Advancing Care in Severe Asthma: The Art of Switching Biologics
Abstract
Highlights
- Switching between different monoclonal antibodies can be beneficial for patients with severe asthma, especially when the initial biologic therapy does not provide sufficient symptom control or presents severe side effects.
- Considering individual patient factors and biomarkers in determining the effectiveness of biologics is fundamental. This personalized approach helps predict positive responses and optimize treatment efficacy.
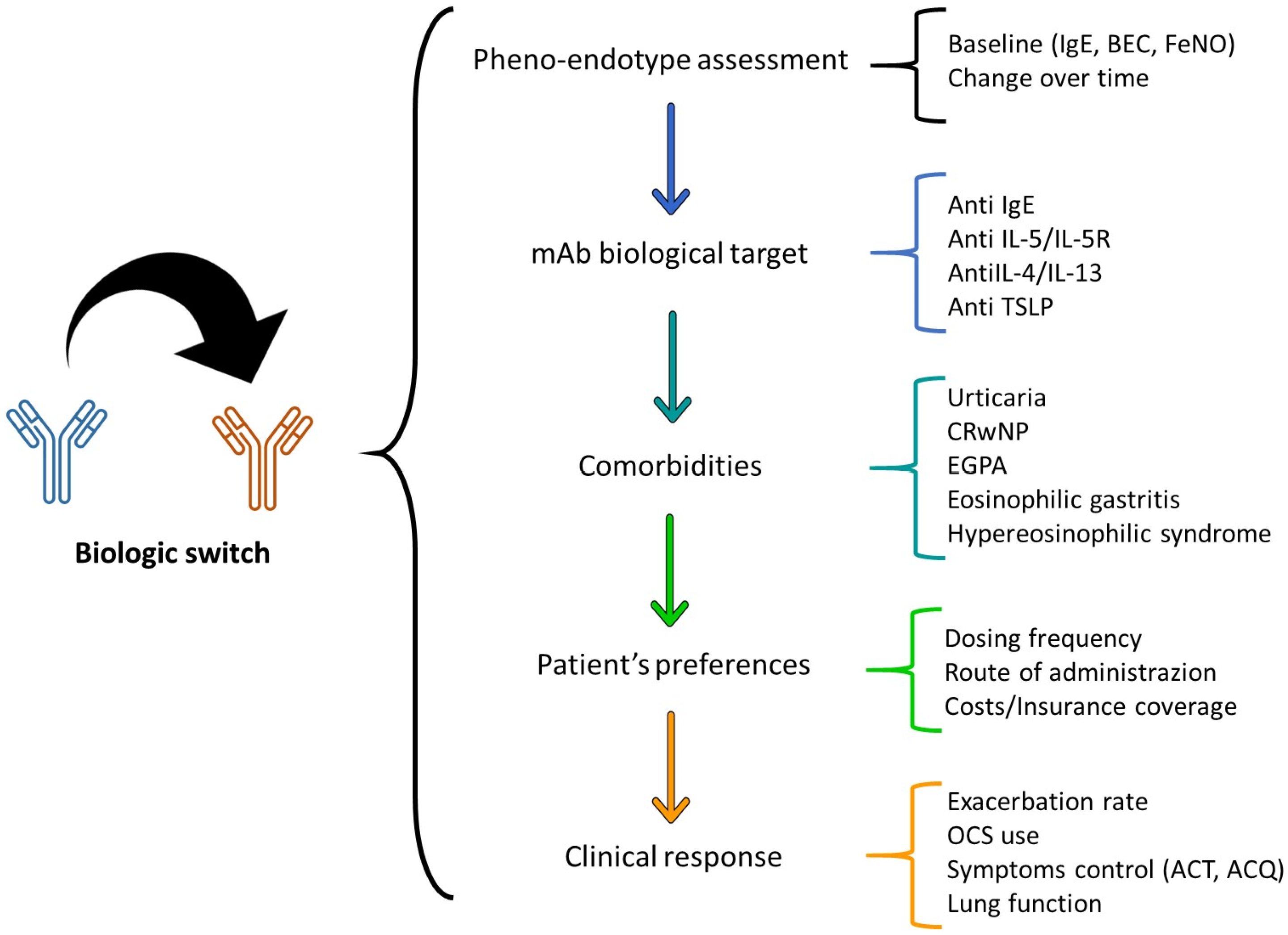
- The need for a more personalized approach in severe asthma treatment, using patient-specific characteristics and biomarker assessment to guide the selection and switching of biologics.
- The importance of flexibility in treatment strategies for severe asthma, acknowledging the dynamic nature of the disease and the evolving landscape of biologic therapies.
Abstract
1. Introduction
2. Initial Selection of Biologics
3. Rationale for Switching Biologics
4. Existing Literature about Switching Biologics in Severe Asthma
Significant Adverse Effects and Considerations in Switching Biologics
5. Molecular and Immunological Considerations
6. Patient-Centered Approach
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dragonieri, S.; Carpagnano, G.E. Biological therapy for severe asthma. Asthma Res. Pract. 2021, 13, 7–12. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A.; Akdis, M.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Chu, D.K.; Del Giacco, S.; Eiwegger, T.; et al. EAACI biologicals guidelines-Recommendations for severe asthma. Allergy 2021, 76, 14–44. [Google Scholar] [CrossRef]
- Porsbjerg, C.; Melén, E.; Lehtimäki, L.; Shaw, D. Asthma. Lancet 2023, 401, 858–873. [Google Scholar] [CrossRef]
- Hekking, P.P.W.; Wener, R.R.; Amelink, M.; Zwinderman, A.H.; Bouvy, M.L.; Bel, E.H. The prevalence of severe refractory asthma. J. Allergy Clin. Immunol. 2015, 135, 896–902. [Google Scholar] [CrossRef]
- Lefebvre, P.; Duh, M.S.; Lafeuille, M.H.; Gozalo, L.; Desai, U.; Robitaille, M.N.; Albers, F.; Yancey, S.; Ortega, H.; Forshag, M.; et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J. Allergy Clin. Immunol. 2015, 136, 1488–1495. [Google Scholar] [CrossRef]
- Chapman, K.R.; Albers, F.C.; Chipps, B.; Muñoz, X.; Devouassoux, G.; Bergna, M.; Galkin, D.; Azmi, J.; Mouneimne, D.; Price, R.G.; et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy 2019, 74, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.N.; McBrien, C.; Unni, B.; Porsbjerg, C.M.; Al-Ahmad, M.; Ambrose, C.S.; Dahl Assing, K.; von Bülow, A.; Busby, J.; Cosio, B.G.; et al. Real World Biologic Use and Switch Patterns in Severe Asthma: Data from the International Severe Asthma Registry and the US CHRONICLE Study. J. Asthma Allergy 2022, 15, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Chipps, B.; Munoz, X.; Devouassoux, G.; Bergna, M.; Smith, S.G.; Price, R.G.; Galkin, D.V.; Azmi, J.; Mouneimne, D.; et al. Benefit of switching to mepolizumab from omalizumab in severe eosinophilic asthma based on patient characteristics. Respir. Res. 2021, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Magnan, A.; Bourdin, A.; Prazma, C.M.; Albers, F.C.; Price, R.G.; Yancey, S.W.; Ortega, H. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy 2016, 71, 1335–1344. [Google Scholar] [CrossRef]
- Bagnasco, D.; Menzella, F.; Caminati, M.; Caruso, C.; Guida, G.; Bonavia, M.; Riccio, A.; Milanese, M.; Manfredi, A.; Senna, G.; et al. Efficacy of mepolizumab in patients with previous omalizumab treatment failure: Real-life observation. Allergy 2019, 74, 2539–2541. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Pelaia, C.; D’Amato, M.; Crimi, N.; Scichilone, N.; Scioscia, G.; Resta, O.; Calabrese, C.; Pelaia, G.; Quarato, C.M.I.; et al. Switching from omalizumab to mepolizumab: Real-life experience from Southern Italy. Ther. Adv. Respir. Dis. 2020, 14, 175346662092923. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Resta, E.; Povero, M.; Pelaia, C.; D’Amato, M.; Crimi, N.; Scichilone, N.; Scioscia, G.; Resta, O.; Calabrese, C.; et al. Clinical and economic consequences of switching from omalizumab to mepolizumab in uncontrolled severe eosinophilic asthma. Sci. Rep. 2021, 11, 5453. [Google Scholar] [CrossRef]
- Pelaia, C.; Crimi, C.; Nolasco, S.; Carpagnano, G.E.; Brancaccio, R.; Buonamico, E.; Campisi, R.; Gagliani, C.; Patella, V.; Pelaia, G.; et al. Switch from Omalizumab to Benralizumab in Allergic Patients with Severe Eosinophilic Asthma: A Real-Life Experience from Southern Italy. Biomedicines 2021, 9, 1822. [Google Scholar] [CrossRef] [PubMed]
- O’reilly, E.; Casey, D.; Ibrahim, H.; McGrath, A.; McHugh, T.; Vairamani, P.; Murphy, J.; Plant, B.; Murphy, D.M. Real-World Clinical Outcomes in Asthmatic Patients Switched from Omalizumab to Anti-Interleukin-5 Therapy. J. Asthma Allergy 2022, 15, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.G.B.; Gallardo, J.F.M.; Romero, J.D.; Falcón, A.R.; Bernáldez, C.B.; Borrego, J.G.; Álvarez-Gutiérrez, F.J. Effectiveness of Switching to Benralizumab in Severe Refractory Eosinophilic Asthma. J. Asthma Allergy 2022, 15, 727–735. [Google Scholar] [CrossRef]
- Caruso, C.; Cameli, P.; Altieri, E.; Aliani, M.; Bracciale, P.; Brussino, L.; Caiaffa, M.F.; Canonica, G.W.; Centanni, S.; D’Amato, M.; et al. Switching from one biologic to benralizumab in patients with severe eosinophilic asthma: An ANANKE study post hoc analysis. Front. Med. 2022, 9, 2579. [Google Scholar] [CrossRef]
- Numata, T.; Miyagawa, H.; Nishioka, S.; Okuda, K.; Utsumi, H.; Hashimoto, M.; Minagawa, S.; Ishikawa, T.; Hara, H.; Araya, J.; et al. Efficacy of benralizumab for patients with severe eosinophilic asthma: A retrospective, real-life study. BMC Pulm. Med. 2020, 20, 207. [Google Scholar] [CrossRef] [PubMed]
- Drick, N.; Milger, K.; Seeliger, B.; Fuge, J.; Korn, S.; Buhl, R.; Schuhmann, M.; Herth, F.; Kendziora, B.; Behr, J.; et al. Switch from IL-5 to IL-5-Receptor α Antibody Treatment in Severe Eosinophilic Asthma. J. Asthma Allergy 2020, 13, 605–614. [Google Scholar]
- Kavanagh, J.E.; Hearn, A.P.; d’Ancona, G.; Dhariwal, J.; Roxas, C.; Green, L.; Thomson, L.; Fernandes, M.; Kent, B.D.; Nanzer, A.M.; et al. Benralizumab after sub-optimal response to mepolizumab in severe eosinophilic asthma. Allergy 2021, 76, 1890–1893. [Google Scholar] [CrossRef]
- Martínez-Moragón, E.; García-Moguel, I.; Nuevo, J.; Resler, G.; The ORBE study investigators. Real-world study in severe eosinophilic asthma patients refractory to anti-IL5 biological agents treated with benralizumab in Spain (ORBE study). BMC Pulm. Med. 2021, 21, 417. [Google Scholar] [CrossRef]
- Mümmler, C.; Munker, D.; Barnikel, M.; Veit, T.; Kayser, M.Z.; Welte, T.; Behr, J.; Kneidinger, N.; Suhling, H.; Milger, K. Dupilumab Improves Asthma Control and Lung Function in Patients with Insufficient Outcome During Previous Antibody Therapy. J. Allergy Clin. Immunol. Pract. 2021, 9, 1177–1185.e4. [Google Scholar] [CrossRef] [PubMed]
- Campisi, R.; Crimi, C.; Nolasco, S.; Beghè, B.; Antonicelli, L.; Guarnieri, G.; Scichilone, N.; Porto, M.; Macchia, L.; Scioscia, G.; et al. Real-World Experience with Dupilumab in Severe Asthma: One-Year Data from an Italian Named Patient Program. J. Asthma Allergy 2021, 14, 575. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Araya, J.; Miyagawa, H.; Okuda, K.; Takekoshi, D.; Hashimoto, M.; Minagawa, S.; Ishikawa, T.; Hara, H.; Kuwano, K. Real-World Effectiveness of Dupilumab for Patients with Severe Asthma: A Retrospective Study. J. Asthma Allergy 2022, 15, 395. [Google Scholar] [CrossRef] [PubMed]
- Eger, K.; Pet, L.; Weersink, E.J.M.; Bel, E.H. Complications of switching from anti–IL-5 or anti–IL-5R to dupilumab in corticosteroiddependent severe asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 2913–2915. [Google Scholar] [CrossRef]
- Caminati, M.; Marcon, A.; Guarnieri, G.; Miotti, J.; Bagnasco, D.; Carpagnano, G.E.; Pelaia, G.; Vaia, R.; Maule, M.; Vianello, A.; et al. Benralizumab Efficacy in Late Non-Responders to Mepolizumab and Variables Associated with Occurrence of Switching: A Real-Word Perspective. J. Clin. Med. 2023, 12, 1836. [Google Scholar] [CrossRef]
- Higo, H.; Ichikawa, H.; Arakawa, Y.; Mori, Y.; Itano, J.; Taniguchi, A.; Senoo, S.; Kimura, G.; Tanimoto, Y.; Miyake, K.; et al. Switching to Dupilumab from Other Biologics without a Treatment Interval in Patients with Severe Asthma: A Multi-Center Retrospective Study. J. Clin. Med. 2023, 12, 5174. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2014, 15, 57–65. [Google Scholar] [CrossRef]
- Chen, M.L.; Nopsopon, T.; Akenroye, A. Incidence of Anti-Drug Antibodies to Monoclonal Antibodies in Asthma: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2023, 11, 1475–1484.e20. [Google Scholar] [CrossRef]
- Canonica, G.W.; Varricchi, G.; Paoletti, G.; Heffler, E.; Virchow, J.C. Advancing precision medicine in asthma: Evolution of treatment outcomes. J. Allergy Clin. Immunol. 2023, 152, 835–840. [Google Scholar] [CrossRef]
- Schoettler, N.; Strek, M.E. Recent Advances in Severe Asthma: From Phenotypes to Personalized Medicine. Chest 2020, 157, 516–528. [Google Scholar] [CrossRef]
| Biological Drug | Target Mechanism | Indications | Common Side Effects |
|---|---|---|---|
| Mepolizumab | IL-5 pathway | Eosinophilic asthma | Headache, injection site reaction, fatigue, flu symptoms, urinary tract infection, abdominal pain, itching, eczema, muscle spasms |
| Reslizumab | IL-5 pathway | Eosinophilic asthma (≥400 eosinophils/μL) | Cough, dizziness, itching, skin rash, fatigue |
| Benralizumab | IL-5 receptor α | Eosinophilic asthma (≥300 eosinophils/μL) | Fever (after first injection), headache, pharyngitis |
| Dupilumab | IL-4 and IL-13 pathways | Type 2 asthma, eosinophilic asthma, OCS-dependent asthma | Transitory increase of blood eosinophilia, reduction in T2 inflammation markers |
| Omalizumab | IgE pathway | Allergic asthma | Headache, injection site reaction, sore throat, fatigue, joint pain, skin rash |
| Tezepelumab | TSLP pathway | Allergic and eosinophilic asthma, non-type-2 asthma | Similar to other biologics, potential for headache, injection site reactions, etc. |
| Reference | Study | Population | Intervention | Outcome |
|---|---|---|---|---|
| [6] | Chapman et al., 2019 | 138 patients with allergic eosinophilic asthma | Switch from Omalizumab to Mepolizumab | Improved asthma control, health status, reduced exacerbation rates |
| [7] | Menzies-Gow et al. | 3531 patients, 384 switched biologics | Switching biologics, primarily from Omalizumab to anti-IL-5/5R | Changes based on inadequate effectiveness or negative side effects |
| [8] | Liu et al., 2021 | 138 patients from the OSMO study | Transition from Omalizumab to Mepolizumab | Improvements regardless of various baseline characteristics |
| [9] | Magnan et al., 2016 | 120 patients from MENSA and SIRIUS studies | Effectiveness of Mepolizumab after Omalizumab | Positive response to Mepolizumab irrespective of prior Omalizumab use |
| [10] | Bagnasco et al., 2019 | 27 patients with severe allergic eosinophilic asthma | Switch to Mepolizumab due to insufficient control with Omalizumab | Reduced exacerbations, decreased prednisone dosage, improved FEV1 and ACT scores |
| [11] | Carpagnano et al., 2020 | 41 patients with severe allergic eosinophilic asthma | Switch to Mepolizumab without a washout period | Increased ACT scores, improved pre-bronchodilator FEV1, reduced exacerbations and corticosteroid dependency |
| [12] | Carpagnano et al., 2021 | 33 patients with severe eosinophilic asthma | Switch to Mepolizumab from Omalizumab | Decrease in annual exacerbations and adverse events, reduction in lost working days |
| [13] | Pelaia et al., 2021 | 20 patients with severe persistent allergic and eosinophilic asthma | Switch to Benralizumab from Omalizumab | Significant improvements in asthma exacerbation rates, rescue medication usage, ACT scores, FEV1, and blood eosinophil counts |
| [14] | O’Reilly et al., 2022 | 10 patients | Switch to anti-IL-5 therapy from Omalizumab | Significant reductions in community exacerbation rates, serum eosinophil counts, and improvement in FEV1 |
| [15] | Gómez-Baster Fernádez et al., 2022 | 40 patients | Switch from Omalizumab or Mepolizumab to Benralizumab | Significant decrease in exacerbations, emergency department visits, corticosteroid cycles, and improvement in ACT scores |
| [16] | Caruso et al., 2022 | 205 asthma patients (147 biologic-naïve and 58 biologic-experienced) | Switch to Benralizumab from Omalizumab or Mepolizumab | Similar reductions in exacerbations, OCS usage, ACT improvement, and lung function in both groups |
| [17] | Numata et al., 2020 | 24 patients treated with Mepolizumab | Switch to Benralizumab due to inadequate control | Slight improvements in some parameters but no significant differences observed |
| [18] | Drick et al., 2020 | 60 patients receiving anti-IL5 treatment | Switch to Benralizumab | Progressive improvement in symptom control, OCS intake, and lung function |
| [19] | Kavanagh et al., 2021 | 33 asthmatic patients | Switch to Benralizumab from Mepolizumab | 58% reduction in the annualized exacerbation rate, significant improvement in symptom control and quality of life |
| [20] | Martínez-Moragón et al., 2021 | Patients treated with anti-IL5 therapy | Switch to Benralizumab | Significant improvements in ACT scores, annualized asthma exacerbation rates, and OCS intake |
| [21] | Mümmler et al., 2021 | 38 severe asthma patients | Switch to Dupilumab from a previous anti-IgE or anti-IL5/IL5R medication | Improvements in asthma control, lung function, exacerbation rates, FENO, and IgE levels |
| [22] | Campisi et al., 2021 | 5 patients | Switch to Dupilumab from Omalizumab, Mepolizumab, or Benralizumab | Reduction in exacerbations, OCS usage, improvement in FEV1% values, and enhanced asthma control |
| [23] | Numata et al., 2022 | 26 patients (10 Dupilumab as first biologic, 16 switched from other biologics) | Switch to Dupilumab | Reductions in exacerbations, OCS maintenance doses, and improvements in asthma symptoms |
| [24] | Eger et al., 2021 | 4 patients treated with anti-IL-5 or anti-IL-5R biologics | Switch to Dupilumab | Development of hypereosinophilia, sudden deterioration in asthma symptoms, tissue infiltration by eosinophils |
| [25] | Caminati et al., 2023 | 68 patients with severe eosinophilic asthma | Switch from Mepolizumab to Benralizumab | Improved outcomes including oral corticosteroid reduction, lung function, and blood eosinophil levels |
| [26] | Higo et al., 2023 | 27 severe asthma patients | Switch to Dupilumab from other biologics without a gap | Significant improvements in lung function and asthma control, 77.8% response rate to Dupilumab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragonieri, S.; Portacci, A.; Quaranta, V.N.; Carpagnano, G.E. Advancing Care in Severe Asthma: The Art of Switching Biologics. Adv. Respir. Med. 2024, 92, 110-122. https://doi.org/10.3390/arm92020014
Dragonieri S, Portacci A, Quaranta VN, Carpagnano GE. Advancing Care in Severe Asthma: The Art of Switching Biologics. Advances in Respiratory Medicine. 2024; 92(2):110-122. https://doi.org/10.3390/arm92020014
Chicago/Turabian StyleDragonieri, Silvano, Andrea Portacci, Vitaliano Nicola Quaranta, and Giovanna Elisiana Carpagnano. 2024. "Advancing Care in Severe Asthma: The Art of Switching Biologics" Advances in Respiratory Medicine 92, no. 2: 110-122. https://doi.org/10.3390/arm92020014
APA StyleDragonieri, S., Portacci, A., Quaranta, V. N., & Carpagnano, G. E. (2024). Advancing Care in Severe Asthma: The Art of Switching Biologics. Advances in Respiratory Medicine, 92(2), 110-122. https://doi.org/10.3390/arm92020014








