RNA Polymerase Inhibitor Enisamium for Treatment of Moderate COVID-19 Patients: A Randomized, Placebo-Controlled, Multicenter, Double-Blind Phase 3 Clinical Trial
Abstract
Highlights
- Enisamium, administered in conjunction with standard care, demonstrated clinical efficacy in hospitalized adults with moderate COVID-19 requiring supplemental oxygen.
- In patients with moderate COVID-19 requiring supplemental oxygen, treatment with enisamium led to a significant reduction in the time to improvement and a reduction in symptoms compared to placebo, particularly when administered within 4 days of COVID-19 symptom onset.
- Enisamium presents a promising treatment option for individuals with moderate COVID-19, offering faster recovery and shorter hospital stays.
- Early administration of enisamium, within 4 days of symptom onset, may lead to more rapid clinical improvement, underscoring the importance of early intervention in managing COVID-19 cases.
Abstract
1. Introduction
2. Methods
2.1. Study Approval
2.2. Study Design
2.3. Outcomes
2.4. Criteria for Inclusion or Exclusion
2.5. Randomization
2.6. Procedures
2.7. Molecular Testing
2.8. Statistical Analyses
2.9. Role of Funding Source
3. Results
3.1. Patients
3.2. Primary Endpoint
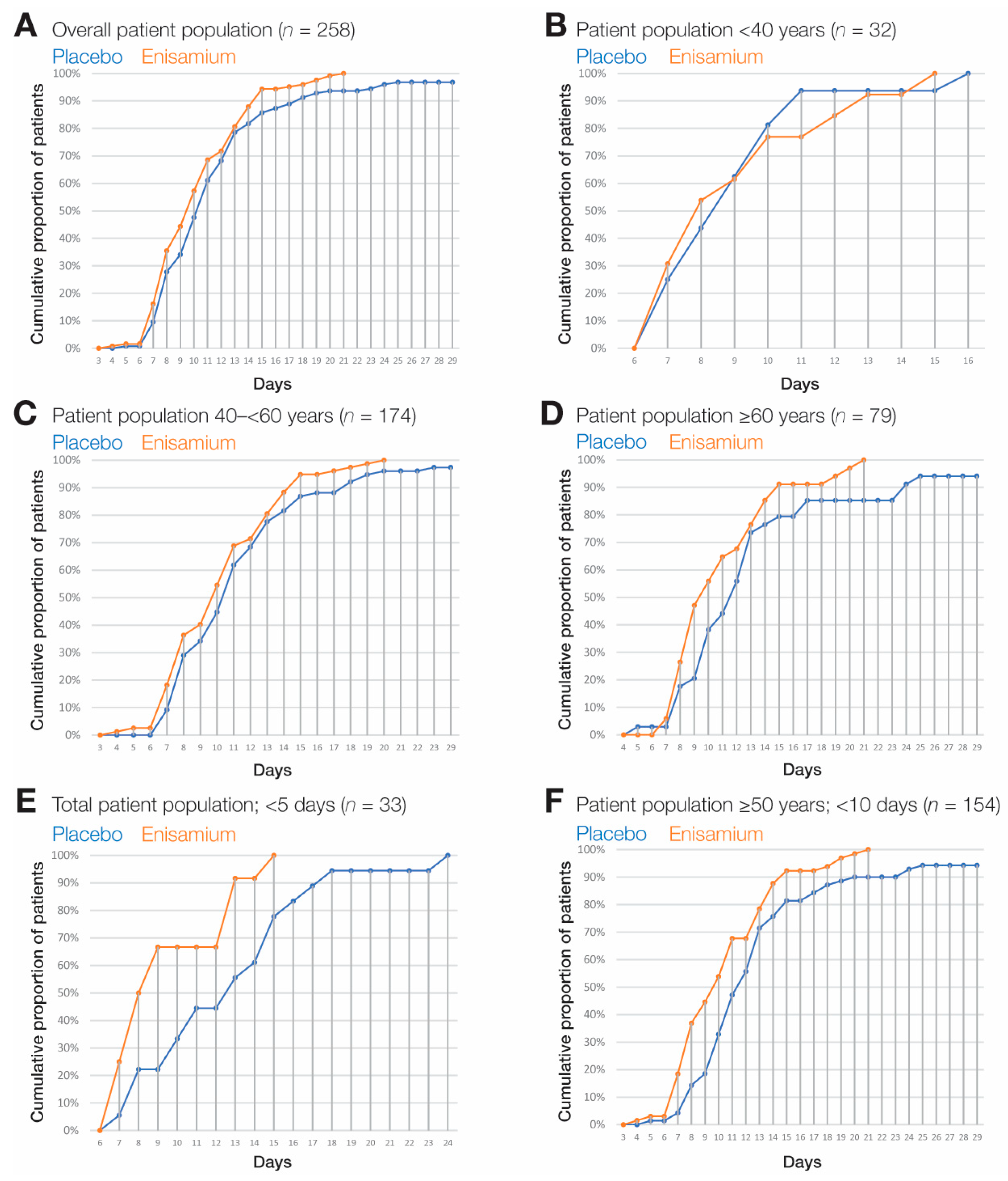
3.3. Subgroup Analyses
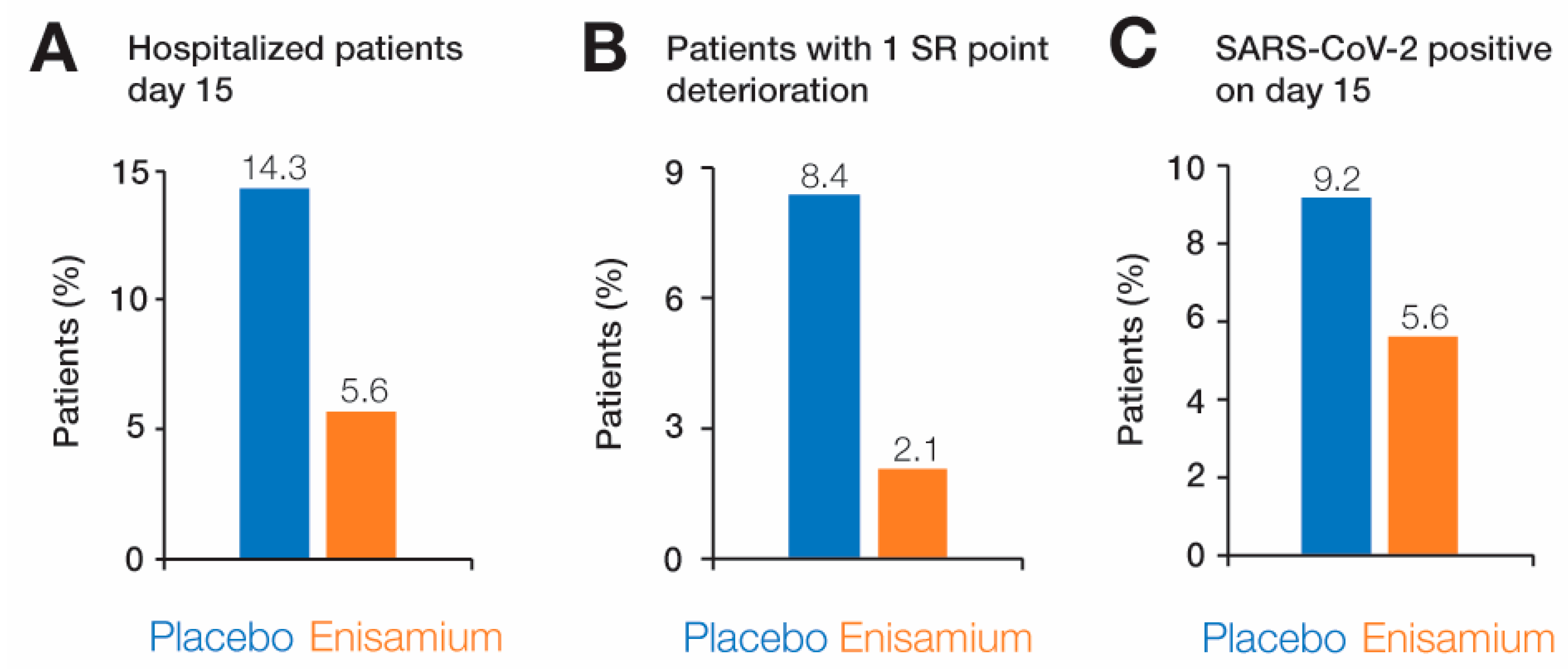
3.4. Secondary Endpoints
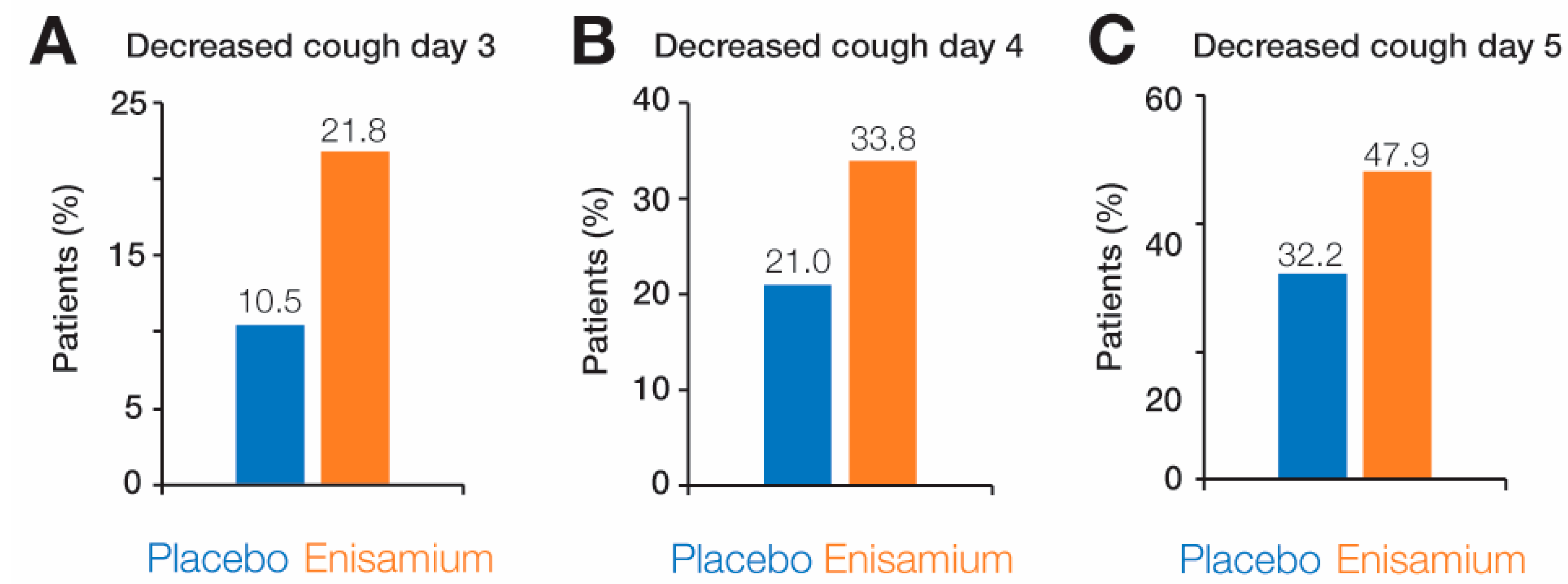
3.5. Symptom Dynamics
3.6. Safety Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, P.A.; Hernandez, J.C.; Galeano, E.; Hincapié-García, J.; Rugeles, M.T.; Zapata-Builes, W. Effectiveness of Drug Repurposing and Natural Products Against SARS-CoV-2: A Comprehensive Review. Clin. Pharmacol. 2024, 16, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Arman, B.Y.; Brun, J.; Hill, M.L.; Zitzmann, N.; von Delft, A. An Update on SARS-CoV-2 Clinical Trial Results-What We Can Learn for the Next Pandemic. Int. J. Mol. Sci. 2023, 25, 354. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Kinoshita, M.; Miyazawa, S.; Seki, M.; Mizuno, T.; Sonoyama, T. Ensitrelvir in Patients with SARS-CoV-2: A Retrospective Chart Review. J. Infect. Chemother. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, M.; Hu, C.; Song, H.; Mei, Y.; Liu, Y.; Zhang, Q. Remdesivir Derivative VV116 Is a Potential Broad-Spectrum Inhibitor of Both Human and Animal Coronaviruses. Viruses 2023, 15, 2295. [Google Scholar] [CrossRef] [PubMed]
- Moshawih, S.; Jarrar, Q.; Bahrin, A.A.; Lim, A.F.; Ming, L.; Goh, H.P. Evaluating NSAIDs in SARS-CoV-2: Immunomodulatory Mechanisms and Future Therapeutic Strategies. Heliyon 2024, 10, e25734. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Nishioka, K.; Yamamoto, C.; Watanabe, Y.; Daidoji, T.; Kawamoto, M.; Uda, S.; Kirito, S.; Nakagawa, Y.; Kasamatsu, Y.; et al. Emergence of SARS-CoV-2 with Dual-Drug Resistant Mutations During a Long-Term Infection in a Kidney Transplant Recipient. Infect. Drug Resist. 2024, 17, 531–541. [Google Scholar] [CrossRef]
- Snijder, E.J.; Decroly, E.; Ziebuhr, J. The Nonstructural Proteins Directing Coronavirus RNA Synthesis and Processing. Adv. Virus Res. 2016, 96, 59–126. [Google Scholar] [CrossRef]
- Snijder, E.J.; Limpens, R.; de Wilde, A.H.; de Jong, A.W.M.; Zevenhoven-Dobbe, J.C.; Maier, H.J.; Faas, F.; Koster, A.J.; Barcena, M. A Unifying Structural and Functional Model of the Coronavirus Replication Organelle: Tracking down RNA Synthesis. PLoS Biol. 2020, 18, e3000715. [Google Scholar] [CrossRef]
- Posthuma, C.C.; Te Velthuis, A.J.W.; Snijder, E.J. Nidovirus RNA Polymerases: Complex Enzymes Handling Exceptional RNA Genomes. Virus Res. 2017, 234, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J. Common and Unique Features of Viral RNA-Dependent Polymerases. Cell Mol. Life Sci. 2014, 71, 4403–4420. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.; Arnold, J.J.; Cameron, C.E.; van den Worm, S.H.; Snijder, E.J. The RNA Polymerase Activity of SARS-Coronavirus Nsp12 Is Primer Dependent. Nucleic Acids Res. 2010, 38, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Boltz, D.; Peng, X.; Muzzio, M.; Dash, P.; Thomas, P.G.; Margitich, V. Activity of Enisamium, an Isonicotinic Acid Derivative, against Influenza Viruses in Differentiated Normal Human Bronchial Epithelial Cells. Antivir. Chem. Chemother. 2018, 26, 2040206618811416. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.W.; Zubkova, T.G.; Shaw, M.; Mehle, A.; Boltz, D.; Gmeinwieser, N.; Stammer, H.; Milde, J.; Muller, L.; Margitich, V. Enisamium Reduces Influenza Virus Shedding and Improves Patient Recovery by Inhibiting Viral RNA Polymerase Activity. Antimicrob. Agents Chemother. 2021, 65, e02605-20. [Google Scholar] [CrossRef] [PubMed]
- Cocking, D.; Cinatl, J.; Boltz, D.A.; Peng, X.; Johnson, W.; Muzzio, M.; Syarkevych, O.; Kostyuk, G.; Goy, A.; Mueller, L.; et al. Antiviral Effect of a Derivative of Isonicotinic Acid Enisamium Iodide (FAV00A) against Influenza Virus. Acta Virol. 2018, 62, 191–195. [Google Scholar] [CrossRef]
- Walker, A.P.; Fan, H.; Keown, J.R.; Margitich, V.; Grimes, J.M.; Fodor, E.; Te Velthuis, A.J.W. Enisamium Is a Small Molecule Inhibitor of the Influenza A Virus and SARS-CoV-2 RNA Polymerases. BioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Elli, S.; Bojkova, D.; Bechtel, M.; Vial, T.; Boltz, D.; Muzzio, M.; Peng, X.; Sala, F.; Cosentino, C.; Goy, A.; et al. Enisamium Inhibits SARS-CoV-2 RNA Synthesis. Biomedicines 2021, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.; Koenig, F. The Reassessment of Trial Perspectives from Interim Data--a Critical View. Stat. Med. 2006, 25, 23–36. [Google Scholar] [CrossRef]
- Bauer, P.; Köhne, K. Evaluation of Experiments with Adaptive Interim Analyses. Biometrics 1994, 50, 1029–1041. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19-Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Naiyer, S.; Mansuri, S.; Soni, N.; Singh, V.; Bhat, K.H.; Singh, N.; Arora, G.; Mansuri, M.S. COVID-19 Diagnosis: A Comprehensive Review of the RT-qPCR Method for Detection of SARS-CoV-2. Diagnostics 2022, 12, 1503. [Google Scholar] [CrossRef] [PubMed]
- Broberg, P. Sample Size Re-Assessment Leading to a Raised Sample Size Does Not Inflate Type I Error Rate under Mild Conditions. BMC Med. Res. Methodol. 2013, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Bege, M.; Borbás, A. The Design, Synthesis and Mechanism of Action of Paxlovid, a Protease Inhibitor Drug Combination for the Treatment of COVID-19. Pharmaceutics 2024, 16, 217. [Google Scholar] [CrossRef] [PubMed]
- Bansode, S.; Singh, P.K.; Tellis, M.; Chugh, A.; Deshmukh, N.; Gupta, M.; Verma, S.; Giri, A.; Kulkarni, M.; Joshi, R.; et al. A Comprehensive Molecular and Clinical Investigation of Approved Anti-HCV Drugs Repurposing against SARS-CoV-2 Infection: A Glaring Gap between Benchside and Bedside Medicine. Vaccines 2023, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bisht, P.; Flamier, A.; Barrasa, M.I.; Friesen, M.; Richards, A.; Hughes, S.H.; Jaenisch, R. LINE1-Mediated Reverse Transcription and Genomic Integration of SARS-CoV-2 mRNA Detected in Virus-Infected but Not in Viral mRNA-Transfected Cells. Viruses 2023, 15, 629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-Transcribed SARS-CoV-2 RNA Can Integrate into the Genome of Cultured Human Cells and Can Be Expressed in Patient-Derived Tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef]
- Azam, M.; Sulistiana, R.; Ratnawati, M.; Fibriana, A.I.; Bahrudin, U.; Widyaningrum, D.; Aljunid, S.M. Recurrent SARS-CoV-2 RNA Positivity after COVID-19: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 20692. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med. 2020, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Urena Neme, A.P.; Tran, A.; Victoria Guerrero, M.; Roa Gomez, G.; Rodriguez Guerra, M.A. A Successful Treatment of COVID-Induced Acute Idiopathic Pancreatitis with an RNA-Polymerase Inhibitor Agent. Cureus 2024, 16, e51992. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalised with COVID-19 during the First Pandemic Wave from the ISARIC WHO CCP-UK Study: A Multicentre, Prospective Cohort Study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]


| SR | Definition |
|---|---|
| 1 | Death |
| 2 | Hospitalized, on invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) |
| 3 | Hospitalized, on non-invasive ventilation or high flow oxygen devices |
| 4 | Hospitalized, requiring supplemental oxygen |
| 5 | Hospitalized, not requiring supplemental oxygen—requiring ongoing medical care (COVID-19 related or otherwise) |
| 6 | Hospitalized, not requiring supplemental oxygen—no longer requires ongoing medical care |
| 7 | Not hospitalized, limitations on activities and/or requiring home oxygen |
| 8 | Not hospitalized, no limitations on activities |
| Characteristic | All N = 285 | Enisamium N = 142 | Placebo N = 143 |
|---|---|---|---|
| Age, years—median (IQR) | 59 (47–65) | 59 (47–65) | 59 (47.5–65) |
| Age category—no. (%) | |||
| —<40 year | 32 (11.2) | 15 (10.6) | 17 (11.9) |
| —40–<65 years | 174 (61.1) | 89 (62.7) | 85 (59.4) |
| —≥65 years | 79 (27.7) | 38 (26.8) | 41 (28.7) |
| Sex—no. (%) | |||
| —male | 134 (47.0) | 64 (45.1) | 70 (49.0) |
| —female | 151 (53.0) | 78 (54.9) | 73 (51.0) |
| Race or ethnic group—no. (%) (b) | |||
| —Caucasian (white) | 284 (99.6) | 141 (99.3) | 143 (100.0) |
| —Asian | 1 (0.4) | 1 (0.7) | 0 (0.0) |
| Median time (IQR) from symptom onset to randomization, days | 8 (6–12) | 8 (5–10) | 7 (5–9) |
| No. of coexisting conditions—no. (%) | |||
| None | 105 (36.8) | 55 (38.7) | 50 (35.0) |
| One | 104 (36.5) | 52 (36.6) | 52 (36.4) |
| Two or more | 76 (26.7) | 35 (24.6) | 41 (28.7) |
| Coexisting conditions—no. (%) | |||
| Hypertension | 140 (49.1) | 67 (47.2) | 73 (51.5) |
| BMI ≥ 30 kg/m2 | 94 (33.0) | 47 (33.1) | 47 (32.9) |
| Type 2 diabetes | 26 (9.1) | 10 (7.0) | 16 (11.2) |
| Severity of symptoms (d) | |||
| Median of cough severity (IQR) (c) | 2 (2–2) | 2 (2–2) | 2 (2–2) |
| Median of sore throat severity (IQR) (c) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Median of shortness in breath severity (IQR) (c) | 2 (2–3) | 2 (2–3) | 2 (2–3) |
| Median of headache severity (IQR) (c) | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| Median of diarrhea (e) severity (IQR) (c) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Median of rhinorrhea (f) severity (IQR) (c) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Median of fatigue severity (IQR) (c) | 3 (2–3) | 3 (2–3) | 3 (2–3) |
| Median of myalgia severity (IQR) (c) | 2 (2–2) | 1 (2–2) | 2 (1–2) |
| Population | Group | n | Median | p-Value (One-Sided) | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | 95% CI | ||||||
| Lower Bound | Upper Bound | |||||||
| All ITT patients (SR = 4 at baseline) with age stratification | Patients <40 years | Placebo | 17 | 9 | 0.65 | 7.73 | 10.27 | 0.009 * |
| Enisamium | 15 | 8 | 0.90 | 6.24 | 9.76 | |||
| Total | 32 | 9 | 0.52 | 7.98 | 10.02 | |||
| Patients 40–<65 years | Placebo | 85 | 11 | 0.33 | 10.36 | 11.64 | ||
| Enisamium | 89 | 10 | 0.40 | 9.22 | 10.78 | |||
| Total | 174 | 11 | 0.27 | 10.46 | 11.54 | |||
| Patients ≥65 years | Placebo | 41 | 12 | 0.72 | 10.58 | 13.42 | ||
| Enisamium | 38 | 10 | 0.58 | 8.87 | 11.13 | |||
| Total | 79 | 11 | 0.65 | 9.73 | 12.27 | |||
| All patients randomized within <5 days of symptom onset | Placebo | 18 | 13 | 2.11 | 8.87 | 17.13 | 0.005 | |
| Enisamium | 15 | 8 | 0.69 | 6.64 | 9.36 | |||
| Total | 33 | 10 | 1.10 | 7.85 | 12.15 | |||
| Patients aged ≥50 years randomized within <10 days of symptom onset | Placebo | 81 | 12 | 0.52 | 10.98 | 13.02 | 0.002 | |
| Enisamium | 73 | 10 | 0.54 | 8.95 | 11.05 | |||
| Total | 154 | 11 | 0.30 | 10.41 | 11.59 | |||
| Variable | Category | Placebo | Enisamium | p-Value (One-Sided) | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Patients discharged on day 8 | Patient not discharged | 92 | 73.0 | 81 | 65.3 | 0.119 |
| Patients discharged on day 15 | Patient not discharged | 18 | 14.3 | 7 | 5.6 | 0.018 |
| Patients discharged on day 22 | Patient not discharged | 8 | 6.3 | 0 | 0.0 | 0.004 |
| Patients discharged on day 29 | Patient not discharged | 4 | 3.2 | 0 | 0.0 | 0.063 |
| Deterioration by 1 SR point | Patients with deterioration of 1 point by SR scale | 12 | 8.4 | 3 | 2.1 | 0.016 |
| RT-qPCR test results on day 8 | SARS-CoV-2 positive on day 8 | 60 | 51.3 | 52 | 45.6 | 0.233 |
| RT-qPCR test results on day 15 | SARS-CoV-2 positive on day 15 | 8 | 9.2 | 5 | 5.6 | 0.289 |
| RT-qPCR test results on day 22 | SARS-CoV-2 positive on day 22 | 2 | 2.4 | 0 | 0.0 | 0.289 |
| RT-qPCR test results on day 29 | SARS-CoV-2 positive on day 29 | 1 | 1.2 | 0 | 0.0 | 0.494 |
| Mortality | Diseased patients | 3 | 2.1 | 0 | 0.0 | 0.125 |
| Parameter | Placebo Group (N = 289) n (%) | Enisamium (N = 293) n (%) |
|---|---|---|
| Subjects evaluated for AR/AE analysis | 289 | 293 |
| Number of ARs/AEs | 172 | 229 |
| Patients with ARs/AEs | 87 (30.1) | 105 (35.8) |
| Number of SAEs | 5 (2.9) | 4 (1.7) |
| Patients with SAEs | 3 (1.04) | 4 (1.37) |
| Patients excluded due to ARs/AEs | 15 (5.2) | 15 (5.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holubovska, O.; Babich, P.; Mironenko, A.; Milde, J.; Lebed, Y.; Stammer, H.; Mueller, L.; te Velthuis, A.J.W.; Margitich, V.; Goy, A. RNA Polymerase Inhibitor Enisamium for Treatment of Moderate COVID-19 Patients: A Randomized, Placebo-Controlled, Multicenter, Double-Blind Phase 3 Clinical Trial. Adv. Respir. Med. 2024, 92, 202-217. https://doi.org/10.3390/arm92030021
Holubovska O, Babich P, Mironenko A, Milde J, Lebed Y, Stammer H, Mueller L, te Velthuis AJW, Margitich V, Goy A. RNA Polymerase Inhibitor Enisamium for Treatment of Moderate COVID-19 Patients: A Randomized, Placebo-Controlled, Multicenter, Double-Blind Phase 3 Clinical Trial. Advances in Respiratory Medicine. 2024; 92(3):202-217. https://doi.org/10.3390/arm92030021
Chicago/Turabian StyleHolubovska, Olga, Pavlo Babich, Alla Mironenko, Jens Milde, Yuriy Lebed, Holger Stammer, Lutz Mueller, Aartjan J. W. te Velthuis, Victor Margitich, and Andrew Goy. 2024. "RNA Polymerase Inhibitor Enisamium for Treatment of Moderate COVID-19 Patients: A Randomized, Placebo-Controlled, Multicenter, Double-Blind Phase 3 Clinical Trial" Advances in Respiratory Medicine 92, no. 3: 202-217. https://doi.org/10.3390/arm92030021
APA StyleHolubovska, O., Babich, P., Mironenko, A., Milde, J., Lebed, Y., Stammer, H., Mueller, L., te Velthuis, A. J. W., Margitich, V., & Goy, A. (2024). RNA Polymerase Inhibitor Enisamium for Treatment of Moderate COVID-19 Patients: A Randomized, Placebo-Controlled, Multicenter, Double-Blind Phase 3 Clinical Trial. Advances in Respiratory Medicine, 92(3), 202-217. https://doi.org/10.3390/arm92030021





