Using a Fibrinolysis Delivery Catheter in Pulmonary Embolism Treatment for Measurement of Pulmonary Artery Hemodynamics
Abstract
Highlights
- What are the main findings?
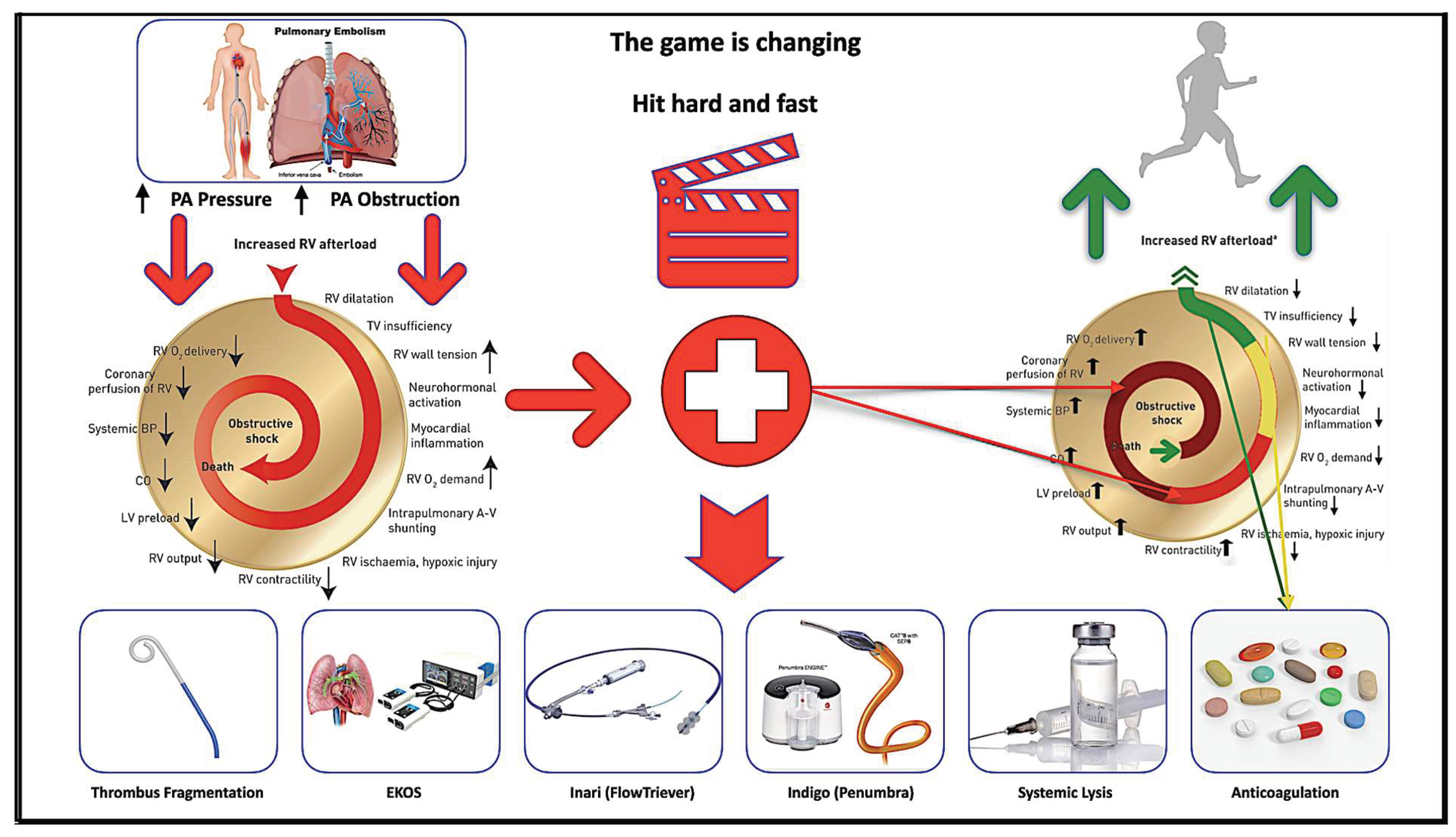
- This study demonstrated that ultrasound-facilitated catheter-directed fibrinolysis for the treatment of 45 patients with intermediate high-risk pulmonary embolism was associated with significant improvements in hemodynamics and low major bleeding events as demonstrated in previous trials (Ultima and Seattle II). However, this protocol used a lower dose and shorter duration of t-PA. The third arm of Optalyse PE-Trial used the same regime (6 mg/6 h/lung) for the treatment of 24 patients.
- It focuses on pulmonary artery hemodynamics as a primary precise surrogate marker for therapy effectiveness in addition to RV/LV ratio that was used in previous trials (Ultima and Seattle II), which has a bias of interobserver variability.
- What is the implication of the main finding?
- It assessed pulmonary artery hemodynamics for the first time by fibrinolysis delivery catheter, without additional need for a right heart catheterization. The results matched the right heart catheter results in EKOS and Heparin arm of Ultima trial, thereby confirming the validity of these diagnostic tools.
- It’s a valid and precise diagnostic tool to assess therapy effectiveness and reduce additional procedure-related complications, hospital residency, and economics. It gives this therapy additional diagnostic potential. It stressed the importance of interdisciplinary teams in the management of PE and evaluated the quality of life of these patients. This protocol shortens ICU stay to 6 h.
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Statistics
2.4. Study Population
- An intermediate–high risk of PE (Simplified Pulmonary Embolism Severity Index (sPESI) ≥ 1, RV strain, positive cardiac markers);
- High risk of PE (with systemic arterial hypotension, cardiogenic shock, or resuscitated);
- Intermediate–low risk of PE with deterioration of symptoms or hemodynamics (systemic arterial hypotension, cardiogenic shock, oxygen Saturation < 90%, heart rate > 100/min, and breathing frequency > 20/min).
- Ischemic stroke within three months;
- Active intracranial or intraspinal disease within 6 months;
- Major surgery within 7 days;
- Recent active bleeding from a major organ;
- Platelets < 50,000/mL;
- Pregnancy.
2.5. Anticoagulation
2.6. Ultrasound-Facilitated and Catheter-Directed Low-Dose Fibrinolysis
2.7. Follow-Up
3. Results
3.1. Outcomes
- The primary efficacy was the mean difference in the right and left pulmonary artery pressures measured invasively using the fibrinolysis delivery catheter at baseline to 6 h after initiation of therapy in the intensive care unit.
- A secondary efficacy outcome was the difference in RV/LV diameter ratio, right ventricular function, and pulmonary artery systolic (measured noninvasively) from baseline to 48 h after the initiation of the procedure and at 90 days.
- The primary safety outcome was death, as well as major and minor bleeding within 48 h of the initiation of the procedure. Bleeding events were classified by the GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) bleeding criteria [9]. Severe or life-threatening bleeding was defined as either intracranial hemorrhage or bleeding that caused hemodynamic compromise and required intervention. Moderate bleeding required a blood transfusion without resulting in hemodynamic compromise. Mild bleeding did not meet criteria for either severe/life-threatening or moderate bleeding. In addition, we assessed all-cause mortality at hospital discharge and through 90 days, the symptomatic recurrent PE up to 90 days after the initiation of the procedure, and procedural complications. Mortality was further classified as related to cancer, PE, stroke, or other causes.
- The secondary safety outcome evaluated quality of life assessment using the German version of the EQ-5D-3L questionnaire [10].
3.2. Baseline Demographics and Clinical Characteristics
3.3. Baseline Characteristics of Pulmonary Embolism
3.4. Procedural Characteristics
4. Discussion
- (1)
- Precapillary pulmonary hypertension (defined as a mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg with a capillary pulmonary pressure ≤15 mmHg) assessed by right cardiac catheterization;
- (2)
- At least one segmental perfusion defect evident on ventilation scan/perfusion;
- (3)
- Signs of chronic thromboembolism on chest CT angiography or conventional pulmonary angiography (ring stenosis, chronic lesions, and/or complete occlusions) in patients receiving effective anticoagulation therapy for at least three months after a pulmonary embolism episode [11]. A recent detected soluble suppression of tumorigenesis 2 (sST2) concentration is higher in CTEPH patients treated with balloon pulmonary angioplasty (BPA) with complications in the postprocedural period. Thus, it could be helpful as an additional noninvasive marker for complications and risk stratification in patients undergoing BPA [12].
Study Limitations and Strengths
5. Conclusions
6. Clinical Perspectives
6.1. Competency in Medical Knowledge
6.2. Translational Outlook 1
6.3. Translational Outlook 2
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RVEDD | Right ventricle end diastolic diameter |
| LVEDD | Left ventricle end diastolic diameter |
| SPAP | Systolic pulmonary artery pressure |
| TAPSE | Tricuspid annular plane systolic excursion |
| ECG | An electrocardiogram |
| AF | Atrial fibrillation |
| RBBB | Right bundle branch block |
| CGY/cm2 | The absorbed dose multiplied by the area irradiated, expressed in gray-centimeters squared |
| EQ-5D-3L | European quality–five dimension–three level |
| USCDT | Ultrasound catheter-directed therapy |
References
- Hobohm, L.; Lankeit, M. Lungenembolie. Pneumologie 2021, 75, 800–818. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, H.; Iordanidis, D.; Mpahara, A.; Mademli, M.; Birmpa, D.; Argentos, S.; Benas, D.; Trivilou, P.; Anagnostopoulos, K.; Mayer, E.; et al. Giant Pulmonary Artery Thrombotic Material, Due to Chronic Thromboembolic Pulmonary Hypertension, Mimics Pulmonary Artery Sarcoma. Medicina 2021, 57, 992. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becat, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.D.; Kabrhel, C.; Courtney, D.M.; Naydenov, S.; Wood, T.; Rosovsky, R.; Rosenfield, K.; Giri, J.; Balan, P.; Barnes, G.; et al. Diversity in the Pulmonary Embolism Response Team Model: An Organizational Survey of the National PERT Consortium Members. Chest 2016, 150, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N.; Boekstegers, P.; Müller, O.J.; Kupatt, C.; Beyer-Westendorf, J.; Heitzer, T.; Tebbe, U.; Horstkotte, J.; Müller, R.; Blessing, E.; et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation 2014, 129, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.W.; Blinc, A.; Lee, S.; Cox, C. Ultrasound Accelerates Transport of Recombinant Tissue Plasminogen Activator into Clots. Ultrasound Med. Biol. 1995, 21, 419–424. [Google Scholar] [CrossRef]
- GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N. Engl. J. Med. 1993, 329, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; von der Schulenburg, J.-M.G.; Greiner, W. German Value Set for the EQ-5D-5L. Pharmacoeconomics 2018, 36, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Baratella, E.; Caforio, G.; Confalonieri, P.; Wade, B.; Marrocchio, C.; Geri, P.; Pozzan, R.; Andrisano, A.G.; Cova, M.A.; et al. Chronic Thromboembolic Pulmonary Hypertension: An Update. Diagnostics 2022, 12, 235. [Google Scholar] [CrossRef]
- Banaszkiewicz, M.; Pietrasik, A.; Florczyk, M.; Kędzierski, P.; Piłka, M.; Mańczak, R.; Kochman, J.; Opolski, G.; Torbicki, A.; Kurzyna, M.; et al. Soluble ST2 as a Biomarker for Early Complications in Patients with Chronic Thromboembolic Pulmonary Hypertension Treated with Balloon Pulmonary Angioplasty. Diagnostics 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.; Hohlfelder, B.; Jaff, M.R.; Ouriel, K.; Engelhardt, T.C.; Sterling, K.M.; Jones, N.J.; Gurley, J.C.; Bhatheja, R.; Kennedy, R.J.; et al. SEATTLE II Investigators. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmo. JACC Cardiovasc. Interv. 2015, 8, 1382–1392. [Google Scholar] [CrossRef]
- Tapson, V.F.; Sterling, K.; Jones, N.; Elder, M.; Tripathy, U.; Brower, J.; Maholic, R.L.; Ross, C.B.; Natarajan, K.; Fong, P.; et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc. Interv. 2018, 11, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Brass, L.M.; Lichtman, J.H.; Wang, Y.; Gurwitz, J.H.; Radford, M.J.; Krumholz, H.M. Intracranial Hemorrhage Associated With Thrombolytic Therapy for Elderly Patients With Acute Myocardial Infarction. Stroke 2000, 31, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Vicaut, E.; Danays, T.; Agnelli, G.; Becattini, C.; Beyer-Westendorf, J.; Bluhmki, E.; Bouvaist, H.; Brenner, B.; Couturaud, F.; et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N. Engl. J. Med. 2014, 370, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chakraborty, A.; Weinberg, I.; Kadakia, M.; Wilensky, R.L.; Sardar, P.; Kumbhani, D.J.; Mukherjee, D.; Jaff, M.R.; Giri, J. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: A meta-analysis. JAMA 2014, 311, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Marti, C.; John, G.; Konstantinides, S.; Combescure, C.; Sanchez, O.; Lankeit, M.; Meyer, G.; Perrier, A. Systemic thrombolytic therapy for acute pulmonary embolism: A systematic review and meta-analysis. Eur. Heart J. 2015, 36, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.; Rosenfield, K.U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT04790370 (accessed on 30 June 2022).



| Age | 66.8 ± 14.7 |
| Height in cm | 173.8 ± 9.7 |
| Weight in Kg | 88.5 ± 21.3 |
| Body mass index kg/m2 | 29.0 ± 6.8 |
| Female | 23 (51.1) |
| Ethnicity/race | 45 (100) Caucasian |
| Comorbid conditions | |
| Hypertension | 18 (41) |
| Diabetes Mellitus | 2 (4.4) |
| Smoking | 3 (6.7) |
| Obesity | 32 (73) |
| Immobility | 7 (16) |
| Surgery | 2 (4.4) |
| Previous TVT | 2 (4.4) |
| Previous PE | 1 (2.2) |
| Atherosclerotic cardiovascular disease | 3 (6.7) |
| Family history of venous thromboembolism | 3 (6.7) |
| Stroke | 5 (11) |
| Active cancer | 10 (22) |
| Chronic pulmonary disease | 9 (20) |
| Renal insufficiency (GFR < 90 mL/min) | 5 (11.1) |
| Hyperlipidaemia | 6 (13.3) |
| Rheumatic disease | 4 (8.8) |
| Estrogen use | 2 (4.4) |
| Congenital Blood disease | 1 (2.2) |
| Clinical presentation of PE | |
| Dyspnoea II | 1 (2.2) |
| Dyspnoea III | 24 (55) |
| Dyspnoea IV | 8 (18) |
| Chest pain | 1 (2.2) |
| Cardiopulmonary resuscitation | 3 (6.7) |
| Syncope | 3 (6.7) |
| Shock | 5 (11) |
| Palpitation | 7 (16) |
| Dizziness | 2 (5) |
| Cough | 1 (2.2) |
| Duration of symptoms/ days (maximum) | <14 days |
| PE risk stratification | |
| High risk | 5 (11) |
| Intermediate high risk | 40 (88) |
| Diagnosed TVT | 22 (48), right 11 (24), left 11 (24) |
| Oxygen saturation | 88.8 ± 7.7 |
| Breathing frequency | 21.2 ± 5 |
| Heart frequency before therapy | 97.3 ± 17.3 |
| Heart frequency after therapy | 81.2 ± 7.1 (mean difference 16) |
| Blood pressure/ mmHg | |
| Systole | 137.6 ± 25.2 |
| Diastole | 82.4 ± 16.6 |
| Computer tomography-PE | |
| Unilateral | |
| Right | 3 (6.7) |
| Left | 1 (2.2) |
| Bilateral | 41 (91.1) |
| ECG | |
| Sinus rhythm | 20 (44) |
| Sinus tachycardia | 16 (36) |
| Atrial flatter | 2 (4.4) |
| Atrial fibrillation | 7 (15.6) |
| Right bundle branch block | 2 (4.4) |
| Laboratory Parameters | |
| Troponin, ng/mL | 504 |
| n terminal-B-Type Natriuretic peptide, pg/ml | 4451 |
| Lactate | 2.6 ± 2.1 |
| Hemoglobin in g/dl before | 12.9 ± 2.2 |
| Hemoglobin in g/dl after Therapy | 11.2 ± 2.1 (mean difference 1.72, (1.27–2.17)) |
| Successful Device Placement | 86 (100) |
| No. of devices per patient | |
| 1 | 4 (8.9) |
| 2 | 41 (91.1) |
| Access site | |
| Right femoral vein | 41 (91.1) |
| Left femoral vein | 4 (8.9) |
| Completed infusion of t-PA | 43 (95.5) |
| Duration of investigation/Minute | 9 (3–35) |
| Radiation dosage in cGY/cm2 | 1405 (305–3916) |
| Outcome | |||
|---|---|---|---|
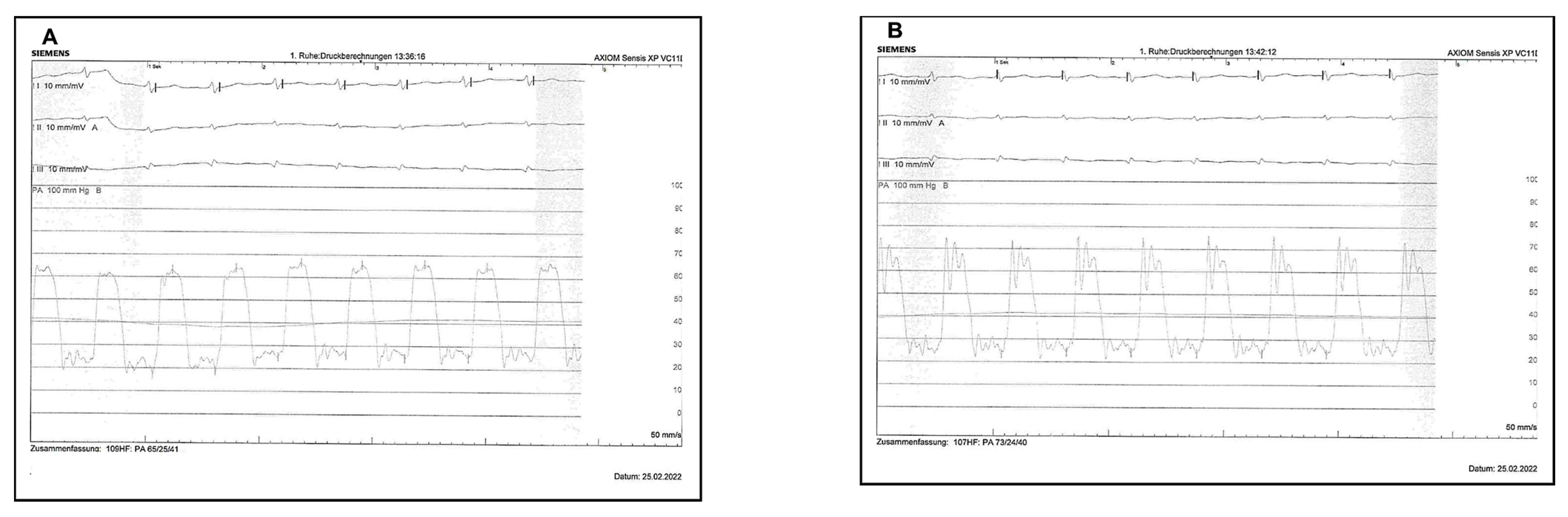
| PA/mmHg | Baseline | 6 h | Mean Difference |
| Right systole | 43.38 ± 14.66 | 27.62 ± 10.31 | 15.76 ± 14 (11.26–20.26) |
| Right diastole | 19.45 ± 7.98 | 13.45 ± 7.27 | 6.0 ± 8.1 (3.2–8.80) |
| Right mean | 27.90 ± 9.05 | 18.10 ± 6.92 | 9.8 ± 9.9 (6.71–12.91) |
| Left systole | 44.89 ± 14.31 | 29.49 ± 12.03 | 15.40 ± 14.4 (10.45–20.35) |
| Left diastole | 21.44 ± 7.28 | 14.97 ± 7.35 | 6.47 ± 9.3 (3.22–9.72) |
| Left mean | 29.91 ± 8.53 | 20.32 ± 8.46 | 9.59 ± 9.4 (6.28–12.90) |
| Outcome | |||||
|---|---|---|---|---|---|
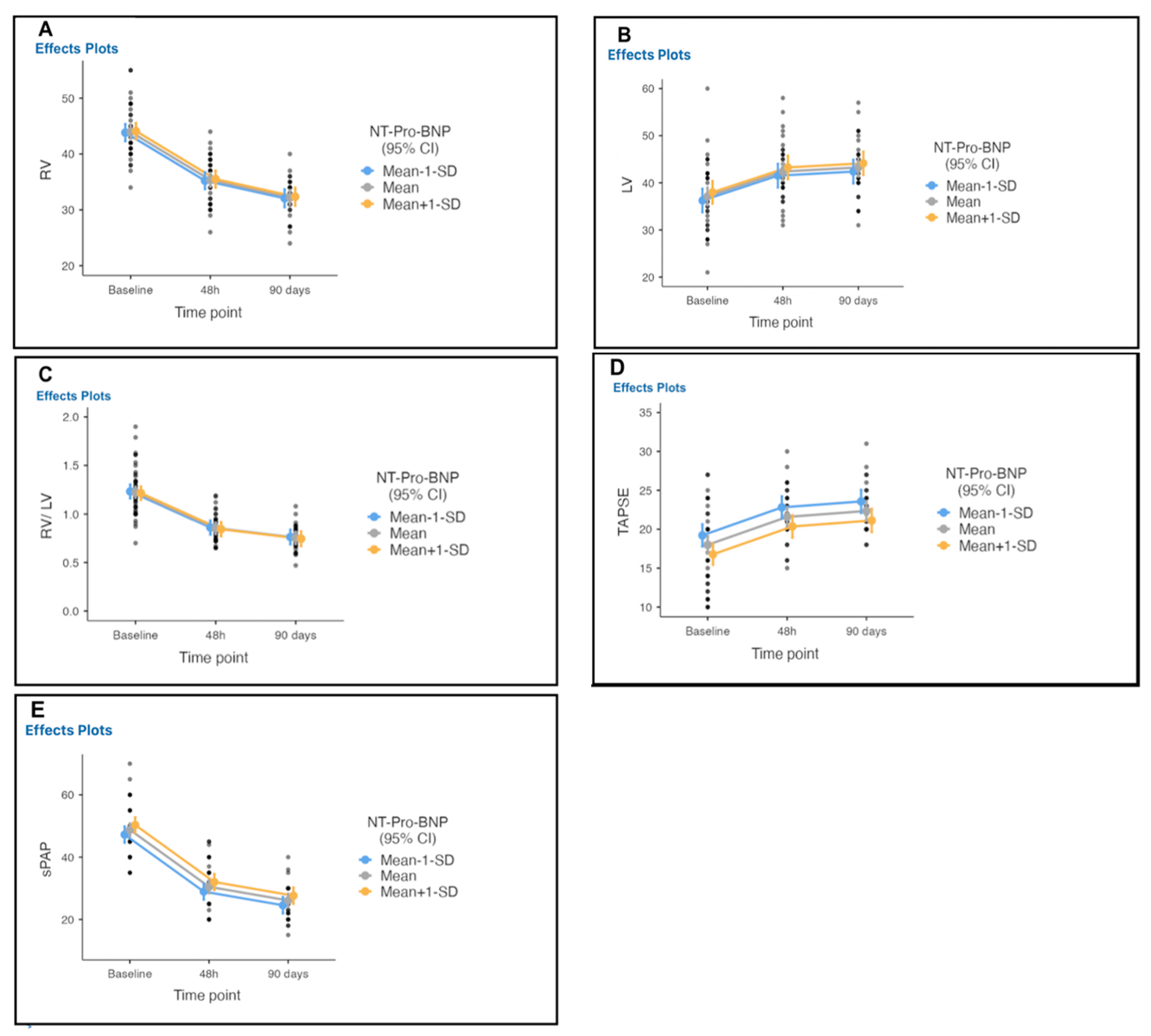
| Echocardiography | Baseline | 48 h | Difference from the Baseline | 90 Days | Difference from 48 h |
| RVEDD, mm | 43.49 ± 4.44 | 35.1 ± 4.1 | 8.4 | 32 ± 3.3 | 3.1 |
| LVEDD, mm | 37.13 ± 7.14 | 42.38 ± 5.99 | −5 | 43.26 ± 5.67 | −6 |
| RV/LV ratio | 1.24 ± 0.28 | 0.85 ± 0.12 | 0.38 (0.27–0.50) | 0.76 ± 0.13 | 0.48 (0.37–0.59) |
| TAPSE, mm | 17.8 ± 5.2 | 21.4 ± 3.2 | −3.6 | 22.3 ± 2.9 | −0.9 |
| SPAP, mmHg | 49 ± 7.8 | 30.1 ± 7.2 | 19 | 26 ± 5.4 | 4 |
| Length of Stay/Days | 6 (1–17) |
| In-hospital death, | 3 (6.7) |
| 90-day mortality, | 5 (11) |
| Serious and severe adverse events potentially related to device | 0 |
| Serious and severe adverse events potentially related to t-PA, | 1 (2.2) |
| Major bleeding within 90 days | |
| GUSTO moderate | 4 (8.8) |
| GUSTO severe | 1 (2.2) |
| Intracranial hemorrhage | 0 |
| EQ-5D-3L, mean ± SD | 0.97 ± 0.05 |
| Patient reported subjective clinical improvement; /%, mean ± SD | 78.71 ± 11.8 |
| No complication (n, %) | 34 (75.6) |
| 6MWT (n, %) | 42 (93.3) |
| Bleeding Event | Site of Bleed | Transfused Blood Products | Hg b (mg/dL) Baseline-24 h | Outcome and Complications |
|---|---|---|---|---|
| Access site hematoma | 4 (8.9) | 11.6–11.3 12.8–10.1 18.7–15.2 17.6–14.1 | Recovered Recovered Recovered Recovered | |
| Access site pseudoaneurysm | 3 (6.7) | 14–11.4 13.4–12.5 15–13 | Thrombin injection Recovered Recovered Recovered | |
| Access site bleeding | 1 (2.2) Retroperitoneal | 2-U PRBCs | 15.5–6.3 | Died of PE, comorbidity, and Shock |
| Mucosal bleeding | 1 (2.2), Unclear | 2-U PRBCs | 7.5–6.8 | Died of PE and comorbidity |
| 1 (2.2) Rectal hematoma | 12.1–10.8 | Rectal drainage, Recovered | ||
| Left Hemothorax | 1 (2.2) 2nd day after Lung resection, under Heparin | 1-U PRBCs | 11–8.7 | Thorax drainage, Recovered |
| Left Knee | 1 (2.2) Left Knee | 14–11.4 | Knee Joint drainage, Recovered | |
| Anemia | 2 (4.4) | 2-U PRBCs | Recovered |
| Study | Ultima Trial (2014) | Seatlle II Trial (2015) | Optalysis Trial (2018) | Current Study (2022) |
|---|---|---|---|---|
| Nummer of Patient | n = 59 30 in USAT 29 Heparin | n = 149 single arm | n = 101 Arm 1 n = 28 Arm 2 n = 27 Arm 3 n = 28 Arm 4 n = 18 | n = 45 single arm |
| Duration of therapy | 15 ± 1 h | 24 h (unilateral) 12 h (bilateral) | 2–6 h | 6 h |
| t-PA total dosis in unilateral PE | 10 mg | 24 mg | 4–12 mg | 6 mg |
| t-PA total dosis In bilateral PE | 20 mg | 24 mg | 8–24 mg | 12 mg |
| Safety: Major bleeding | No major bleeding | 10% major bleeding | 4% major bleeding | 2% major bleeding |
| Intracraniel heamorrahge | No | No | 2 Cases | No |
| Effectiveness | Pulmonary artery pressure (s/d/m) by right heart catheter | Pulmonary artery systolic pressure by right heart catheter | No | Pulmonary artery pressure (s/d/m) by drug delivery catheter |
| RV/LV ratio | RV/LV ratio | RV/LV ratio | RV/LV ratio | |
| No | Control CTPA | Control CTPA | No | |
| Echocardiography | Echocardiography | Echocardiography | Echocardiography | |
| QoL | QoL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhakim, A.; Knauth, M.; Elhakim, M.; Böhmer, U.; Patzelt, J.; Radke, P. Using a Fibrinolysis Delivery Catheter in Pulmonary Embolism Treatment for Measurement of Pulmonary Artery Hemodynamics. Adv. Respir. Med. 2022, 90, 483-499. https://doi.org/10.3390/arm90060055
Elhakim A, Knauth M, Elhakim M, Böhmer U, Patzelt J, Radke P. Using a Fibrinolysis Delivery Catheter in Pulmonary Embolism Treatment for Measurement of Pulmonary Artery Hemodynamics. Advances in Respiratory Medicine. 2022; 90(6):483-499. https://doi.org/10.3390/arm90060055
Chicago/Turabian StyleElhakim, Abdelrahman, Martin Knauth, Mohamed Elhakim, Ulrich Böhmer, Johannes Patzelt, and Peter Radke. 2022. "Using a Fibrinolysis Delivery Catheter in Pulmonary Embolism Treatment for Measurement of Pulmonary Artery Hemodynamics" Advances in Respiratory Medicine 90, no. 6: 483-499. https://doi.org/10.3390/arm90060055
APA StyleElhakim, A., Knauth, M., Elhakim, M., Böhmer, U., Patzelt, J., & Radke, P. (2022). Using a Fibrinolysis Delivery Catheter in Pulmonary Embolism Treatment for Measurement of Pulmonary Artery Hemodynamics. Advances in Respiratory Medicine, 90(6), 483-499. https://doi.org/10.3390/arm90060055





