1. Introduction
Despite decreasing over time, the morbidity and mortality of aneurysmal subarachnoid hemorrhage (aSAH) remain high. The development of symptomatic vasospasm and delayed cerebral ischemia (DCI) contributes significantly to morbidity in survivors of aSAH [
1]. Symptomatic vasospasm and DCI occur in approximately 30% of aSAH patients, and are associated with worse prognosis [
2,
3].
The Practice Guideline for Critical Care Management of SAH recommends multimodality monitoring of aSAH patients to improve patient outcomes. Imaging modalities for vasospasm detection include computed tomography angiography (CTA), which reaches nearly 100% accuracy for macrovascular spasm, as well as catheter angiography, which is the gold standard for vasospasm diagnosis but carries procedural risks [
4]. Transcranial dopplers can accurately detect middle cerebral artery vasospasm, but are less reliable in other vascular territories and in detecting microvascular spasm [
5].
While the above tests detect vessel narrowing, alternative monitoring methods can be used to determine whether brain tissue is experiencing or at risk for ischemia. Quantitative electroencephalography (qEEG) can detect various patterns, including relative slowing or decreased variability of brain activity in specific regions, that correlate with structural vasospasm or DCI, as reviewed in [
6,
7]. In addition, cerebral blood flow and mean transit time values on CT perfusion (CTP) imaging can diagnose DCI with sensitivities of 70–78% and specificities of 63–81%, as reviewed in [
8]. Invasive monitoring can also provide ancillary information about brain perfusion but can be cost-prohibitive and limited by its invasiveness and ability to sample only small brain regions [
4].
The above monitoring methods must be correlated, however, with the patient’s neurologic exam in order to understand the clinical impact of any changes detected. Therefore, aSAH patients require frequent neurologic examinations to detect sometimes subtle and fluctuating changes that may be attributable to vasospasm in the cerebral vessels perfusing particular brain regions [
4]. While changes in strength or sensation can be represented numerically, objectively quantifying changes in frontal processes such as attention is more challenging. In awake patients with a GCS of 15, two rapid tests of attention had high interrater reliability (kappa > 0.8), and an adjusted sensitivity of 62% in detecting changes in a patient’s level of consciousness [
9]. One of these attention tests, counting backwards from twenty to one (TTO), was part of the Mental Test Score developed in 1972, but has not been incorporated broadly into targeted neurologic assessments of attention [
10].
This retrospective study investigates the use of TTO as a test of attention in patients with aSAH in order to detect vasospasm. We evaluate risk factors for the development of inattention in aSAH patients, assess hospital outcomes in those with inattention compared to controls, perform accuracy testing comparing inattention to radiographic vasospasm, and report the vascular territories affected by vasospasm.
2. Materials and Methods
2.1. Study Design, Data Source, and Variables
This retrospective case–control study was conducted using a quality assurance clinical report of all SAH patients treated in the Neurosciences Intensive Care Unit (NSICU) at a single academic center from September 2018 through August 2019. As part of a quality initiative (QI), every patient’s performance on the TTO task was documented on the day of ICU admission, daily during morning rounds, and as needed for changes in a patient’s level of alertness, throughout their vasospasm window. The vasospasm window spanned post-bleed days 3 through 14, except in high-risk patients, for whom testing continued through day 21. If a patient’s TTO test changed during a given day, these changes were recorded in our SAH patient clinical report for the appropriate day.
In addition to TTO testing, the following information was collected from clinical reports and by review of patient charts by two study investigators: patient demographics, medical comorbidities, ICU and hospital length of stay, aneurysm location and type of treatment, clinical and radiographic grades, daily neurologic exam, other possible reasons for inattention (including fever, infection, seizures, hydrocephalus, and use of sedating medications), treatment with systemic medications (intravenous milrinone or blood pressure augmentation), treatment with intra-arterial verapamil, improvement in attention after treatment, and functional ability (modified Rankin score, mRS) at discharge.
Symptomatic vasospasm was defined based on consensus definitions as a new focal neurologic deficit, a decrease in Glasgow Coma Scale by at least 2 points, lasting greater than 1 h and not obviously attributable to another cause by clinical assessment or diagnostic studies [
11]. The determination of symptomatic vasospasm was recorded in the SAH patient clinical report at the discretion of the attending neuro-intensivist, and then reviewed by study personnel with mediation of conflicting diagnoses by supervising authors. We defined radiographic vasospasm as any reduction in vessel caliber on computed tomography (CT) or conventional angiogram [
12].
2.2. Case–Control Selection
Among patients with aSAH admitted to the NSICU during the pre-specified one-year period, those with non-aneurysmal subarachnoid hemorrhage, ICU mortality, inability to perform TTO, or missing TTO data were excluded. Cases were defined as patients who made errors in the TTO task without self-correction or who were unable to complete the task at any time during their ICU stay. Controls were defined as patients who never made errors on the TTO task. For accuracy testing and determination of vascular territory affected, we further excluded patients who did not undergo any angiographic testing (CTA or DSA) during their vasospasm window.
2.3. Outcomes and Statistical Analysis
Our primary outcome was the odds of symptomatic vasospasm versus other clinical conditions that can cause inattention. Secondary outcomes included mean ICU and hospital length of stay, ICU disposition, discharge disposition, and modified Rankin scale (mRS) score at discharge. For the limited case–control groups who underwent angiographic testing during their vasospasm window, we additionally performed accuracy testing and compared the affected intracranial vessels between cases and controls for those with radiographic vasospasm.
Primary and secondary outcomes are described as odds ratios or relative risk, with 95% confidence intervals in brackets, or differences between medians. Statistical analyses included chi square and Fisher’s exact tests for categorical variables, and Wilcoxon rank sum tests for continuous variables. Analyses were performed using the SAS statistics software packages, Version 9.4 (SAS Institute Inc 2013). Figures were created in Microsoft Excel [
13].
3. Results
3.1. Patient Selection and Characteristics
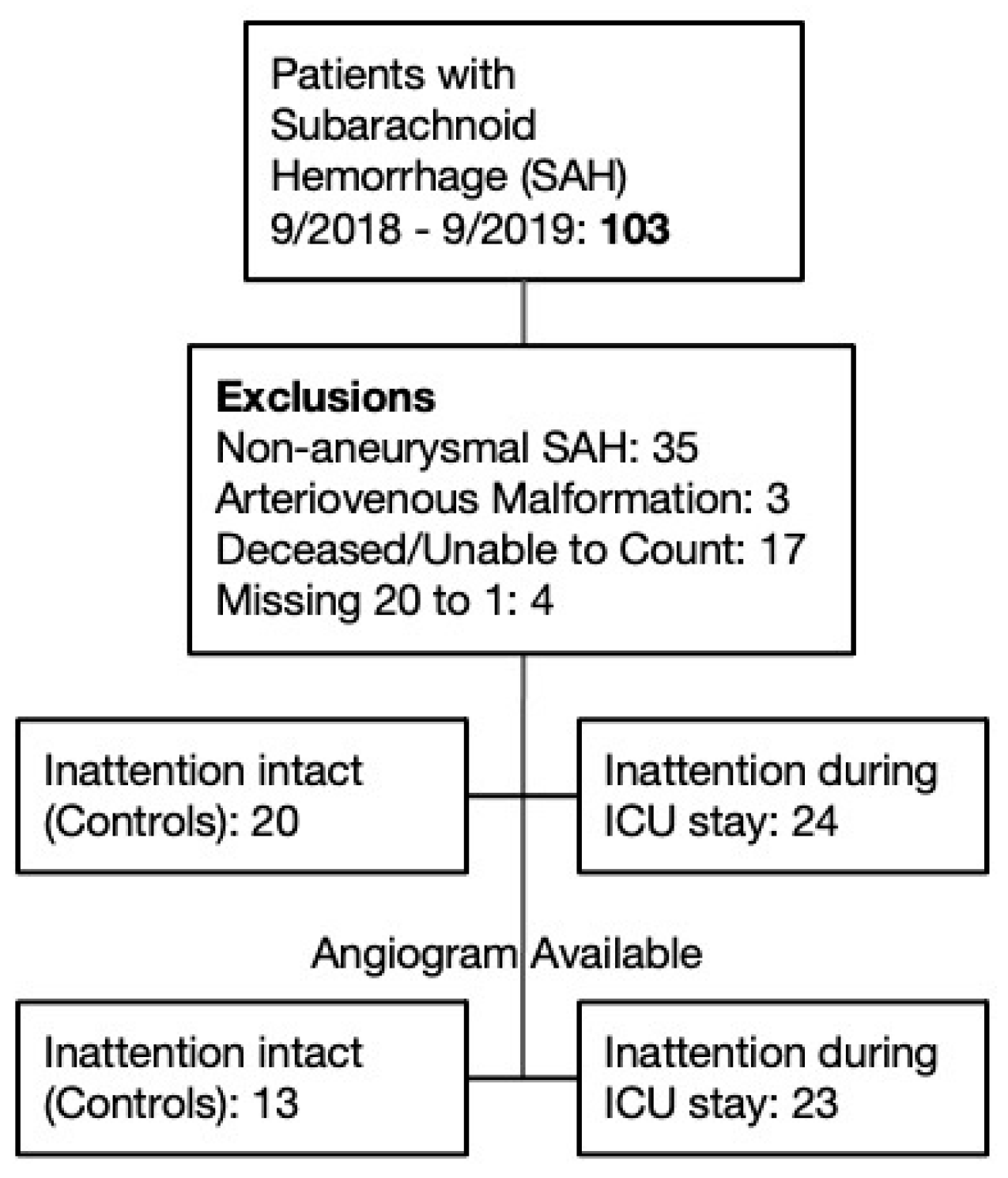
Over the study period, 103 patients with subarachnoid hemorrhage were admitted to the NSICU. A total of 35 patients were excluded for non-aneurysmal subarachnoid hemorrhage, 3 patients with arteriovenous malformation without flow-related aneurysm, 17 patients who died during the NSICU stay or were unable to ever perform the TTO task, and 4 patients because TTO data was missing. The remaining 44 patients were divided into controls (n = 20) who never made TTO errors, and cases (n = 24) who made TTO errors during their ICU stay. For accuracy testing, we further excluded patients who did not undergo angiographic testing during their vasospasm window, resulting in subgroups of 23 cases and 13 controls (
Figure 1).
Demographics and clinical characteristics of cases and controls are shown in
Table 1. The groups showed no significant difference in age, sex, or comorbidities that predispose to subarachnoid hemorrhage. Although not statistically significant, 25% (n = 6) of patients in the inattention group had a high SAH clinical grade (Hunt Hess Scale 4 or 5), compared to 5% (n = 1) of patients in the control group. Although patients with a high HHS were not able to participate in TTO testing at initial presentation, they improved enough to participate by post-bleed day 3, when routine TTO testing began.
Radiographic grade, quantified by the modified Fisher Scale (mFS), differed significantly between the two groups, with the cases having a higher score than controls [
14]. Furthermore, the odds ratio for inattention in patients with a high mFS score (3 or 4) was 9.0 [95% CI 1.7–50.0,
p = 0.012].
The two groups had no significant difference with regard to aneurysm location when comparing anterior to posterior circulation aneurysms. Furthermore, the odds ratio of impaired attention with an ACA territory aneurysm was not significantly greater than with an MCA territory aneurysm [OR 0.4, 95% CI 0.08–2.27, p = 0.20].
3.2. Vasospasm Risk and Hospital Outcomes in Patients with Inattention
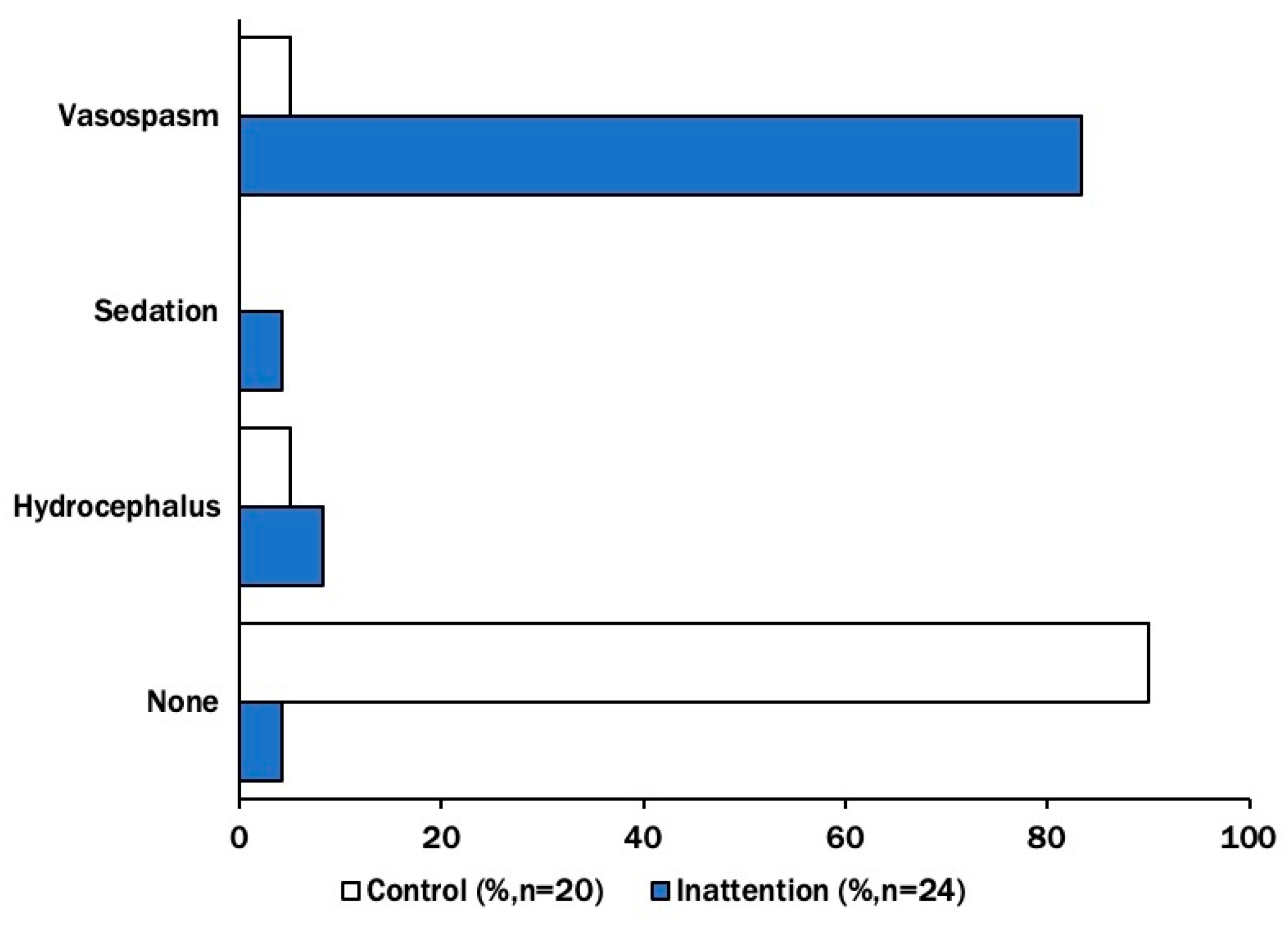
Symptomatic vasospasm was diagnosed in 83% of patients with inattention, compared to 5% in controls. We additionally compared the occurrence of sedation (4% vs. 0%) and hydrocephalus (8% vs. 5%) between the two groups. Neither group had isolated fever, infection, or seizure (in the absence of concurrent vasospasm) documented during their vasospasm window (
Figure 2). The odds of symptomatic vasospasm compared to other inattention correlates in the inattention group were significantly higher than in the control group (OR 72 [7.6–677.7],
p = 0.001). This remained true when data were stratified by the modified Fisher scale (mFS > 2), in which case the relative risk of symptomatic vasospasm was 4.4 (95% CI [2.0–9.5],
p < 0.001).
Patients with inattention had significantly worse hospital outcomes than controls, with longer lengths of stay in the ICU (median 5.9 days,
p = 0.001) and hospital (median 6.6 days,
p < 0.001). Inattentive patients had higher rates of discharge to rehabilitation or other healthcare facilities (OR 11.4 [2.8 to 46.8],
p < 0.001). Although the two groups had a statistically significant difference in functional disability at discharge defined as mRS >2 (0.11 [0.03–0.44],
p = 0.001), the median mRS in both groups was 3. Hospital disposition did not differ significantly between the two groups as patients in both groups were discharged at similar rates to lower levels of care within the hospital (
Table 2).
3.3. Inattention Testing for Radiographic Vasospasm and Cerebral Vessels Affected
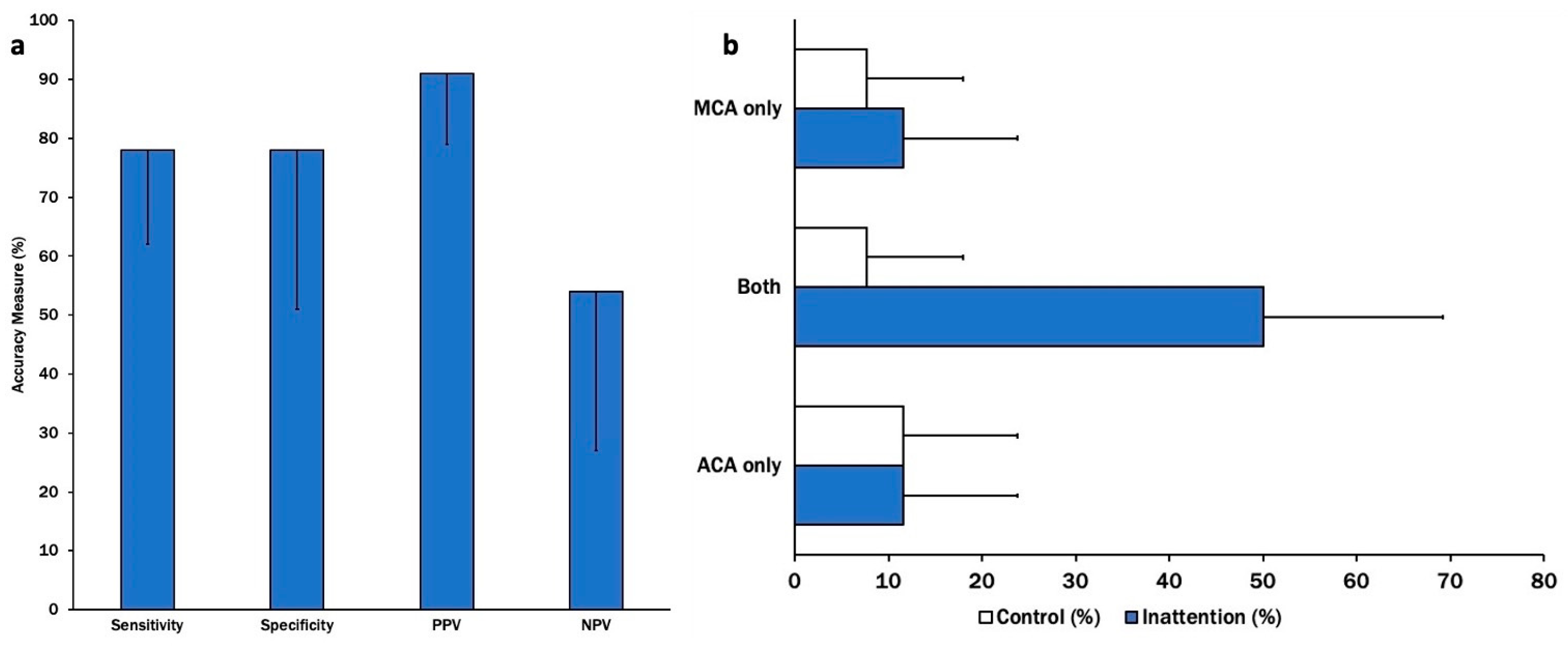
Errors on TTO testing had a specificity and sensitivity of 78% for radiographic vasospasm detection. The positive predictive value was the most robust at 91% [0.80–1.03]. The test had a low negative predictive value at only 54% [0.27–0.81] (
Figure 3a). The test correlated most strongly with vasospasm in both the middle and anterior cerebral arteries, without any preference for one territory over the other (
Figure 3b).
4. Discussion
This study found that patients with aSAH who developed inattention, as identified by TTO testing, during their hospital stay were more likely to have cerebral vasospasm than controls with intact attention throughout their ICU admission. This held true when stratifying our cohort for the confounder of modified Fisher scale, which is known to correlate with increased vasospasm risk. Although aneurysm site and craniotomy could also impact attention, these characteristics were similar between the two groups and likely did not contribute to the increased odds of vasospasm in the inattention group. In addition to sedation, hydrocephalus, fever, infection, and seizure, cortical spreading depolarization could be assessed as a contributor to inattention in aSAH patients in future studies but was not evaluated in our cohort [
4].
For the subgroup of patients who underwent angiographic testing, the attention test had >75% sensitivity and specificity with a high positive predictive value. For a screening test that has high reproducibility and can be easily performed by all members of the critical care team, we would prefer a test with higher sensitivity. Because this is a small study and angiographic testing was not performed in all control patients, future studies are needed to better define the accuracy of the TTO attention test for vasospasm in aSAH patients.
With regard to the vasospasm territory, we hypothesized that inattention, as a marker of frontal lobe dysfunction, might correlate more with vasospasm in the anterior cerebral artery that supplies the medial frontal lobe. In this cohort, however, inattention correlated with vasospasm in both the ACA and MCA territories, and did not select for one over the other. Some possible explanations for this finding include that attention is a complex process that can rely on contributions from MCA-supplied brain regions as well as ACA, that diffuse subarachnoid blood can cause simultaneous vasospasm in multiple vascular territories, or that our cohort of patients happened to have high rates of multi-territory vasospasm despite being a consecutive series of patients admitted to a single-center neuroscience ICU.
Finally, hospital outcomes were worse for aSAH patients with inattention than those with intact attention. This difference may be a result of the higher grade of SAH for the inattention group, which is a known risk factor for vasospasm and is generally associated with higher mortality. However, because vasospasm and DCI are complex processes, it may be helpful for providers and families to have an additional, simple clinical test that can offer some predictive information about a patient’s hospital course and outcomes.
Strengths of this study include its use of a highly reproducible, easy-to-perform, and relatively quantitative bedside assessment of attention. It utilized data from consecutive patients with aSAH admitted to an NSICU, and included any patient who was able to perform the TTO task during their ICU stay, thereby minimizing bias through any further selection. Limitations include the single-center design, as results may not be broadly generalizable, the retrospective nature as patients were not randomized in any manner, and the lack of angiographic data for all patients, particularly in the control group. Because symptomatic vasospasm can differ from radiographic vasospasm, our choice to use radiographic spasm as the gold standard in our accuracy testing may have over-represented spasm in the control group. However, any bias introduced in this way should decrease accuracy measures.
Further studies can overcome the limitations here by utilizing a prospective study design and randomizing patients to use TTO in neurologic monitoring vs. standard care, and/or performing angiographic imaging on all patients regardless of a clinical suspicion for vasospasm in order to have a more robust control group for comparison.
5. Conclusions
This study provides support for the use of TTO testing in clinical monitoring of aSAH patients for vasospasm in the anterior cerebral circulation. We find that patients with inattention on TTO testing have increased odds of vasospasm and worse hospital outcomes than those with intact attention.
Author Contributions
H.L., conceptualization, methodology, investigation, data curation, formal analysis, writing, visualization; A.B., conceptualization, methodology, investigation, data curation, writing, review and editing; N.M., conceptualization, methodology, investigation, data curation, formal analysis; A.M., formal analysis, validation; A.S.R. and J.L., resources, supervision, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Icahn School of Medicine’s Institutional Review Board (IF2785796, approved 10/2022), and performed in compliance with all ethical standards for human subject research.
Informed Consent Statement
The need for informed consent was waived given the retrospective nature of the study.
Data Availability Statement
Data will be made available by request of the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| aSAH | aneurysmal subarachnoid hemorrhage |
| TTO | twenty to one |
| DCI | delayed cerebral ischemia |
| PPV | positive predictive value |
| LOS | length of stay |
| mFS | modified Fisher scale |
| mRS | modified Rankin score |
| HHS | Hunt Hess scale |
| qEEG | quantitative electroencephalopathy |
| CTA | computed tomography angiogram |
| CTP | computed tomography perfusion imaging |
References
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, N. A clinical review of cerebral vasospasm and delayed ischaemia following aneurysm rupture. Acta Neurochir. Suppl. 2011, 110, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Rosengart, A.J.; Schultheiss, K.E.; Tolentino, J.; Macdonald, R.L. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007, 38, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Abdulazim, A.; Heilig, M.; Rinkel, G.; Etminan, N. Diagnosis of Delayed Cerebral Ischemia in Patients with Aneurysmal Subarachnoid Hemorrhage and Triggers for Intervention. Neurocrit. Care 2023, 39, 311–319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vora, Y.Y.; Suarez-Almazor, M.; Steinke, D.E.; Martin, M.L.; Findlay, J.M. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery 1999, 44, 1237–1247. [Google Scholar] [PubMed]
- Baang, H.Y.; Chen, H.Y.; Herman, A.L.; Gilmore, E.J.; Hirsch, L.J.; Sheth, K.N.; Petersen, N.H.; Zafar, S.F.; Rosenthal, E.S.; Westover, M.B.; et al. The Utility of Quantitative EEG in Detecting Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. J. Clin. Neurophysiol. 2022, 39, 207–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scherschinski, L.; Catapano, J.S.; Karahalios, K.; Koester, S.W.; Benner, D.; Winkler, E.A.; Graffeo, C.S.; Srinivasan, V.M.; Jha, R.M.; Jadhav, A.P.; et al. Electroencephalography for detection of vasospasm and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: A retrospective analysis and systematic review. Neurosurg. Focus 2022, 52, E3. [Google Scholar] [CrossRef] [PubMed]
- Cremers, C.H.; van der Schaaf, I.C.; Wensink, E.; Greving, J.P.; Rinkel, G.J.; Velthuis, B.K.; Vergouwen, M.D. CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2014, 34, 200–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, S.A.; Dennis, L.J.; Peery, S.; Fitsimmons, B.F.; Du, Y.E.; Bernardini, G.L.; Commichau, C.; Eldaief, M. Quantification of lethargy in the neuro-ICU: The 60-Second Test. Neurology 2003, 61, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, H.M. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972, 1, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H. Subarachnoid Hemorrhage. Continuum 2021, 27, 1201–1245. [Google Scholar] [CrossRef] [PubMed]
- Microsoft Corporation. Microsoft Excel. 2019. Available online: https://office.microsoft.com/excel (accessed on 11 April 2023).
- Frontera, J.A.; Claassen, J.; Schmidt, J.M.; Wartenberg, K.E.; Temes, R.; Connolly, E.S., Jr.; MacDonald, R.L.; Mayer, S.A. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified fisher scale. Neurosurgery 2006, 59, 21–27. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).