Imaging and Laboratory Results as Predictors of the Course of COVID-19
Abstract
Highlights
- Inflammatory changes involving more than 50% of the lung parenchyma on chest CT scans proved to be the best predictor of severe COVID-19 disease.
- The results of imaging and laboratory tests are useful in predicting the need for non-invasive ventilation support.
- Chest CT and laboratory tests should be performed upon hospital admission in patients with COVID-19
- Despite the decreased incidence of COVID-19 following the pandemic, early identification of patients at risk for severe infection remains essential.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Tests
2.3. Computed Tomography
2.4. Patients
2.5. Oxygen Supplementation
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths of the Study
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease 2019 |
| NETs | Neutrophil extracellular traps |
| HFNOT | High-flow nasal oxygen therapy |
| CPAP | Continuous positive airway pressure |
| BPAP | Bilevel positive airway pressure |
| NIRS | Non-invasive respiratory support |
| IMV | Invasive mechanical ventilation |
| PCR | Real time polymerase chain reaction test |
| CT | Computed tomography |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| LDH | Lactate dehydrogenase |
| CRP | C-reactive protein |
| PCT | Procalcitonin |
| IL-6 | Interleukin-6 |
| CBC | Complete blood count |
| HRCT | High resolution computed tomography |
| ICU | Intensive Care Unit |
| NS | Not significant |
| WBC | White blood cells |
| NEUT | Neutrophils |
| LYMPH | Lymphocytes |
| NIV | Non-invasive ventilation |
References
- Ghebreyesus, T.A. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 11 March 2020).
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Doudin, A.; Sulaiman, T.O.; Jamil, O.; Arif, R.; Sada, F.A.; Yassine, H.M.; Elrayess, M.A.; Elzouki, A.N.; Emara, M.M.; et al. Metabolic predictors of COVID-19 mortality and severity: A survival analysis. Front. Immunol. 2024, 15, 1353903. [Google Scholar] [CrossRef]
- Robinson, J.I.; Marks, L.R.; Hinton, A.L.; O’Halloran, J.A.; Goss, C.W.; Mucha, P.J.; Henderson, J.P. Development of a metabolome-based respiratory infection prognostic during COVID-19 arrival. mBio 2025, 16, e0334323. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, T.; Yang, J.; Li, X.; Xuan, P.; Wang, H. Predictive Value of dsDNA and Nucleosomes as Neutrophil Extracellular Traps-Related Biomarkers for COVID-19 in Older Patients. J. Inflamm. Res. 2024, 17, 8831–8838. [Google Scholar] [CrossRef] [PubMed]
- Zali, M.; Sadat Larijani, M.; Bavand, A.; Moradi, L.; Ashrafian, F.; Ramezani, A. Circulatory microRNAs as potential biomarkers for different aspects of COVID-19. Arch. Virol. 2024, 170, 8. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, P.; Abate, A.C.; Izzo, C.; Toni, A.L.; Rusciano, M.R.; Folliero, V.; Dell’Annunziata, F.; Granata, G.; Visco, V.; Motta, B.M.; et al. Plasma miR-1-3p levels predict severity in hospitalized COVID-19 patients. Br. J. Pharmacol. 2025, 182, 451–467. [Google Scholar] [CrossRef]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.M.O.T.S.C.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef]
- Czajkowska-Malinowska, M.; Kania, A.; Kuca, P.J.; Nasiłowski, J.; Skoczyński, S.; Sokołowski, R.; Śliwiński, P.S. Treatment of acute respiratory failure in the course of COVID-19. Practical hints from the expert panel of the Assembly of Intensive Care and Rehabilitation of the Polish Respiratory Society. Adv. Respir. Med. 2020, 88, 245–266. [Google Scholar] [CrossRef]
- Ferreyro, B.L.; Angriman, F.; Munshi, L.; Del Sorbo, L.; Ferguson, N.D.; Rochwerg, B.; Ryu, M.J.; Saskin, R.; Wunsch, H.; da Costa, B.R.; et al. Association of Noninvasive Oxygenation Strategies With All-Cause Mortality in Adults With Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-analysis. JAMA 2020, 324, 57–67. [Google Scholar] [CrossRef]
- Aretha, D.; Kefala, S.; Nikolopoulou, A.; Karamouzos, V.; Valta, M.; Mplani, V.; Georgakopoulou, A.; Papamichail, C.; Sklavou, C.; Fligou, F. Intubation Time, Lung Mechanics and Outcome in COVID-19 Patients Suffering Acute Respiratory Distress Syndrome: A Single-Center Study. J. Clin. Med. Res. 2024, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Koh, Y.; Lim, C.M.; Huh, J.W.; Baek, S.; Han, M.; Seo, H.S.; Suh, H.J.; Seo, G.J.; Kim, E.Y.; et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015, 41, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef]
- Kangelaris, K.N.; Ware, L.B.; Wang, C.Y.; Janz, D.R.; Zhuo, H.; Matthay, M.A.; Calfee, C.S. Timing of Intubation and Clinical Outcomes in Adults With Acute Respiratory Distress Syndrome. Crit. Care Med. 2016, 44, 120–129. [Google Scholar] [CrossRef]
- Weerakkody, S.; Arina, P.; Glenister, J.; Cottrell, S.; Boscaini-Gilroy, G.; Singer, M.; Montgomery, H.E. Non-invasive respiratory support in the management of acute COVID-19 pneumonia: Considerations for clinical practice and priorities for research. Lancet Respir. Med. 2022, 10, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Baridah, I.; Setyowireni, D.K.; Citta, A.N.; Arguni, E. The severity of pediatric COVID-19 during hospitalization is not associated with mortality within six months of discharge. BMC Pediatr. 2025, 25, 199. [Google Scholar] [CrossRef]
- Novelli, L.; Raimondi, F.; Carioli, G.; Carobbio, A.; Pappacena, S.; Biza, R.; Trapasso, R.; Anelli, M.; Amoroso, M.; Allegri, C.; et al. One-year mortality in COVID-19 is associated with patients’ comorbidities rather than pneumonia severity. Respir. Med. Res. 2023, 83, 100976. [Google Scholar] [CrossRef]
- Kusza, K.; Kuebler, A.; Maciejewski, D.; Mikstacki, A.; Owczuk, R.; Wujtewicz, M.; Piechota, M. Guidelines of the Polish Society of Anaesthesiology and Intensive Therapy determining principles, conditions and organisational aspects of anaesthesiology and intensive therapy services. Anaesthesiol. Intensive Ther. 2012, 44, 201–212. [Google Scholar]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Yuan, M.; Yin, W.; Tao, Z.; Tan, W.; Hu, Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE 2020, 15, e0230548. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, A.; Nikolic, S.; Elek, Z.; Aritonovic Pribakovic, J.; Ilic, A.; Bulatovic, K.; Gasic, M.; Jaksic, B.; Stojanovic, M.; Miljkovic Jaksic, D.; et al. Significance of Initial Chest CT Severity Score (CTSS) and Patient Characteristics in Predicting Outcomes in Hospitalized COVID-19 Patients: A Single Center Study. Viruses 2024, 16, 1683. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.F.; Azevedo, E.; Paes, A.T.; Lima, E.; Campos Guerra, J.C.; Ingham, S. Chronic diseases, chest computed tomography, and laboratory tests as predictors of severe respiratory failure and death in elderly Brazilian patients hospitalized with COVID-19: A prospective cohort study. BMC Geriatr. 2022, 22, 132. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, Y.; Zhang, J.; Li, J.; Li, W.; Guo, K.; Wang, Y.; Chen, J.; Yan, W.; Wang, B.; et al. Early predictors of severe COVID-19 among hospitalized patients. J. Clin. Lab. Anal. 2022, 36, e24177. [Google Scholar] [CrossRef]
- Li, L.; Sun, W.; Han, M.; Ying, Y.; Wang, Q. A Study on the Predictors of Disease Severity of COVID-19. Med. Sci. Monit. 2020, 26, e927167. [Google Scholar] [CrossRef] [PubMed]
- Saeed, G.A.; Gaba, W.; Shah, A.; Al Helali, A.A.; Raidullah, E.; Al Ali, A.B.; Elghazali, M.; Ahmed, D.Y.; Al Kaabi, S.G.; Almazrouei, S. Correlation between Chest CT Severity Scores and the Clinical Parameters of Adult Patients with COVID-19 Pneumonia. Radiol. Res. Pract. 2021, 2021, 6697677. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef]
- Paciorek, M.; Bieńkowski, C.; Kowalska, J.D.; Skrzat-Klapaczyńska, A.; Bednarska, A.; Krogulec, D.; Cholewińska, G.; Kowalski, J.; Podlasin, R.; Ropelewska-Łącka, K.; et al. Hospital Admission Factors Independently Affecting the Risk of Mortality of COVID-19 Patients. J. Clin. Med. 2023, 12, 6264. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Calle-Peña, S.T.; Diaz Tavara, E.D.; Aguirre-Milachay, E.; León-Figueroa, D.A.; Valladares-Garrido, M.J. Predictors of high-flow nasal cannula failure in COVID-19 patients in a northern Peruvian hospital. BMC Pulm. Med. 2024, 24, 414. [Google Scholar] [CrossRef]
- Liu, L.; Xie, J.; Wu, W.; Chen, H.; Li, S.; He, H.; Yu, Y.; Hu, M.; Li, J.; Zheng, R.; et al. A simple nomogram for predicting failure of non-invasive respiratory strategies in adults with COVID-19: A retrospective multicentre study. Lancet Digit. Health 2021, 3, e166–e174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Feng, X.; Jiang, C.; Mi, S.; Yang, L.; Zhao, Z.; Zhang, Y.; Zhang, L. Correlation between white blood cell count at admission and mortality in COVID-19 patients: A retrospective study. BMC Infect. Dis. 2021, 21, 574. [Google Scholar] [CrossRef]
- He, R.; Lu, Z.; Zhang, L.; Fan, T.; Xiong, R.; Shen, X.; Feng, H.; Meng, H.; Lin, W.; Jiang, W.; et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J. Clin. Virol. 2020, 127, 104361. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, X.; Chen, X.; Liu, C. Early decrease in blood lymphocyte count is associated with poor prognosis in COVID-19 patients: A retrospective cohort study. BMC Pulm. Med. 2023, 23, 453. [Google Scholar] [CrossRef]
- Palladino, M. Complete blood count alterations in COVID-19 patients: A narrative review. Biochem. Med. 2021, 31, 030501. [Google Scholar] [CrossRef]
- Reyes, L.F.; Murthy, S.; Garcia-Gallo, E.; Merson, L.; Ibáñez-Prada, E.D.; Rello, J.; Fuentes, Y.V.; Martin-Loeches, I.; Bozza, F.; Duque, S.; et al. Respiratory support in patients with severe COVID-19 in the International Severe Acute Respiratory and Emerging Infection (ISARIC) COVID-19 study: A prospective, multinational, observational study. Crit. Care 2022, 26, 276. [Google Scholar] [CrossRef] [PubMed]
- Varpaei, H.A.; Bayraktar, N.; Mohammadi, M. Predictors of Non-invasive Ventilation Failure and Associated Factors Among the COVID-19 Patients Admitted to Intensive Care Unit. Anesth. Pain. Med. 2023, 13, e140847. [Google Scholar] [CrossRef]
- Suardi, L.R.; Pallotto, C.; Esperti, S.; Tazzioli, E.; Baragli, F.; Salomoni, E.; Botta, A.; Covani Frigieri, F.; Pazzi, M.; Stera, C.; et al. Risk factors for non-invasive/invasive ventilatory support in patients with COVID-19 pneumonia: A retrospective study within a multidisciplinary approach. Int. J. Infect. Dis. 2020, 100, 258–263. [Google Scholar] [CrossRef]
- Dyrbuś, M.; Oraczewska, A.; Szmigiel, S.; Gawęda, S.; Kluszczyk, P.; Cyzowski, T.; Jędrzejek, M.; Dubik, P.; Kozłowski, M.; Kwiatek, S.; et al. Mallampati Score Is an Independent Predictor of Active Oxygen Therapy in Patients with COVID-19. J. Clin. Med. 2022, 11, 2958. [Google Scholar] [CrossRef]
- Singh, J.; Malik, P.; Patel, N.; Pothuru, S.; Israni, A.; Chakinala, R.C.; Hussain, M.R.; Chidharla, A.; Patel, H.; Patel, S.K.; et al. Kidney disease and COVID-19 disease severity-systematic review and meta-analysis. Clin. Exp. Med. 2022, 22, 125–135. [Google Scholar] [CrossRef]
- Gendrel, D.; Bohuon, C. Procalcitonin as a marker of bacterial infection. Pediatr. Infect. Dis. J. 2000, 19, 679–687; quiz 688. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Jafri, L.; Hoodbhoy, Z.; Siddiqui, I. Prognostic Value of Serum Procalcitonin in COVID-19 Patients: A Systematic Review. Indian. J. Crit. Care Med. 2021, 25, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Karn, E.; Trivedi, K.; Kumar, P.; Chauhan, G.; Kumari, A.; Pant, P.; Munisamy, M.; Prakash, J.; Sarkar, P.G.; et al. Procalcitonin as a predictive marker in COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272840. [Google Scholar] [CrossRef] [PubMed]
- Won, J.H.; Hong, Y.; Kim, S.; Lee, H. One-year post-acute COVID-19 syndrome and mortality in South Korea: A nationwide matched cohort study using claims data. Front. Public Health 2024, 12, 1403153. [Google Scholar] [CrossRef]
- Lomba, G.S.B.; Silva, P.; Rosário, N.F.D.; Medeiros, T.; Alves, L.S.; Silva, A.A.; Almeida, J.R.; Lugon, J.R. Post-discharge all-cause mortality in COVID-19 recovered patients hospitalized in 2020: The impact of chronic kidney disease. Rev. Inst. Med. Trop. Sao Paulo 2024, 66, e1. [Google Scholar] [CrossRef] [PubMed]
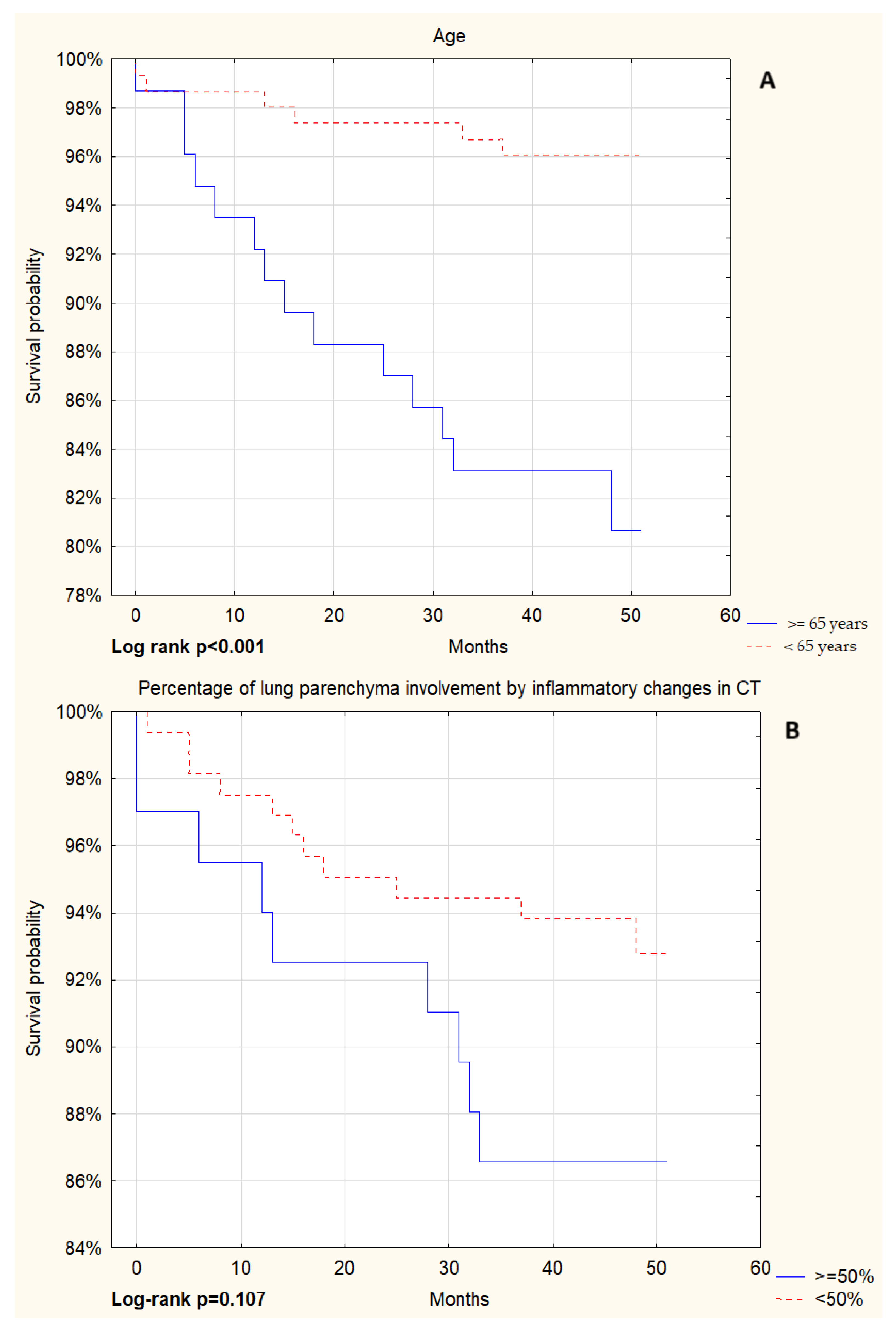
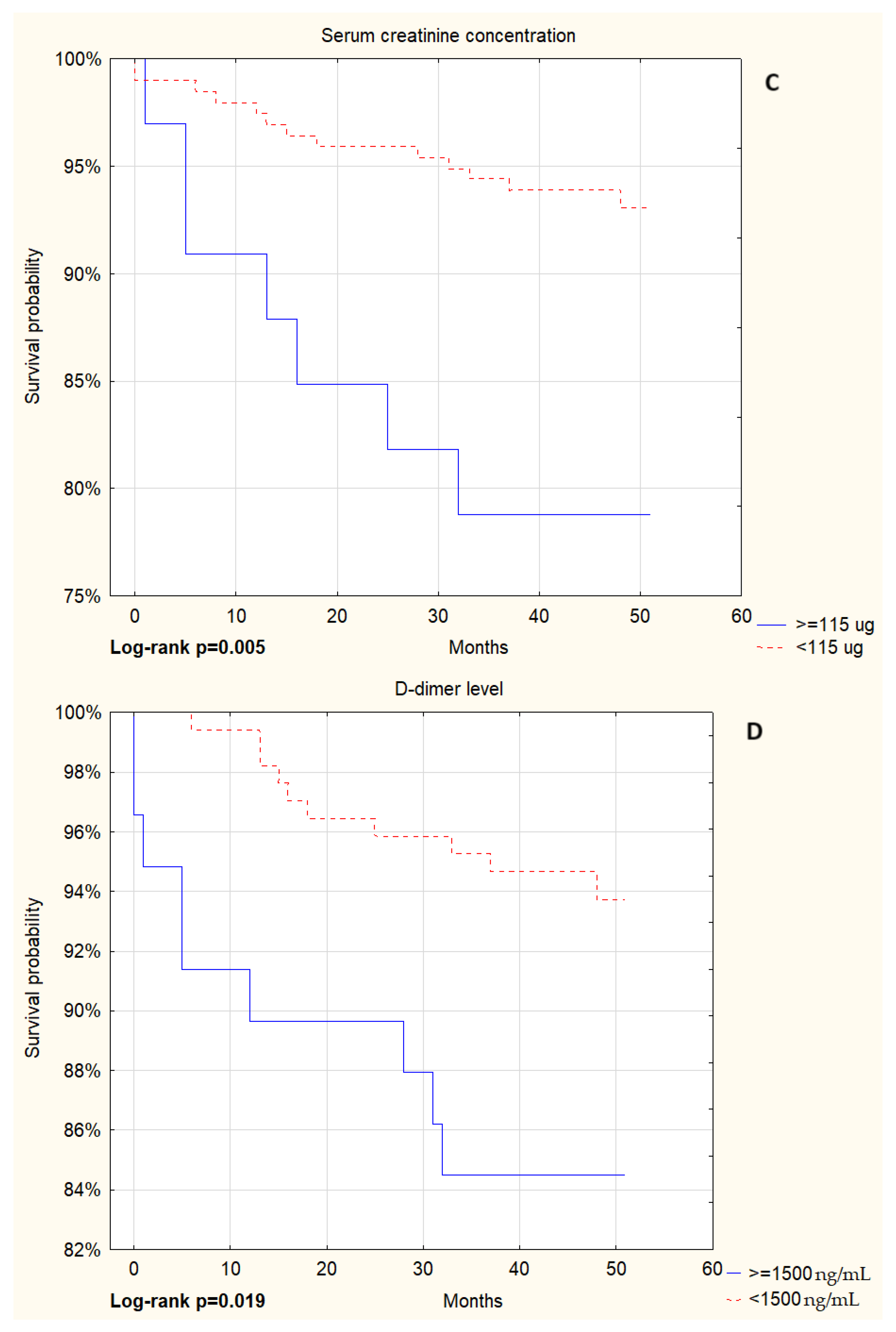
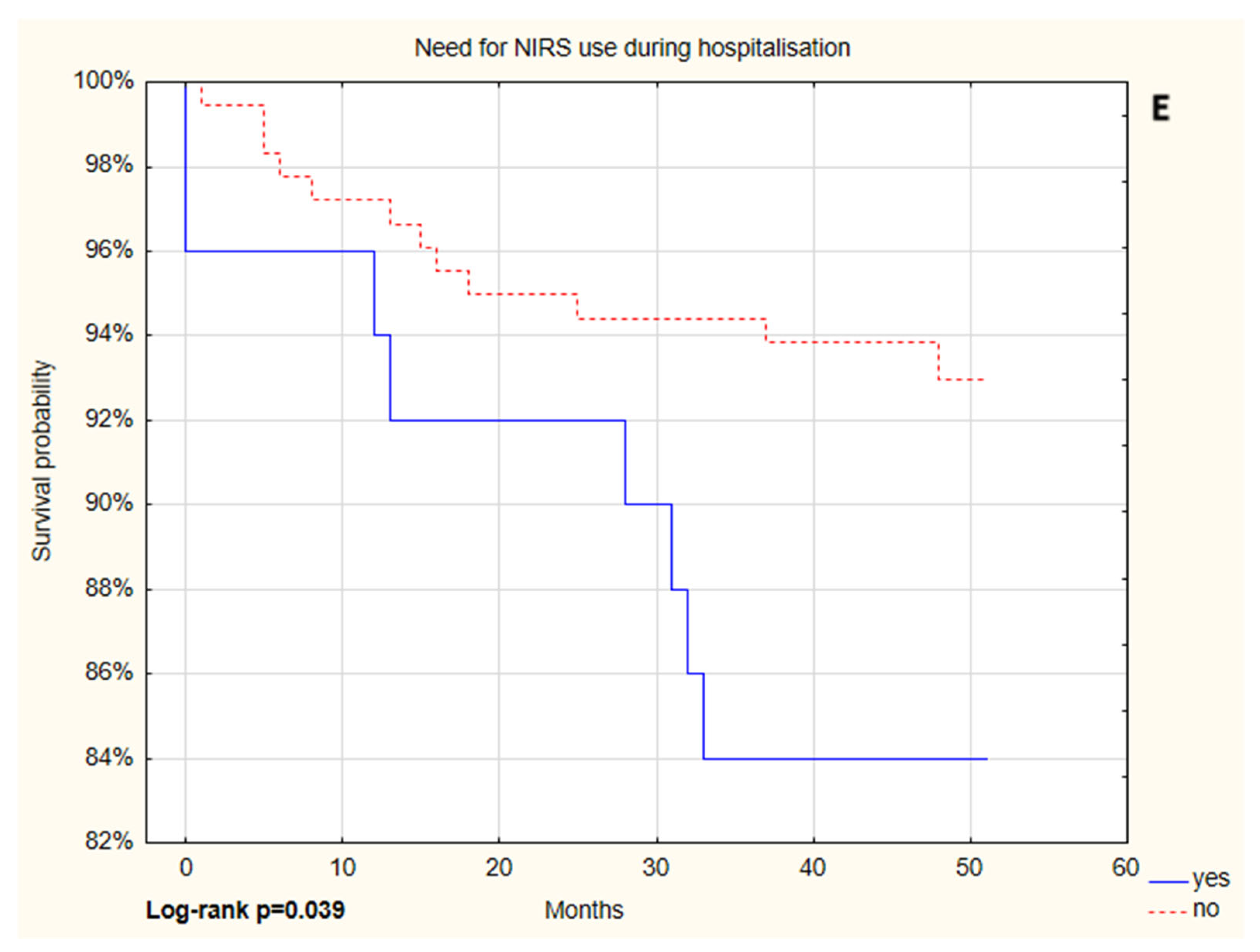
| Parameter | Patients Without NIRS (N = 181) | Patients on NIRS (N = 101) | p Value |
|---|---|---|---|
| Average length of hospitalisation in the department [days] | 7 (5–10) | 13 (8–18) | <0.001 |
| Age [years] | 58 (46–67) | 66 (60–72) | <0.001 |
| Lung parenchyma percentage involvement in CT [%] | 25 (15–40) | 60 (50–70) | <0.001 |
| Duration of oxygen therapy [percentage of hospitalisation time] | 63 (0–81) | 100 (93–100) | <0.001 |
| WBC [109/L] | 6.1 (4.5–7.8) | 8.1 (6.1–10.3) | <0.001 |
| NEUT [109/L] | 4.7 (3.0–6.2) | 7.0 (5.01–9.2) | <0.001 |
| LYMPH [109/L] | 0.8 (0.6–1.2) | 0.6 (0.5–0.8) | <0.001 |
| ALT [U/L] | 39.0 (25.0–60.0) | 46.0 (29.0–66.0) | 0.25 |
| AST [U/L] | 43.0 (30.0–63.0) | 58.0 (44.0–97.0) | <0.001 |
| Creatinine [umol/L] | 83.0 (70.0–100.0) | 89.0 (70.0–115.0) | 0.06 |
| D-dimer [ng/mL] | 706.0 (484.0–1221.5) | 1417.0 (766.0–5831.0) | <0.001 |
| LDH [U/L] | 345.0 (266.0–434.0) | 527.0 (407.0–647.0) | <0.001 |
| CRP [mg/L] | 67.0 (31.0–122.0) | 136.0 (80.0–200.0) | <0.001 |
| PCT [ng/mL] | 0.07 (0.04–0.14) | 0.19 (0.11–0.38) | <0.001 |
| IL-6 [pg/mL] | 25.7 (7.5–47.3) | 44.1 (21.2–104.4) | <0.001 |
| Parameter | NIRS-Effective Group (N = 47) | NIRS-Ineffective Group (N = 54) | p Value |
|---|---|---|---|
| Hospitalisation time in the department [days] | 17 (12–24) | 9 (4–13) | <0.001 |
| Age [years] | 64 (55–68) | 69 (63–79) | <0.001 |
| Lung parenchyma involvement on CT [%] | 65 (55–70) | 60 (45–70) | 0.09 |
| Duration of oxygen therapy [percentage of hospitalisation time] | 91.7 (83.3–100.0) | 100.0 | <0.001 |
| WBC [109/L] | 9.0 (6.5–11.3) | 7.5 (5.4–9.2) | 0.042 |
| NEUT [109/L] | 7.8 (5.6–10.1) | 6.2 (3.9–8.2) | 0.034 |
| LYMPH [109/L | 0.6 (0.5–0.9) | 0.5 (0.4–0.8) | 0.62 |
| ALT [U/L] | 47.0 (29.0–71.0) | 46.0 (25–62) | 0.71 |
| AST [U/L] | 55.0 (36.0–82.0) | 67.5 (49.0–109.0) | 0.041 |
| Creatinine [umol/L] | 83.0 (62.0–108.0) | 92.0 (80.0–129.0) | 0.012 |
| D-dimer [ng/mL] | 1426.0 (668.0–5357.0) | 1417.0 (886.0–6768.0) | 0.74 |
| LDH [U/L] | 478.0 (390.0–644.0) | 559.5 (436.0–665.0) | 0.16 |
| CRP [mg/L] | 155.0 (79.0–272.0) | 125.0 (80.0–191.0) | 0.35 |
| PCT [ng/mL] | 0.21 (0.1–0.36) | 0.17 (0.11–0.28) | 0.93 |
| IL-6 [pg/mL] | 43.4 (19.9–104.4) | 44.1 (21.2–88.7) | 0.71 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% of CI) | p Value | Odds Ratio (95% of CI) | p Value |
| Inflammatory changes in CT involving ≥50% of the lung parenchyma | 4.52 (3.30–6.18) | <0.001 | 4.38 (3.06–6.28) | <0.001 |
| Gender (male) | 0.88 (0.53–1.48) | 0.63 | ||
| Age ≥ 65 years | 1.60 (1.24–2.05) | <0.001 | ||
| WBC ≥ 10 × 109/L | 1.85 (1.35–2.55) | <0.001 | 1.68 (1.07–2.64) | 0.023 |
| NEUT ≥ 7 × 109/L | 2.18 (1.66–2.87) | <0.001 | ||
| LYMPH ≤ 0.5 × 109/L | 1.91 (1.41–2.58) | <0.001 | 1.50 (1.00–2.24) | 0.051 |
| AST ≥ 45 U/L | 1.74 (1.34–2.27) | <0.001 | ||
| Creatinine ≥ 115 umol/L | 1.44 (1.06–1.95) | 0.024 | ||
| D-dimer ≥ 1500 ng/mL | 2.04 (1.56–2.68) | <0.001 | ||
| LDH ≥ 350 U/L | 2.63 (1.90–3.65) | <0.001 | ||
| CRP ≥ 80 mg/L | 2.05 (1.57–2.69) | <0.001 | ||
| PCT ≥ 0.1 ng/mL | 2.62 (1.96–3.50) | <0.001 | 2.27 (1.56–3.31) | <0.001 |
| IL-6 ≥ 40 pg/mL | 1.64 (1.27–2.11) | <0.001 |
| Variables | p Value | HR * (95% of CI) | p Value | HR ** (95% of CI) | |
|---|---|---|---|---|---|
| Age | <0.001 | 1.08 (1.04–1.12) | 0.001 | 1.09 (1.04–1.13) | |
| NIRS | no | 1 | 1 | ||
| yes | 0.043 | 2.51 (1.03–6.15) | 0.013 | 2.53 (1.03–6.26) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobiczyk, E.; Winiarska, H.M.; Springer, D.; Ludziejewska, A.; Wysocka, E.; Skoczyński, S.; Cofta, S. Imaging and Laboratory Results as Predictors of the Course of COVID-19. Adv. Respir. Med. 2025, 93, 22. https://doi.org/10.3390/arm93040022
Tobiczyk E, Winiarska HM, Springer D, Ludziejewska A, Wysocka E, Skoczyński S, Cofta S. Imaging and Laboratory Results as Predictors of the Course of COVID-19. Advances in Respiratory Medicine. 2025; 93(4):22. https://doi.org/10.3390/arm93040022
Chicago/Turabian StyleTobiczyk, Ewelina, Hanna Maria Winiarska, Daria Springer, Aleksandra Ludziejewska, Ewa Wysocka, Szymon Skoczyński, and Szczepan Cofta. 2025. "Imaging and Laboratory Results as Predictors of the Course of COVID-19" Advances in Respiratory Medicine 93, no. 4: 22. https://doi.org/10.3390/arm93040022
APA StyleTobiczyk, E., Winiarska, H. M., Springer, D., Ludziejewska, A., Wysocka, E., Skoczyński, S., & Cofta, S. (2025). Imaging and Laboratory Results as Predictors of the Course of COVID-19. Advances in Respiratory Medicine, 93(4), 22. https://doi.org/10.3390/arm93040022






