Physically Active Lifestyle Attenuates Impairments on Lung Function and Mechanics in Hypertensive Older Adults
Abstract
Highlights
- Systemic arterial hypertension negatively affects not only the lung function but also lung mechanics.
- A physically active lifestyle by older adults prevents the impairment of lung function and mechanics induced by systemic arterial hypertension.
- Medical doctors who diagnose their patients, especially older adults with systemic arterial hypertension, should also check for lung function and mechanics.
- Medical doctors should advise their patients with systemic arterial hypertension to keep an active lifestyle to avoid hypertension-induced loss of lung function.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric Evaluations
2.3. Blood Pressure Evaluation
2.4. Lung Function and Lung Mechanics Evaluation
2.5. Peripheral and Respiratory Muscle Strength Measurements
2.6. International Physical Activity Questionnaire (IPAQ)
2.7. Health-Related Quality of Life (HRQoL)
2.8. Dyspnea Evaluation
2.9. Statistical Analysis
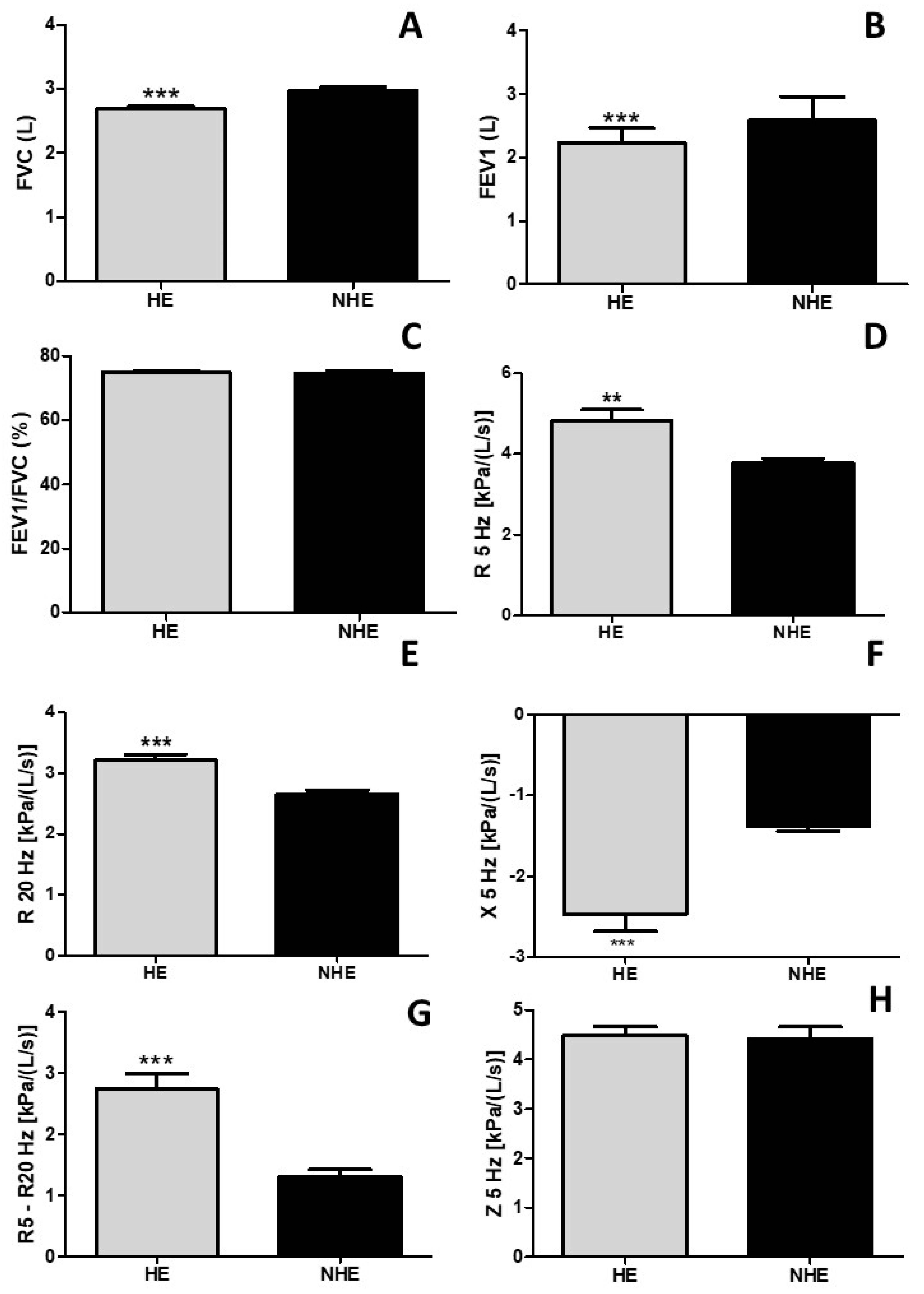
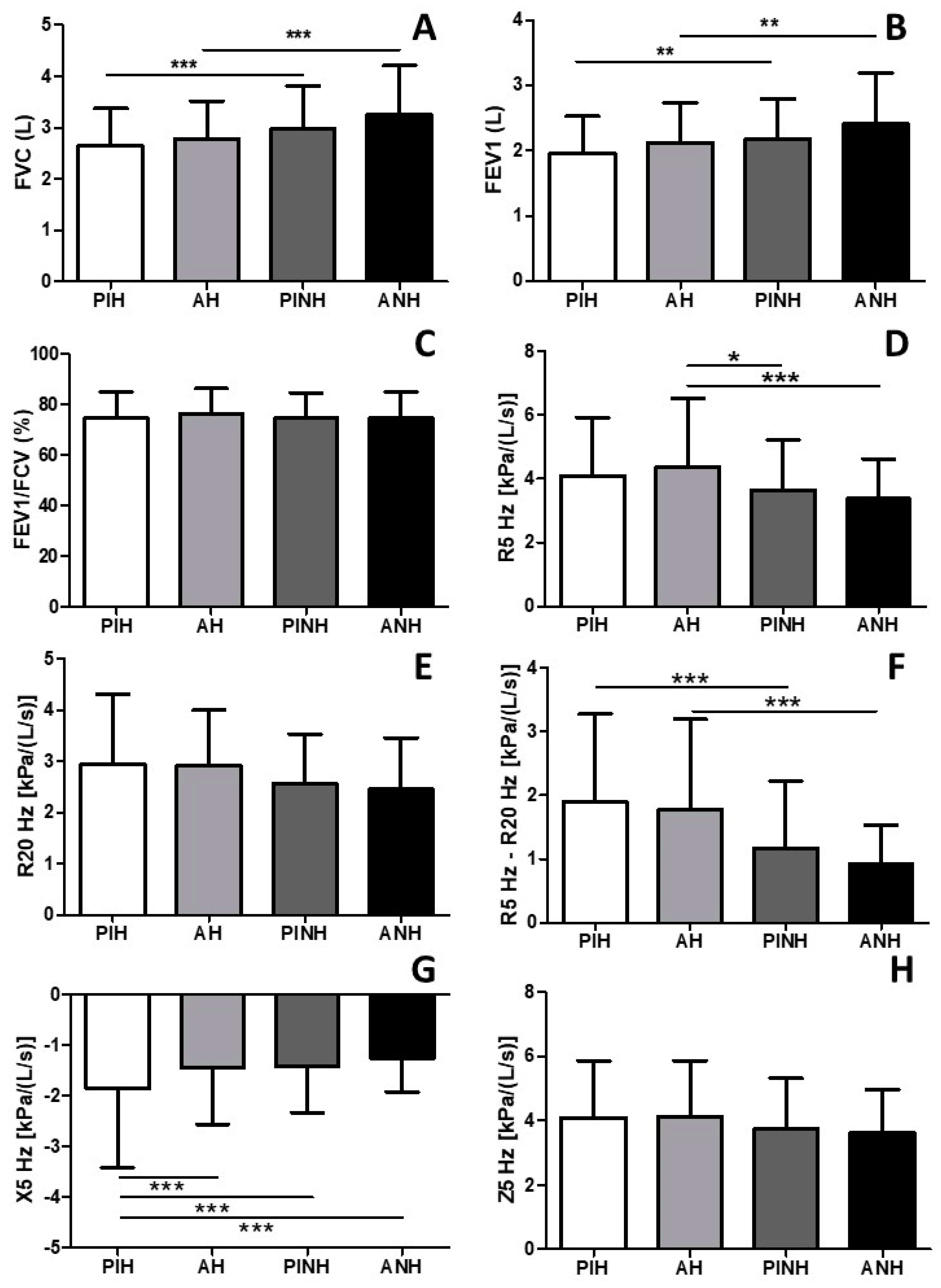
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensah, G.A.; Croft, J.B.; Giles, W.H. The heart, kidney, and brain as target organs in hypertension. Cardiol. Clin. 2002, 20, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Kim, K.I.; Cho, M.C. Current status and therapeutic considerations of hypertension in the elderly. Korean J. Intern. Med. 2019, 34, 687–695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Enright, P.L.; Kronmal, R.A.; Smith, V.E.; Gardin, J.M.; Schenker, M.B.; Manolio, T.A. Reduced vital capacity in elderly persons with hypertension, coronary heart disease, or left ventricular hypertrophy. The Cardiovascular Health Study. Chest 1995, 107, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.I.; Kuo, C.S. Relationship between respiratory muscle function and age, sex, and other factors. J. Appl. Physiol. 1989, 66, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Sardeli, A.V.; Griffth, G.J.; Dos Santos, M.V.M.A.; Ito, M.S.R.; Chacon-Mikahil, M.P.T. The effects of exercise training on hypertensive older adults: An umbrella meta-analysis. Hypertens. Res. 2021, 44, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Takahashi, K. Clinical Impacts of Interventions for Physical Activity and Sedentary Behavior on Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2023, 12, 1631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Margretardottir, O.B.; Thorleifsson, S.J.; Gudmundsson, G.; Olafsson, I.; Benediktsdottir, B.; Janson, C.; Buist, A.S.; Gislason, T. Hypertension, systemic inflammation and body weight in relation to lung function impairment-an epidemiological study. COPD 2009, 6, 250–255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Childs, A.; Zullo, A.R.; Joyce, N.R.; McConeghy, K.W.; van Aalst, R.; Moyo, P.; Bosco, E.; Mor, V.; Gravenstein, S. The burden of respiratory infections among older adults in long-term care: A systematic review. BMC Geriatr. 2019, 19, 210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakker, E.A.; Sui, X.; Brellenthin, A.G.; Lee, D.C. Physical activity and fitness for the prevention of hypertension. Curr. Opin. Cardiol. 2018, 33, 394–401. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, E.S.; Sampaio, L.M.; Peixoto-Souza, F.S.; Dias, F.D.; Gomes, E.L.; Greiffo, F.R.; Ligeiro de Oliveira, A.P.; Stirbulov, R.; Vieira, R.P.; Costa, D. Home-based pulmonary rehabilitation improves clinical features and systemic inflammation in chronic obstructive pulmonary disease patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 645–653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moraes-Ferreira, R.; Brandao-Rangel, M.A.R.; Gibson-Alves, T.G.; Silva-Reis, A.; Souza-Palmeira, V.H.; Aquino-Santos, H.C.; Frison, C.R.; Oliveira, L.V.F.; Albertini, R.; Vieira, R.P. Physical Training Reduces Chronic Airway Inflammation and Mediators of Remodeling in Asthma. Oxid. Med. Cell. Longev. 2022, 2022, 5037553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pereira, C.A.; Sato, T.; Rodrigues, S.C. New reference values for forced spirometry in white adults in Brazil. J. Bras. Pneumol. 2007, 33, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Mineshita, M.; Shikama, Y.; Nakajima, H.; Nishihira, R.; Komatsu, S.; Kubota, M.; Kobayashi, H.; Kokubu, F.; Shinkai, M.; Kaneko, T.; et al. The application of impulse oscillation system for the evaluation of treatment effects in patients with COPD. Respir. Physiol. Neurobiol. 2014, 202, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shi, Z.; Cui, Y.; Mi, J.; Ma, Z.; Ren, J.; Li, J.; Xu, S.; Guo, Y. Impulse oscillometry system as an alternative diagnostic method for chronic obstructive pulmonary disease. Medicine 2017, 96, e8543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brandao-Rangel, M.A.R.; Moraes-Ferreira, R.; Oliveira-Junior, M.C.; Santos-Dias, A.; Bachi, A.L.L.; Gabriela-Pereira, G.; de Oliveira Freitas, S.; Araújo-Rosa, A.C.; Oliveira, L.V.F.; Frison, C.R.; et al. Pulmonary function changes in older adults with and without metabolic syndrome. Sci. Rep. 2021, 11, 17337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, M.J.; Bland, J.M.; Gahbauer, E.A.; Ekström, M.; Sinnarajah, A.; Gill, T.M.; Currow, D.C. Breathlessness in Elderly Adults During the Last Year of Life Sufficient to Restrict Activity: Prevalence, Pattern, and Associated Factors. J. Am. Geriatr. Soc. 2016, 64, 73–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos, C.A.F.; Amirato, G.R.; Paixão, V.; Almeida, E.B.; Do Amaral, J.B.; Monteiro, F.R.; Roseira, T.; Juliano, Y.; Novo, N.F.; Rossi, M.; et al. Association among inflammaging, body composition, physical activity, and physical function tests in physically active women. Front. Med. 2023, 10, 1206989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahler, D.A. Evaluation of Dyspnea in the Elderly. Clin. Geriatr. Med. 2017, 33, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.A.; Rossiter, H.B.; Casaburi, R. Exercise, ageing and the lung. Eur. Respir. J. 2016, 48, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Rosiello, R.A.; Loke, J. The aging lung. Clin. Geriatr. Med. 1986, 2, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.A.; Saif, H.F.A.E.A.; Taha, M.M. Effect of alternate nostril breathing exercise on autonomic functions, ocular hypertension, and quality of life in elderly with systemic hypertension and high-tension primary open-angle glaucoma. Geriatr. Nurs. 2023, 52, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.A.; El-Azeim, A.S.A.; Saif, H.F.A.E.A. Effect of aerobic exercise alone or combined with Mediterranean diet on dry eye in obese hypertensive elderly. Ir. J. Med. Sci. 2023, 192, 3151–3161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| PIH (n = 182) | AH (n = 110) | PINH (n = 104) | ANH (n = 65) | p Value | F Value | |
|---|---|---|---|---|---|---|
| Age (years) | 70.07 ± 7.49 | 69.39 ± 5.49 | 67.42 ± 6.34 | 66.83 ± 5.18 | p > 0.05 | 5.073 |
| SBP (mmHg) | 140.28 ± 16.07 * | 144.44 ± 18.46 * | 140.94 ± 21.35 * | 129.75 ± 16.74 | p < 0.05 | 9.084 |
| DBP (mmHg) | 75.96 ± 9.14 | 72.56 ± 18.12 | 79.61 ± 9.78 | 79.25 ± 8.06 | p > 0.05 | 2.471 |
| Weight (kg) | 70.86 ± 15.15 | 68.76 ± 10.75 | 66.57 ± 13.89 | 67.52 ± 12.88 | p > 0.05 | 2.084 |
| Height (m) | 1.56 ± 0.08 | 1.57 ± 0.07 | 1.57 ± 0.08 | 1.59 ± 0.10 | p > 0.05 | 2.056 |
| BMI (kg/m2) | 26.92 ± 8.83 | 27.88 ± 4.34 | 26.76 ± 4.52 | 26.60 ± 3.53 | p > 0.05 | 1.980 |
| HGS left (kg) | 22.50 ± 10.08 | 24.78 ± 7.38 | 24.07 ± 8.69 | 25.72 ± 9.95 | p > 0.05 | 2.073 |
| HGS right (kg) | 23.37 ± 11.02 | 25.35 ± 7.45 | 24.99 ± 8.95 | 27.55 ± 10.51 | p > 0.05 | 4.744 |
| MIP (cm H2O) | 50.69 ± 23.48 | 59.95 ± 22.90 # | 62.71 ± 28.46 | 69.68 ± 35.24 # | p < 0.05 | 8.026 |
| MEP (cm H2O) | 64.66 ± 34.50 | 81.36 ± 42.92 & | 76.77 ± 49.82 | 82.13 ± 35.44 & | p < 0.05 | 6.589 |
| HC (cm) | 96.59 ± 12.38 | 92.62 ± 10.90 | 90.79 ± 11.63 | 90.92 ± 10.58 | p > 0.05 | 2.214 |
| WC (cm) | 104.58 ± 10.92 | 101.12 ± 14.77 | 100.19 ± 9.63 | 101.73 ± 8.23 | p > 0.05 | 3.470 |
| WHR | 0.93 ± 0.09 | 0.90 ± 0.08 | 0.90 ± 0.08 | 0.89 ± 0.07 | p > 0.05 | 2.811 |
| PA (years) | - | 4.04 ± 3.98 | - | 4.69 ± 4.13 | p > 0.05 | 2.416 |
| PIH (n = 182) | AH (n = 110) | PINH (n = 104) | ANH (n = 65) | p Value | F Value | |
|---|---|---|---|---|---|---|
| IPAQ Physical activity time (min/week) | 20.27 ± 8.71 | 184.83 ± 20.62 * | 24.32 ± 10.46 | 258.76 ± 28.87 * | <0.001 | 9.98 |
| SF-36 Physical functioning | 62.84 ± 20.45 | 74.28 ± 23.30 * | 67.14 ± 23.66 | 69.59 ± 22.27 | <0.001 | 10.54 |
| SF-36 Bodily pain | 49.75 ± 29.85 | 63.45 ± 43.21 * | 50.66 ± 30.94 | 36.29 ± 28.28 | <0.001 | 15.66 |
| SF-36 Role-physical | 86.12 ± 29.97 | 87.59 ± 32.39 | 91.16 ± 26.42 | 92.85 ± 24.41 | >0.05 | 0.8514 |
| SF-36 General health | 40.35 ± 22.88 | 55.80 ± 24.22 * | 46.77 ± 23.37 | 60.59 ± 25.00 * | <0.001 | 20.11 |
| SF-36 Vitality | 40.86 ± 31.47 | 69.91 ± 27.35 * | 55.04 ± 33.60 | 55.66 ± 32.05 * | <0.001 | 14.85 |
| SF-36 Social functioning | 46.87 ± 32.49 * | 77.01 ± 30.75 | 63.2 ± 35.73 | 70.69 ± 33.42 | <0.001 | 11.99 |
| SF-36 Role-emotional | 91.08 ± 26.13 | 86.93 ± 31.29 | 94.27 ± 22.74 | 98.03 ± 14.07 | >0.05 | 2.954 |
| SF-36 Mental health | 38.71 ± 30.11 | 69.84 ± 27.21 * | 55.07 ± 31.92 | 56.97 ± 31.25 | <0.001 | 20.99 |
| MRC Dyspnea scale | 9.37 ± 2.54 | 9.89 ± 2.02 | 9.69 ± 2.13 | 9.23 ± 1.93 | >0.05 | 2.132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandao-Rangel, M.A.R.; Brill, B.; de Souza Carvalho, E.; Melamed, D.; Moraes-Ferreira, R.; Silva-Reis, A.; Leonardo, P.S.; Frison, C.R.; De Angelis, K.; Vieira, R.P. Physically Active Lifestyle Attenuates Impairments on Lung Function and Mechanics in Hypertensive Older Adults. Adv. Respir. Med. 2024, 92, 278-290. https://doi.org/10.3390/arm92040027
Brandao-Rangel MAR, Brill B, de Souza Carvalho E, Melamed D, Moraes-Ferreira R, Silva-Reis A, Leonardo PS, Frison CR, De Angelis K, Vieira RP. Physically Active Lifestyle Attenuates Impairments on Lung Function and Mechanics in Hypertensive Older Adults. Advances in Respiratory Medicine. 2024; 92(4):278-290. https://doi.org/10.3390/arm92040027
Chicago/Turabian StyleBrandao-Rangel, Maysa Alves Rodrigues, Boris Brill, Edilson de Souza Carvalho, Dobroslav Melamed, Renilson Moraes-Ferreira, Anamei Silva-Reis, Patricia Sardinha Leonardo, Claudio Ricardo Frison, Kátia De Angelis, and Rodolfo P. Vieira. 2024. "Physically Active Lifestyle Attenuates Impairments on Lung Function and Mechanics in Hypertensive Older Adults" Advances in Respiratory Medicine 92, no. 4: 278-290. https://doi.org/10.3390/arm92040027
APA StyleBrandao-Rangel, M. A. R., Brill, B., de Souza Carvalho, E., Melamed, D., Moraes-Ferreira, R., Silva-Reis, A., Leonardo, P. S., Frison, C. R., De Angelis, K., & Vieira, R. P. (2024). Physically Active Lifestyle Attenuates Impairments on Lung Function and Mechanics in Hypertensive Older Adults. Advances in Respiratory Medicine, 92(4), 278-290. https://doi.org/10.3390/arm92040027







