, F.; Mangioni, D.; Migliorino, G.M.; et al. Changes in upper airways microbiota in ventilator-associated pneumonia. Intensive Care Med. Exp. 2023, 11, 17. [Google Scholar] [CrossRef]
- Kitsios, G.D.; Yang, H.; Yang, L.; Qin, S.; Fitch, A.; Wang, X.-H.; Fair, K.; Evankovich, J.; Bain, W.; Shah, F.; et al. Respiratory Tract Dysbiosis Is Associated with Worse Outcomes in Mechanically Ventilated Patients. Am. J. Respir. Crit. Care Med. 2020, 202, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Otsuji, K.; Fukuda, K.; Ogawa, M.; Fujino, Y.; Kamochi, M.; Saito, M. Dynamics of microbiota during mechanical ventilation in aspiration pneumonia. BMC Pulm. Med. 2019, 19, 260. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qu, H.; Yang, D.; Zhou, L.; He, Y.-W.; Yu, Y.; Qu, J.; Liu, J. Lower respiratory tract microbial composition was diversified in Pseudomonas aeruginosa ventilator-associated pneumonia patients. Respir. Res. 2018, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Cotoia, A.; Paradiso, R.; Ferrara, G.; Borriello, G.; Santoro, F.; Spina, I.; Mirabella, L.; Mariano, K.; Fusco, G.; Cinnella, G.; et al. Modifications of lung microbiota structure in traumatic brain injury ventilated patients according to time and enteral feeding formulas: A prospective randomized study. Crit. Care 2023, 27, 244. [Google Scholar] [CrossRef]
- De Pascale, G.; De Maio, F.; Carelli, S.; De Angelis, G.; Cacaci, M.; Montini, L.; Bello, G.; Cutuli, S.L.; Pintaudi, G.; Tanzarella, E.S.; et al. Staphylococcus aureus ventilator-associated pneumonia in patients with COVID-19: Clinical features and potential inference with lung dysbiosis. Crit. Care 2021, 25, 197. [Google Scholar] [CrossRef]
- Woo, S.; Park, S.-Y.; Kim, Y.; Jeon, J.P.; Lee, J.J.; Hong, J.Y. The Dynamics of Respiratory Microbiota during Mechanical Ventilation in Patients with Pneumonia. J. Clin. Med. 2020, 9, 638. [Google Scholar] [CrossRef]
- Shajiei, A.; Liu, L.; Seinen, J.; Dieperink, W.; Hammerschmidt, S.; van Dijl, J.M.; Harmsen, H.J. Specific associations between fungi and bacteria in broncho-alveolar aspirates from mechanically ventilated intensive care unit patients. Virulence 2022, 13, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Lloréns-Rico, V.; Gregory, A.C.; Van Weyenbergh, J.; Jansen, S.; Van Buyten, T.; Qian, J.; Braz, M.; Menezes, S.M.; Van Mol, P.; Vanderbeke, L.; et al. Clinical practices underlie COVID-19 patient respiratory microbiome composition and its interactions with the host. Nat. Commun. 2021, 12, 6243. [Google Scholar] [CrossRef]
- Xiao, T.; Guo, Q.; Zhou, Y.; Shen, P.; Wang, Y.; Fang, Q.; Li, M.; Zhang, S.; Guo, L.; Yu, X.; et al. Comparative Respiratory Tract Microbiome Between Carbapenem-Resistant Acinetobacter baumannii Colonization and Ventilator Associated Pneumonia. Front. Microbiol. 2022, 13, 782210. [Google Scholar] [CrossRef]
- Algethamy, H.; Bokhary, D.H.; Abushoshah, I.; Alalyani, A.A.; Baamer, M.K.; Attallah, D.M.; Alotaibi, R.M.; Amin, S.N.; Mass, S.A.; Tashkandy, N.R. Distinct relative abundances in pathogens detected in mechanically ventilated patients with suspected pneumonia in the intensive care unit at King Abdulaziz University Hospital. Sci. Rep. 2025, 15, 3291. [Google Scholar] [CrossRef]
- Shimizu, K.; Hirata, H.; Tokuhira, N.; Motooka, D.; Nakamura, S.; Ueda, A.; Tachino, J.; Koide, M.; Uchiyama, A.; Ogura, H.; et al. Dysbiosis of gut microbiota in patients with severe COVID-19. Acute Med. Surg. 2024, 11, e923. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Posteraro, B.; De Maio, F.; Pafundi, P.C.; Tanzarella, E.S.; Cutuli, S.L.; Lombardi, G.; Grieco, D.L.; Franchini, E.; Santarelli, G.; et al. Lung microbiota composition, respiratory mechanics, and outcomes in COVID-19-related ARDS. Microbiol. Spectr. 2024, 12, e0357423. [Google Scholar] [CrossRef] [PubMed]
- Molina, F.J.; Botero, L.E.; Isaza, J.P.; Cano, L.E.; López, L.; Valdés, L.; Arbeláez, A.J.A.; Moreno, I.; Restrepo, L.S.P.; Usuga, J.; et al. Deciphering the lung microbiota in COVID-19 patients: Insights from culture analysis, FilmArray pneumonia panel, ventilation impact, and mortality trends. Sci. Rep. 2024, 14, 30035. [Google Scholar] [CrossRef] [PubMed]
- Xue-Meng, C.; Gao-Wang, L.; Xiao-Mei, L.; Fan-Fang, Z.; Jin-Fang, X. Effect of mechanical ventilation under intubation on respiratory tract change of bacterial count and alteration of bacterial flora. Exp. Lung Res. 2023, 49, 165–177. [Google Scholar] [CrossRef]
- Alibeik, N.; Pishgar, E.; Bozorgmehr, R.; Aghaaliakbari, F.; Rahimian, N. Potential role of gut microbiota in patients with COVID-19, its relationship with lung axis, central nervous system (CNS) axis, and improvement with probiotic therapy. Iran. J. Microbiol. 2022, 14, 1–9. [Google Scholar] [CrossRef]
| Base Data | Search Date | Search Equation | Articles Found |
|---|---|---|---|
| PubMed | 3 October 2025 | ((((humans) AND (artificial respiration)) OR (mechanical ventilation)) AND (microbiota)) OR (dysbiosis) | 183 |
| SCOPUS | 3 October 2025 | ((((humans) AND (artificial respiration)) OR (mechanical ventilation)) AND (microbiota)) | 169 |
| Web Of Science | 3 December 2025 | ((((humans) AND (artificial respiration)) OR (mechanical ventilation)) AND (microbiota)) | 140 |
| Author/Year | Methodology | Population | Pathology upon ICU Admission | Mortality | Microorganisms Detected |
|---|---|---|---|---|---|
| Lamarche et al. (2018) [18] | Prospective and observational study of critically ill patients with invasive ventilatory support showing a high incidence of pneumonia in two ICUs in Hamilton, Canada. | Cohort of cases: 34 patients (14 women and 20 men). Mean age 66.6 years. Control group: 35 healthy patients. | Medical, surgical, or trauma conditions | ICU: 14.7% HOSPITAL: 35.3%. | Enterococcus, Pseudomonas, and Staphylococcus |
| Man et al. (2019) [19] | Prospective matched case–control study of hospitalized children. | Cohort of patients in the PICU: 29 patients. Cases: 154 patients (61 women and 93 men). Average age 13.6 months. Controls: 307 patients (122 women and 185 men). Average age 14.1 months. | Lower respiratory tract infections | No deaths reported. | Viruses: rhinovirus, coronavirus, respiratory syncytial virus, adenovirus. Bacteria: PICU cohort: H. influenzae/H. haemolyticus, S. pneumoniae, Actinomyces spp., and Prevotella spp. Controls: Moraxella spp, C. propinquum, D. pigrum, and Helcococcus spp. |
| Alagna et al. (2023) [20] | Exploratory analysis of data from a prospective observational study in intubated patients. | Cohort of cases: 13 patients with VAP (4 women and 9 men). Average age: 40 years. Control cohort: 22 patients with NO-VAP (9 women and 13 men). Average age: 62.5 years. | Nonpulmonary pathologies | No deaths reported. | Streptococcus, Prevotella, Actinomyces |
| Kitsios et al. (2020) [21] | Cross-sectional study from March 2015 to December 2018. | 301 patients with invasive mechanical ventilation (156 men and 145 women). Average age is 59 years. | Severe respiratory insufficiency | No deaths reported. | Streptococcus, Staphylococcus, Pseudomonadaceae, Stenotrophomonas, Prevotella, and Veillonella |
| Outsuji et al. (2019) [22] | Prospective study conducted at the hospital of Japan University of Occupational and Environmental Health between March 2016 and March 2018. | 22 patients with invasive ventilatory support (13 men and 9 women). | Aspiration pneumonia | 13.6%. | Streptococcus agalactiae, Enterobacter aerogene, Klebsiella pneumoniae |
| Qi et al. (2018) [23] | Prospective study of patients hospitalized in an ICU of Ruijin Hospital, China. | Cases: 36 patients with ventilator-associated pneumonia due to P. aeruginosa (15 women and 21 men). Average age 68.50 years. Control: 18 healthy subjects (12 women and 6 men). The average age is 57.78 years. | Ventilator-associated pneumonia due to P. aeruginosa | 29.6% | COHORT OF CASES: 1. Pro Group: Proteobacteria, Pseudomonas, Citrobacter, Enterobacter, Klebsiella, Enterococcus, and Acinetobacter. 2. Fir-Bac Group: Firmicutes, Bacteroidetes, Lachnospiraceae, Bacteroides, Blautia, and Alloprevotella. HEALTHY COHORT: Proteobacteria, Firmicutes, Bacteroidetes, Prevotella, Streptococcus, and Alloprevotella |
| Cotoia et al. (2023) [24] | Prospective randomized study between February 2021 and March 2022 in the Intensive Care Department of the University Hospital of Foggia, Italy. | 31 patients divided into 2 groups: 15 enteral nutrition (11 men and 4 women). Average age: 56 years. 16 specialized nutrition (13 men and 3 women). Average age: 51 years. | Head trauma | At 28 days Enteral nutrition: 45%. Specialized nutrition: 25%. | Staphylococcus and Acinetobacter |
| De Pascale et al. (2021) [25] | Prospective observational study of patients hospitalized in two medical ICUs of Fondazione Policlínico Universitario A. Gemelli IRCCS (Rome, Italy), who developed SA-VAP between 20 March 2020, and 30 October 2020. | Total cohort: 120 patients Cases: 40 patients with COVID-19 (33 men and 7 women). Average age: 64 years Controls: 80 patients (59 men and 21 women). Average age: 62 years. | COVID-19 | ICU: 25.0%. HOSPITAL: 26.7%. | S. aureus, Streptococcus anginosus, and Olsenella |
| Woo et al. (2020) [26] | Prospective study conducted in the ICU of the Sacred Heart Hospital of Chuncheon, South Korea. | Cases: 41 patients with pneumonia divided into 2 groups: Successful extubation: 22 patients (16 men and 6 women). Average age: 72 years. Failed extubation: 19 patients (12 men and 7 women). Average age: 76 years. Control group: 19 patients without pneumonia (12 men and 7 women). Average age: 76 years. | Pneumonia | Failed extubation: 63.2% Control group: 31.6% | Pseudomonas, Corynebacterium, Streptoccocus, Prevotella, and Alloprevotella |
| Shajiei et al. (2022) [27] | Pilot study between February and August 2015 of patients with mechanical ventilation was admitted to the UMCG Critical Care department. | 53 patients: (32 men and 23 women). Average age: 58 years. | Neurological, respiratory, medical, cardiological, gastroenterological conditions | 26.4% | Candida |
| Lloréns-Rico et al. (2021) [28] | Observational clinical trial. | 58 patients whose upper respiratory tract microbiota was evaluated (13 women and 45 men). Mean age: 61 years. 35 patients whose lower respiratory tract microbiota was evaluated (12 women and 23 men). Mean age: 64 years. | COVID-19 | No deaths reported. | Staphylococcus and Corynebacterium |
| Tingting et al. (2022) [29] | Prospective study in patients with mechanical ventilation from July 2018 to December 2019 in a tertiary hospital in adult ICUs. | 52 patients: 24 with carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia (CRAB-I) (16 men and 8 women). Mean age: 60.7 years. 22 with carbapenem-resistant Acinetobacter baumannii (CRAB-C) (14 men and 8 women). Mean age: 58.5 years. 6 without infection (CRAB-N) (4 men and 2 women). Mean age: 51.8 years. | Ventilator-associated pneumonia | CRAB-I: 33.3% CRAB-C: 4.5% | Acinetobacter baumannii |
| Algethamy et al. (2025) [30] | Prospective observational study in 83 ICU with invasive mechanical ventilation. | 83 patients G1: VP (Pneumonia-Positive Group): 51 patients who developed VAP. G2:VN (Pneumonia-Negative Group): 32 patients who did not develop pneumonia. | Pneumonia | 51 pneumonia-positive (VP) patients: 28 deaths (54.9%) 32 pneumonia-negative (VN) patients: 12 deaths (37.5%) | Klebsiella pneumoniae Stenotrophomonas maltophilia Haemophilus influenzae Pseudomonas aeruginosaStaphylococcus aureus Acinetobacter baumannii |
| Shimizu K et al. (2024) [31] | Retrospective, observational cohort study 76 adult ICU with invasive mechanical ventilation | Sepsis Group: 40 patients Control Group: 36 patients | Sepsis (Pulmonary origin of infection) | Sepsis Group (n = 40): Mortality: 18 patients (45%) Control Group (n = 36): Mortality: 7 patients (19.4%) | Enterobacteriaceae (e.g., Escherichia coli, Klebsiella spp.) Streptococcus spp. Staphylococcus aureus Pseudomonas aeruginosa Acinetobacter spp. |
| De Pascale et al. (2024) [32] | Prospective observational study with 70 C-ARDS patients requiring IMV | 70 mechanically ventilated COVID-19 ARDS patients admitted to the ICU | C-ARDS with IVM | ICU Mortality: 48.6% (34 out of 70 patients). | Acinetobacter, Staphylococcus, Lactobacillus, Klebsiella, Pseudomonas Paenibacillus |
| Molina FJ et al. (2024) [33] | Retrospective Study 67 COVID-19 ICU Patient. | 67 COVID-19 patients had severe pneumonia and were invasive mechanical ventilation. | COVID-19 | ICU mortality 32.4% (22 patients) | Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estela-Zape, J.L.; Sanclemente-Cardoza, V.; Espinosa-Cifuentes, M.A.; Ordoñez-Mora, L.T. Impact of Invasive Mechanical Ventilation on the Lung Microbiome. Adv. Respir. Med. 2025, 93, 23. https://doi.org/10.3390/arm93040023
Estela-Zape JL, Sanclemente-Cardoza V, Espinosa-Cifuentes MA, Ordoñez-Mora LT. Impact of Invasive Mechanical Ventilation on the Lung Microbiome. Advances in Respiratory Medicine. 2025; 93(4):23. https://doi.org/10.3390/arm93040023
Chicago/Turabian StyleEstela-Zape, Jose Luis, Valeria Sanclemente-Cardoza, Maria Alejandra Espinosa-Cifuentes, and Leidy Tatiana Ordoñez-Mora. 2025. "Impact of Invasive Mechanical Ventilation on the Lung Microbiome" Advances in Respiratory Medicine 93, no. 4: 23. https://doi.org/10.3390/arm93040023
APA StyleEstela-Zape, J. L., Sanclemente-Cardoza, V., Espinosa-Cifuentes, M. A., & Ordoñez-Mora, L. T. (2025). Impact of Invasive Mechanical Ventilation on the Lung Microbiome. Advances in Respiratory Medicine, 93(4), 23. https://doi.org/10.3390/arm93040023






