Bridging Bench to Bedside for Brain Health: Non-Invasive Brain Stimulation for Neurodegenerative Diseases
Abstract
1. Introduction
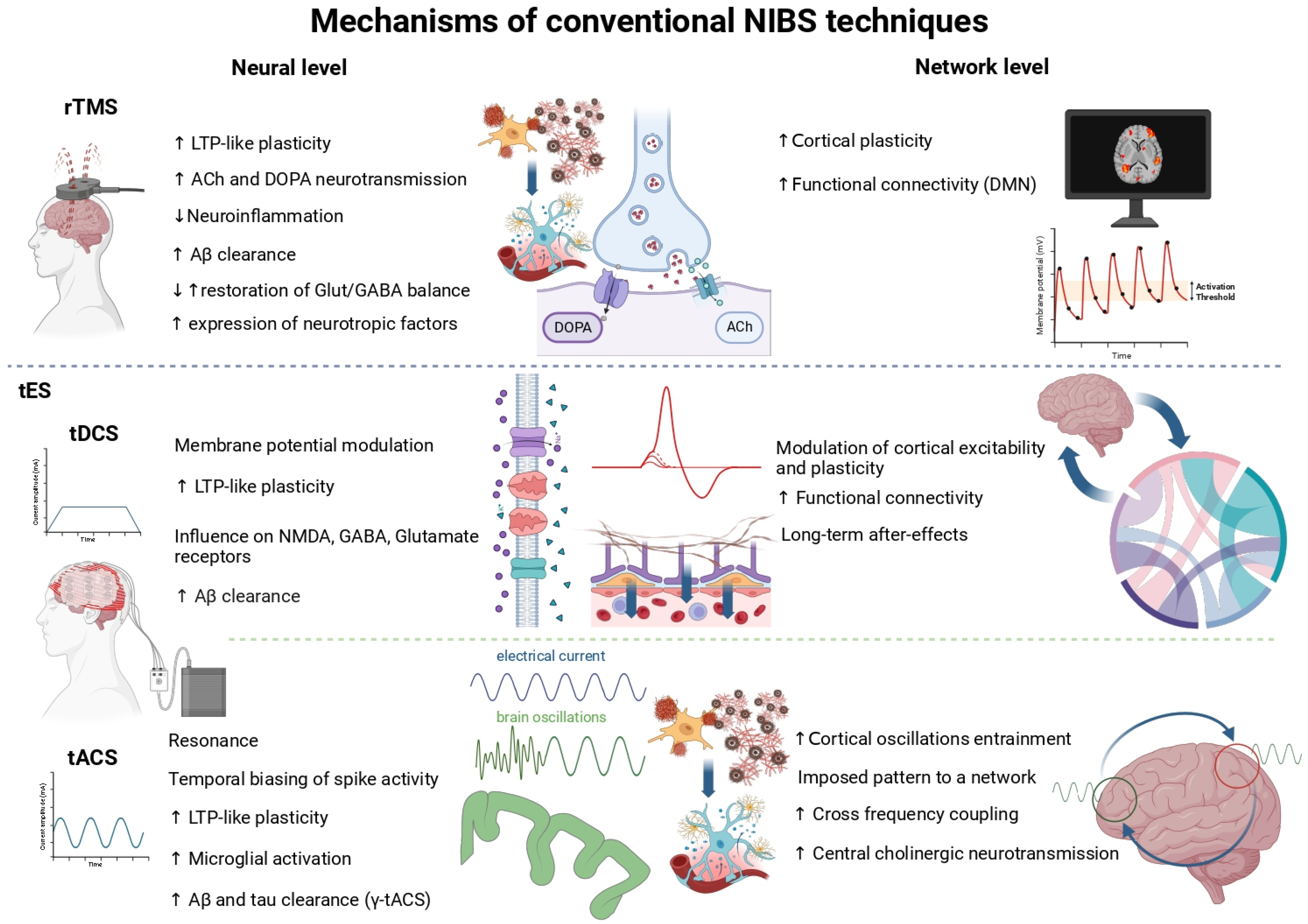
2. NIBS as a Therapeutic Tool for Cognitive Rehabilitation in Dementias
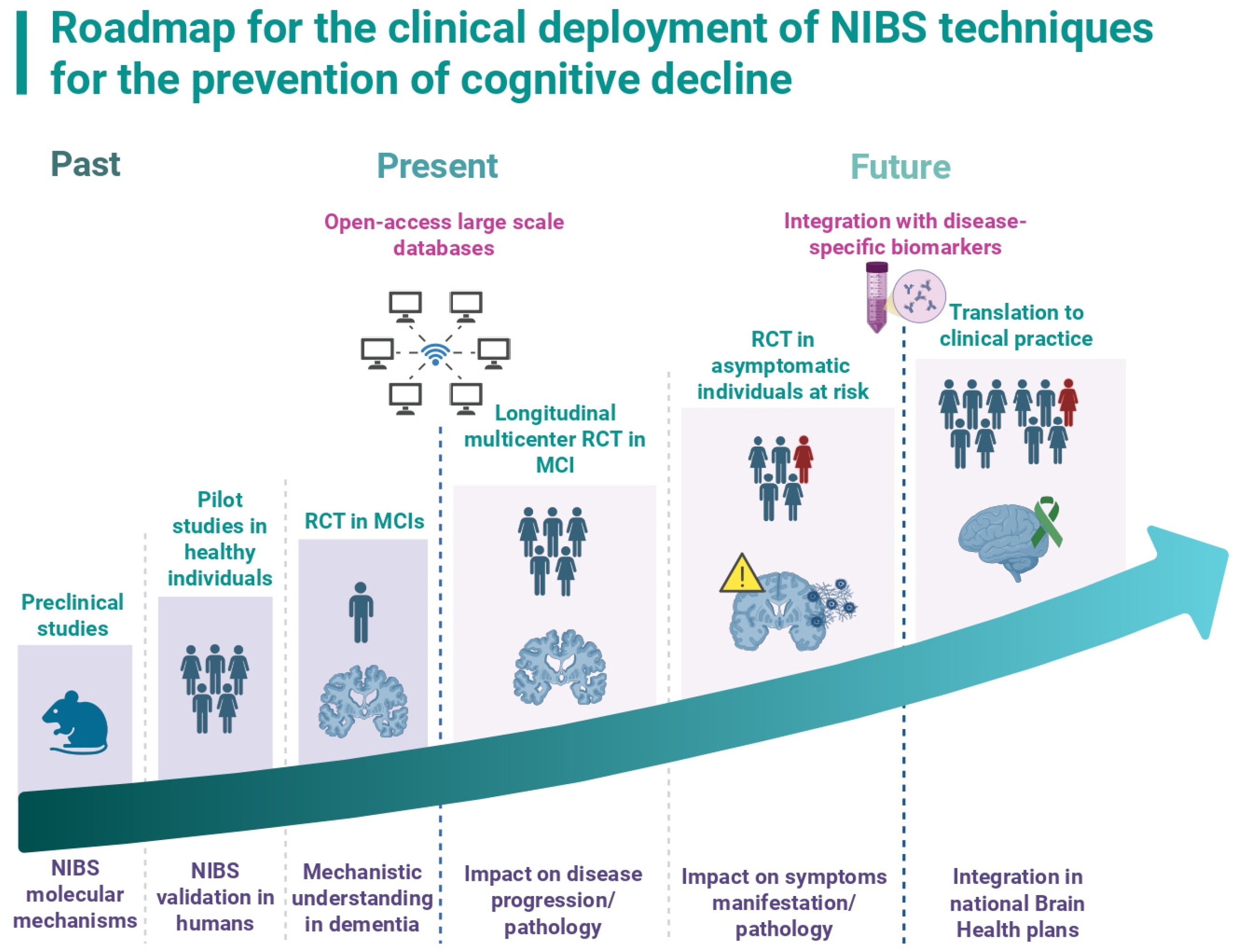
3. Next Steps for Clinical Implementation of NIBS in the Treatment and Prevention of Dementia
3.1. Building Large, Multicenter, Population-Based Longitudinal Neurophysiological Databases
3.2. Understanding the Differential Impact of NIBS on Specific Types of Dementias and Probing Their Values as Possible Disease-Modifying Therapies
3.3. Validate the Use of NIBS for the Prevention of Cognitive Decline in At-Risk Populations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Altomare, D.; Ribaldi, F.; Villain, N.; Brayne, C.; Mukadam, N.; Abramowicz, M.; Barkhof, F.; Berthier, M.; Bieler-Aeschlimann, M.; et al. Dementia prevention in memory clinics: Recommendations from the European task force for brain health services. Lancet Reg. Health—Eur. 2023, 26, 100576. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Delrieu, J.; Payoux, P.; Carrié, I.; Cantet, C.; Weiner, M.; Vellas, B.; Andrieu, S. Multidomain intervention and/or omega-3 in nondemented elderly subjects according to amyloid status. Alzheimer’s Dement. 2019, 15, 1392–1401. [Google Scholar] [CrossRef]
- Chhetri, J.K.; De Souto Barreto, P.; Cantet, C.; Pothier, K.; Cesari, M.; Andrieu, S.; Coley, N.; Vellas, B. Effects of a 3-Year Multi-Domain Intervention with or without Omega-3 Supplementation on Cognitive Functions in Older Subjects with Increased CAIDE Dementia Scores. J. Alzheimer’s Dis. 2018, 64, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Villain, N.; Ayton, S.; Ackley, S.F.; Planche, V.; Howard, R.; Thambisetty, M. Key questions for the evaluation of anti-amyloid immunotherapies for Alzheimer’s disease. Brain Commun. 2023, 5, fcad175. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Donohue, M.C.; Raman, R.; Rafii, M.S.; Johnson, K.; Masters, C.L.; Van Dyck, C.; Iwatsubo, T.; Marshall, G.A.; Yaari, R.; et al. Trial of Solanezumab in Preclinical Alzheimer’s Disease. N. Engl. J. Med. 2023, 389, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- AHEAD 3-45 Study: A Phase 3 Study Evaluating the Efficacy and Safety of Aducanumab in Preclinical Alzheimer’s Disease. ClinicalTrials.gov, Registration No. NCT04465898. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04465898 (accessed on 22 August 2025).
- Wei, N.; Liu, H.; Ye, W.; Xu, S.; Lu, C.; Dai, A.; Hou, T.; Zeng, X.; Wu, J.; Chen, J. Repetitive transcranial magnetic stimulation may be superior to drug therapy in the treatment of Alzheimer’s disease: A systematic review and Bayesian network meta-analysis. CNS Neurosci. Ther. 2023, 29, 2912–2924. [Google Scholar] [CrossRef]
- Draaisma, L.R.; Wessel, M.J.; Hummel, F.C. Non-invasive brain stimulation to enhance cognitive rehabilitation after stroke. Neurosci. Lett. 2020, 719, 133678. [Google Scholar] [CrossRef]
- Lanni, I.; Chiacchierini, G.; Papagno, C.; Santangelo, V.; Campolongo, P. Treating Alzheimer’s disease with brain stimulation: From preclinical models to non-invasive stimulation in humans. Neurosci. Biobehav. Rev. 2024, 165, 105831. [Google Scholar] [CrossRef]
- Antal, A.; Luber, B.; Brem, A.K.; Bikson, M.; Brunoni, A.R.; Cohen Kadosh, R.; Dubljević, V.; Fecteau, S.; Ferreri, F.; Flöel, A.; et al. Non-invasive brain stimulation and neuroenhancement. Clin. Neurophysiol. Pract. 2022, 7, 146–165. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.; Huang, H.; Chen, X.; Zeng, P.; Xu, Y. Comparative efficacy of non-invasive brain stimulation on cognition function in patients with mild cognitive impairment: A systematic review and network meta-analysis. Ageing Res. Rev. 2024, 101, 102508. [Google Scholar] [CrossRef]
- Cespón, J.; Miniussi, C.; Pellicciari, M.C. Interventional programmes to improve cognition during healthy and pathological ageing: Cortical modulations and evidence for brain plasticity. Ageing Res. Rev. 2018, 43, 81–98. [Google Scholar] [CrossRef]
- Nissim, N.R.; Pham, D.V.H.; Poddar, T.; Blutt, E.; Hamilton, R.H. The impact of gamma transcranial alternating current stimulation (tACS) on cognitive and memory processes in patients with mild cognitive impairment or Alzheimer’s disease: A literature review. Brain Stimul. 2023, 16, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Zimerman, M.; Nitsch, M.; Giraux, P.; Gerloff, C.; Cohen, L.G.; Hummel, F.C. Neuroenhancement of the aging brain: Restoring skill acquisition in old subjects. Ann. Neurol. 2013, 73, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.D.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Serap Mokul nery, E.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. J. Am. Med. Assoc. 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; Von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Ross, E.L.; Weinberg, M.S.; Arnold, S.E. Cost-effectiveness of Aducanumab and Donanemab for Early Alzheimer Disease in the US. JAMA Neurol. 2022, 79, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Apostolova, L.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R.C.; et al. Lecanemab: Appropriate Use Recommendations. J. Prev. Alzheimer’s Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef]
- Tatti, E.; Rossi, S.; Innocenti, I.; Rossi, A.; Santarnecchi, E. Non-invasive brain stimulation of the aging brain: State of the art and future perspectives. Ageing Res. Rev. 2016, 29, 66–89. [Google Scholar] [CrossRef]
- Reinhart, R.M.G.; Nguyen, J.A. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat. Neurosci. 2019, 22, 820–827. [Google Scholar] [CrossRef]
- Menardi, A.; Dotti, L.; Ambrosini, E.; Vallesi, A. Transcranial magnetic stimulation treatment in Alzheimer’s disease: A meta-analysis of its efficacy as a function of protocol characteristics and degree of personalization. J. Neurol. 2022, 269, 5283–5301. [Google Scholar] [CrossRef] [PubMed]
- Šimko, P.; Kent, J.A.; Rektorova, I. Is non-invasive brain stimulation effective for cognitive enhancement in Alzheimer’s disease? An updated meta-analysis. Clin. Neurophysiol. 2022, 144, 23–40. [Google Scholar] [CrossRef]
- Gu, L.; Xu, H.; Qian, F. Effects of Non-Invasive Brain Stimulation on Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 410–424. [Google Scholar] [CrossRef]
- Vacas, S.M.; Stella, F.; Loureiro, J.C.; Simões do Couto, F.; Oliveira-Maia, A.J.; Forlenza, O.V. Noninvasive brain stimulation for behavioural and psychological symptoms of dementia: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2019, 34, 1336–1345. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Yu, X. The role of noninvasive brain stimulation for behavioral and psychological symptoms of dementia: A systematic review and meta-analysis. Neurol. Sci. 2020, 41, 1063–1074. [Google Scholar] [CrossRef]
- Chu, C.S.; Li, C.T.; Brunoni, A.R.; Yang, F.C.; Tseng, P.T.; Tu, Y.K.; Stubbs, B.; Carvalho, A.F.; Thompson, T.; Rajji, T.K.; et al. Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: A component network meta-analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Teselink, J.; Bawa, K.K.; Koo, G.K.; Sankhe, K.; Liu, C.S.; Rapoport, M.; Oh, P.; Marzolini, S.; Gallagher, D.; Swardfager, W.; et al. Efficacy of non-invasive brain stimulation on global cognition and neuropsychiatric symptoms in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review. Ageing Res. Rev. 2021, 72, 101499. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Bonnì, S.; Pellicciari, M.C.; Casula, E.P.; Mancini, M.; Esposito, R.; Ponzo, V.; Picazio, S.; Di Lorenzo, F.; Serra, L.; et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 2018, 169, 302–311. [Google Scholar] [CrossRef]
- Koch, G.; Casula, E.P.; Bonnì, S.; Borghi, I.; Assogna, M.; Di Lorenzo, F.; Esposito, R.; Maiella, M.; D’Acunto, A.; Ferraresi, M.; et al. Effects of 52 weeks of precuneus rTMS in Alzheimer’s disease patients: A randomized trial. Alzheimer’s Res. Ther. 2025, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, N.; Hu, G.; Nousayhah, A.; Xue, C.; Qi, W.; Xu, W.; Chen, S.; Rao, J.; Liu, W.; et al. rTMS modulates precuneus-hippocampal subregion circuit in patients with subjective cognitive decline. Aging 2020, 13, 1314–1331. [Google Scholar] [CrossRef]
- Liang, X.; Xue, C.; Zheng, D.; Yuan, Q.; Qi, W.; Ruan, Y.; Chen, S.; Song, Y.; Wu, H.; Lu, X.; et al. Repetitive transcranial magnetic stimulation regulates effective connectivity patterns of brain networks in the spectrum of preclinical Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1343926. [Google Scholar] [CrossRef]
- Antonioni, A.; Martorana, A.; Santarnecchi, E.; Hampel, H.; Koch, G. The neurobiological foundation of effective repetitive transcranial magnetic brain stimulation in Alzheimer’s disease. Alzheimer’s Dement. 2025, 21, e70337. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Lv, C.; Pang, J.; Han, B.; Wang, M.; Gen, Y. The role of hippocampal structural synaptic plasticity in repetitive transcranial magnetic stimulation to improve cognitive function in male SAMP8 mice. Cell. Physiol. Biochem. 2017, 41, 137–144. [Google Scholar] [CrossRef]
- Cao, H.; Zuo, C.; Gu, Z.; Huang, Y.; Yang, Y.; Zhu, L.; Jiang, Y.; Wang, F. High frequency repetitive transcranial magnetic stimulation alleviates cognitive deficits in 3xTg-AD mice by modulating the PI3K/Akt/GLT-1 Axis. Redox Biol. 2022, 54, 102354. [Google Scholar] [CrossRef]
- Windy McNerney, M.; Heath, A.; Narayanan, S.K.; Yesavage, J. Repetitive Transcranial Magnetic Stimulation Improves Brain-Derived Neurotrophic Factor and Cholinergic Signaling in the 3xTgAD Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 86, 499–507. [Google Scholar] [CrossRef]
- Choung, J.S.; Kim, J.M.; Ko, M.H.; Cho, D.S.; Kim, M.Y. Therapeutic efficacy of repetitive transcranial magnetic stimulation in an animal model of Alzheimer’s disease. Sci. Rep. 2021, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, C.; Huang, Z.; Ji, X.; Cui, R.; Kang, Y.; Zhang, G.; Wang, Y.; Zhang, T. Repetitive Transcranial Magnetic Stimulation-Mediated Neuroprotection in the 5xFAD Mouse Model of Alzheimer’s Disease Through GABRG2 and SNAP25 Modulation. Mol. Neurobiol. 2025, 62, 1971–1997. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Liang, W.; Ba, F. Administration of repetitive transcranial magnetic stimulation attenuates A β 1-42 -induced Alzheimer’s disease in mice by activating β-catenin signaling. Biomed Res. Int. 2019, 2019, 1431760. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Lei, B.; Zhu, Y.; Fang, X.; Liao, L.; Chen, D.; Gao, C. Repetitive Transcranial Magnetic Stimulation Decreases Serum Amyloid-β and Increases Ectodomain of p75 Neurotrophin Receptor in Patients with Alzheimer’s Disease. J. Integr. Neurosci. 2022, 21, 140. [Google Scholar] [CrossRef]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Memory Enhancement: Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef]
- Vidal-Piñeiro, D.; Martín-Trias, P.; Falcón, C.; Bargalló, N.; Clemente, I.C.; Valls-Solé, J.; Junqué, C.; Pascual-Leone, A.; Bartres-Faz, D. Neurochemical modulation in posteromedial default-mode network cortex induced by transcranial magnetic stimulation. Brain Stimul. 2015, 8, 937–944. [Google Scholar] [CrossRef]
- Li, X.; Qi, G.; Yu, C.; Lian, G.; Zheng, H.; Wu, S.; Yuan, T.F.; Zhou, D. Cortical plasticity is correlated with cognitive improvement in Alzheimer’s disease patients after rTMS treatment. Brain Stimul. 2021, 14, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Dayan, E.; Censor, N.; Buch, E.R.; Sandrini, M.; Cohen, L.G. Noninvasive brain stimulation: From physiology to network dynamics and back. Nat. Neurosci. 2013, 16, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Cantoni, V.; Cotelli, M.S.; Cotelli, M.; Brattini, C.; Datta, A.; Thomas, C.; Santarnecchi, E.; Pascual-Leone, A.; Borroni, B. Exposure to gamma tACS in Alzheimer’s disease: A randomized, double-blind, sham-controlled, crossover, pilot study. Brain Stimul. 2021, 14, 531–540. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Grassi, M.; Brechet, L.; Michel, C.M.; Datta, A.; Thomas, C.; Gazzina, S.; Cotelli, M.S.; Bianchi, M.; et al. Increasing Brain Gamma Activity Improves Episodic Memory and Restores Cholinergic Dysfunction in Alzheimer’s Disease. Ann. Neurol. 2022, 92, 322–334. [Google Scholar] [CrossRef]
- Dhaynaut, M.; Sprugnoli, G.; Cappon, D.; Macone, J.; Sanchez, J.S.; Normandin, M.D.; Guehl, N.J.; Koch, G.; Paciorek, R.; Connor, A.; et al. Impact of 40 Hz Transcranial Alternating Current Stimulation on Cerebral Tau Burden in Patients with Alzheimer’s Disease: A Case Series. J. Alzheimer’s Dis. 2022, 85, 1667–1676. [Google Scholar] [CrossRef]
- Majdi, A.; van Boekholdt, L.; Sadigh-Eteghad, S.; Mc Laughlin, M. A systematic review and meta-analysis of transcranial direct-current stimulation effects on cognitive function in patients with Alzheimer’s disease. Mol. Psychiatry 2022, 27, 2000–2009. [Google Scholar] [CrossRef]
- Wang, H.; Qin, N.; Maimaitiaili, D.; Wu, J.; Wang, S.; Zhou, Y.; Lu, J.; Li, Y. Transcranial electrical stimulation as a therapeutic strategy for Alzheimer’s disease: Current uses and challenges. J. Alzheimer’s Dis. 2025, 104, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ardolino, G.; Bossi, B.; Barbieri, S.; Priori, A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J. Physiol. 2005, 568, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N. Action mechanisms of transcranial direct current stimulation in Alzheimer’s disease and memory loss. Front. Psychiatry 2012, 3, 48. [Google Scholar] [CrossRef]
- Paulus, W. Chapter 75 Outlasting excitability shifts induced by direct current stimulation of the human brain. Suppl. Clin. Neurophysiol. 2004, 57, 708–714. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, H.; Yan, X.; Wu, Y.; Wei, G.; Wu, X.; Tian, X.; Xiong, Y.; Wu, G.; Wen, H. Transcranial Direct Current Stimulation Alleviates Neurovascular Unit Dysfunction in Mice With Preclinical Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 857415. [Google Scholar] [CrossRef]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.J.; Reiser, M.; Padberg, F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef] [PubMed]
- Pini, L.; Pizzini, F.B.; Boscolo-Galazzo, I.; Ferrari, C.; Galluzzi, S.; Cotelli, M.; Gobbi, E.; Cattaneo, A.; Cotelli, M.S.; Geroldi, C.; et al. Brain network modulation in Alzheimer’s and frontotemporal dementia with transcranial electrical stimulation. Neurobiol. Aging 2022, 111, 24–34. [Google Scholar] [CrossRef]
- Antonenko, D.; Fromm, A.E.; Thams, F.; Kuzmina, A.; Backhaus, M.; Knochenhauer, E.; Li, S.C.; Grittner, U.; Flöel, A. Cognitive training and brain stimulation in patients with cognitive impairment: A randomized controlled trial. Alzheimer’s Res. Ther. 2024, 16, 6. [Google Scholar] [CrossRef]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef]
- Vossen, A.; Gross, J.; Thut, G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (a-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 2015, 8, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Adaikkan, C.; Middleton, S.J.; Marco, A.; Pao, P.C.; Mathys, H.; Kim, D.N.W.; Gao, F.; Young, J.Z.; Suk, H.J.; Boyden, E.S.; et al. Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection. Neuron 2019, 102, 929–943.e8. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef]
- Martorell, A.J.; Paulson, A.L.; Suk, H.J.; Abdurrob, F.; Drummond, G.T.; Guan, W.; Young, J.Z.; Kim, D.N.W.; Kritskiy, O.; Barker, S.J.; et al. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell 2019, 177, 256–271.e22. [Google Scholar] [CrossRef]
- Duecker, F.; Sack, A.T. Rethinking the role of sham TMS. Front. Psychol. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, H.J.; Van Den Eynde, F.; Guillaume, S.; Hanif, E.L.; Stahl, D.; David, A.S.; Campbell, I.C.; Schmidt, U. Blinding success of rTMS applied to the dorsolateral prefrontal cortex in randomised sham-controlled trials: A systematic review. World J. Biol. Psychiatry 2011, 12, 240–248. [Google Scholar] [CrossRef]
- Sheffield, J.G.; Ramerpresad, S.; Brem, A.K.; Mansfield, K.; Orhan, U.; Dillard, M.; McKanna, J.; Plessow, F.; Thomposon, T.; Santarnecchi, E.; et al. Blinding efficacy and adverse events following repeated transcranial alternating current, direct current, and random noise stimulation. Cortex 2022, 154, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.M.; Oostendorp, T.F.; Stegeman, D.F. The coil orientation dependency of the electric field induced by TMS for M1 and other brain areas. J. Neuroeng. Rehabil. 2015, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage 2015, 109, 140–150. [Google Scholar] [CrossRef]
- Kasten, F.H.; Duecker, K.; Maack, M.C.; Meiser, A.; Herrmann, C.S. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat. Commun. 2019, 10, 5427. [Google Scholar] [CrossRef]
- Caulfield, K.A.; Fleischmann, H.H.; Cox, C.E.; Wolf, J.P.; George, M.S.; McTeague, L.M. Neuronavigation maximizes accuracy and precision in TMS positioning: Evidence from 11,230 distance, angle, and electric field modeling measurements. Brain Stimul. 2022, 15, 1192–1205. [Google Scholar] [CrossRef]
- Windel, F.; Gardier, R.M.M.; Fourchard, G.; Viñals, R.; Bavelier, D.; Padberg, F.J.; Rancans, E.; Bonne, O.; Nahum, M.; Thiran, J.P.; et al. Computer vision-based algorithm to sUppoRt coRrect electrode placemeNT (CURRENT) for home-based electric non-invasive brain stimulation. Clin. Neurophysiol. 2023, 153, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Yeagle, E.; Thielscher, A.; Schroeder, C.; Mehta, A.D.; Milham, M.P. On the importance of precise electrode placement for targeted transcranial electric stimulation. Neuroimage 2018, 181, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Casula, E.P.; Bonnì, S.; Borghi, I.; Assogna, M.; Minei, M.; Pellicciari, M.C.; Motta, C.; D’Acunto, A.; Porrazzini, F.; et al. Precuneus magnetic stimulation for Alzheimer’s disease: A randomized, sham-controlled trial. Brain 2022, 145, 3776–3786. [Google Scholar] [CrossRef]
- Deng, Z.D.; Lisanby, S.H.; Peterchev, A.V. Electric field depth-focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimul. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Wagner, T.; Fregni, F.; Fecteau, S.; Grodzinsky, A.; Zahn, M.; Pascual-Leone, A. Transcranial direct current stimulation: A computer-based human model study. Neuroimage 2007, 35, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e16. [Google Scholar] [CrossRef]
- Wessel, M.J.; Beanato, E.; Popa, T.; Windel, F.; Vassiliadis, P.; Menoud, P.; Beliaeva, V.; Violante, I.R.; Abderrahmane, H.; Dzialecka, P.; et al. Noninvasive theta-burst stimulation of the human striatum enhances striatal activity and motor skill learning. Nat. Neurosci. 2023, 26, 2005–2016. [Google Scholar] [CrossRef]
- Violante, I.R.; Alania, K.; Cassarà, A.M.; Neufeld, E.; Acerbo, E.; Carron, R.; Williamson, A.; Kurtin, D.L.; Rhodes, E.; Hampshire, A.; et al. Non-invasive temporal interference electrical stimulation of the human hippocampus. Nat. Neurosci. 2023, 26, 1994–2004. [Google Scholar] [CrossRef]
- Vassiliadis, P.; Beanato, E.; Popa, T.; Windel, F.; Morishita, T.; Neufeld, E.; Duque, J.; Derosiere, G.; Wessel, M.J.; Friedhelm, F.C. Non-invasive stimulation of the human striatum disrupts reinforcement learning of motor skills. Nat. Hum. Behav. 2024, 8, 1581–1598. [Google Scholar] [CrossRef]
- Beanato, E.; Moon, H.-J.; Windel, F.; Vassiliadis, P.; Wessel, M.J.; Popa, T.; Menoud, P.; Neufeld, E.; De Falco, E.; Gauthier, B.; et al. Noninvasive modulation of the hippocampal-entorhinal complex during spatial navigation in humans. Sci. Adv. 2024, 10, eado4103. [Google Scholar] [CrossRef]
- Mirzakhalili, E.; Barra, B.; Capogrosso, M.; Lempka, S.F. Biophysics of Temporal Interference Stimulation. Cell Syst. 2020, 11, 557–572.e5. [Google Scholar] [CrossRef]
- Caldas-Martinez, S.; Goswami, C.; Forssell, M.; Cao, J.; Barth, A.L.; Grover, P. Cell-specific effects of temporal interference stimulation on cortical function. Commun. Biol. 2024, 7, 1076. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Y.; Feng, X.; Wang, B.; Du, Y.; Wang, K.; Lü, J.; Huang, L.; Qian, Z.; Wang, Z.; et al. Transcranial Temporal Interference Stimulation of the Right Globus Pallidus in Parkinson’s Disease. Mov. Disord. 2024, 40, 1061–1069. [Google Scholar] [CrossRef]
- Hummel, F.C.; Wessel, M.J. Non-invasive deep brain stimulation: Interventional targeting of deep brain areas in neurological disorders. Nat. Rev. Neurol. 2024, 20, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Lamoš, M.; Bočková, M.; Missey, F.; Lubrano, C.; De Araújo e Silva, M.; Trajlínek, J.; Studnička, O.; Daniel, P.; Carron, R.; Jirsa, V.; et al. Noninvasive Temporal Interference Stimulation of the Subthalamic Nucleus in Parkinson’s Disease Reduces Beta Activity. Mov. Disord. 2025, 40, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Ploumitsakou, M.; Beanato, E.; Windel, F.; Nencha, U.; Maceira-Elvira, P.; Di Natale, M.; Vassiliadis, P.; Leger, B.; Wessel, M.; Nouri, A.; et al. Effects of striatal transcranial temporal interference stimulation on motor learning after a traumatic brain injury. Brain Stimul. 2025, 18, 401–402. [Google Scholar] [CrossRef]
- Temporal Interference for Drug Resistant Epilepsy (TIE). Available online: https://clinicaltrials.gov/study/NCT06708143 (accessed on 22 August 2025).
- Temporal Interference for Essential Tremor. Available online: https://clinicaltrials.gov/study/NCT07016425 (accessed on 22 August 2025).
- The Efficacy of Temporal Interference Stimulation in the Treatment of Obsessive-Compulsive Disorder. Available online: https://clinicaltrials.gov/study/NCT07113652 (accessed on 22 August 2025).
- Amygdala MRI-TIS for Depression. Available online: https://clinicaltrials.gov/study/NCT06461260 (accessed on 22 August 2025).
- Temporal Interference Neurostimulation and Addiction. Available online: https://clinicaltrials.gov/study/NCT04432064 (accessed on 22 August 2025).
- Proulx, C.E.; Hummel, F.C. Beyond the surface: Advancing neurorehabilitation with transcranial temporal interference stimulation—Clinical applications and future prospects. Neural Regen. Res. 2025, 10, 4103. [Google Scholar] [CrossRef] [PubMed]
- Wessel, M.J.; Egger, P.; Hummel, F.C. Predictive models for response to non-invasive brain stimulation in stroke: A critical review of opportunities and pitfalls. Brain Stimul. 2021, 14, 1456–1466. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Zhou, J.; Qian, Z.; Lü, J.; Li, L.; Liu, Y. Temporal interference stimulation targeting right frontoparietal areas enhances working memory in healthy individuals. Front. Hum. Neurosci. 2022, 16, 918470. [Google Scholar] [CrossRef]
- Vassiliadis, P.; Stiennon, E.; Windel, F.; Wessel, M.J.; Beanato, E.; Hummel, F.C. Safety, tolerability and blinding efficiency of non-invasive deep transcranial temporal interference stimulation: First experience from more than 250 sessions. J. Neural Eng. 2024, 21, 24001. [Google Scholar] [CrossRef]
- Piao, Y.; Ma, R.; Weng, Y.; Fan, C.; Xia, X.; Zhang, W.; Qinghong Zeng, G.; Wang, Y.; Lu, Z.; Cui, J.; et al. Safety Evaluation of Employing Temporal Interference Transcranial Alternating Current Stimulation in Human Studies. Brain Sci. 2022, 12, 1194. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Kronberg, G.; Reato, D.; Parra, L.C.; Bikson, M. Temporal interference stimulation targets deep brain regions by modulating neural oscillations. Brain Stimul. 2021, 14, 55–65. [Google Scholar] [CrossRef]
- Missey, F.; Acerbo, E.; Dickey, A.S.; Trajlinek, J.; Studnička, O.; Lubrano, C.; De Araújo e Silva, M.; Brady, E.; Vsiansky, V.; Szabo, J.; et al. Non-invasive Temporal Interference Stimulation of the Hippocampus Suppresses Epileptic Biomarkers in Patients with Epilepsy: Biophysical Differences between Kilohertz and Amplitude Modulated Stimulation. medRxiv 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, G.Q.; Wang, M.; Zhang, M.; Chang, C.; Liu, Q.; Wang, K.; Ma, R.; Wang, Y.; Zhang, X. The safety and efficacy of applying a high-current temporal interference electrical stimulation in humans. Front. Hum. Neurosci. 2024, 18, 1484593. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhu, G.; Wu, Z.; Gan, Y.; Zhang, J.; Liu, J.; Wang, L. Temporal interference stimulation targets deep primate brain. Neuroimage 2024, 291, 120581. [Google Scholar] [CrossRef] [PubMed]
- Corp, D.T.; Bereznicki, H.G.K.; Barham, M.P.; Clark, G.M.; Chadwick, B.J.; Jain, S.; Khalajzadeh, H.; Pascual-Leone, A.; Entincott, P.G. Big non-invasive brain stimulation data (Big NIBS data): An open-access platform and repository for NIBS data. Brain Stimul. 2025, 18, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.G.; Weiner, M.W.; Thal, L.J.; Petersen, R.C.; Jack, C.R.; Jagust, W.; Trojanowski, J.Q.; Toga, A.W.; Beckett, L. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s Dement. 2005, 1, 55–66. [Google Scholar] [CrossRef]
- Lopes Alves, I.; Collij, L.E.; Altomare, D.; Frisoni, G.B.; Saint-Aubert, L.; Payoux, P.; Kivipelto, M.; Jessen, F.; Drzezga, A.; Leeuwis, A.; et al. Quantitative amyloid PET in Alzheimer’s disease: The AMYPAD prognostic and natural history study. Alzheimer’s Dement. 2020, 16, 750–758. [Google Scholar] [CrossRef]
- Elder, G.J.; Taylor, J.P. Transcranial magnetic stimulation and transcranial direct current stimulation: Treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimers. Res. Ther. 2014, 6, 74. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Salvadó, G.; Janelidze, S.; Pichet Binette, A.; Bali, D.; Karlsson, L.; Palmqvist, S.; Mattson-Calgren, N.; Stomrud, E.; Therriault, J.; et al. Plasma p-tau217 and tau-PET predict future cognitive decline among cognitively unimpaired individuals: Implications for clinical trials. Nat. Aging 2025, 5, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Salvadó, G.; Ashton, N.J.; Tideman, P.; Stomrud, E.; Zetterberg, H.; Ossenkoppele, R.; Betthauser, T.J.; Cody, K.A.; Jonaitis, E.M.; et al. Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma Biomarkers. JAMA Neurol. 2023, 80, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Brum, W.S.; Cullen, N.C.; Therriault, J.; Janelidze, S.; Rahmouni, N.; Stevenson, J.; Servaes, S.; Benedet, A.L.; Zimmer, E.R.; Stomrud, E.; et al. A blood-based biomarker workflow for optimal tau-PET referral in memory clinic settings. Nat. Commun. 2024, 15, 2311. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nencha, U.; Hummel, F.C. Bridging Bench to Bedside for Brain Health: Non-Invasive Brain Stimulation for Neurodegenerative Diseases. Clin. Transl. Neurosci. 2025, 9, 43. https://doi.org/10.3390/ctn9030043
Nencha U, Hummel FC. Bridging Bench to Bedside for Brain Health: Non-Invasive Brain Stimulation for Neurodegenerative Diseases. Clinical and Translational Neuroscience. 2025; 9(3):43. https://doi.org/10.3390/ctn9030043
Chicago/Turabian StyleNencha, Umberto, and Friedhelm C. Hummel. 2025. "Bridging Bench to Bedside for Brain Health: Non-Invasive Brain Stimulation for Neurodegenerative Diseases" Clinical and Translational Neuroscience 9, no. 3: 43. https://doi.org/10.3390/ctn9030043
APA StyleNencha, U., & Hummel, F. C. (2025). Bridging Bench to Bedside for Brain Health: Non-Invasive Brain Stimulation for Neurodegenerative Diseases. Clinical and Translational Neuroscience, 9(3), 43. https://doi.org/10.3390/ctn9030043







