Unclosing Clinical Criteria and the Role of Cytokines in the Pathogenesis of Persistent Post-COVID-19 Headaches: A Pilot Case-Control Study from Egypt
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Post-COVID Headache Characteristics
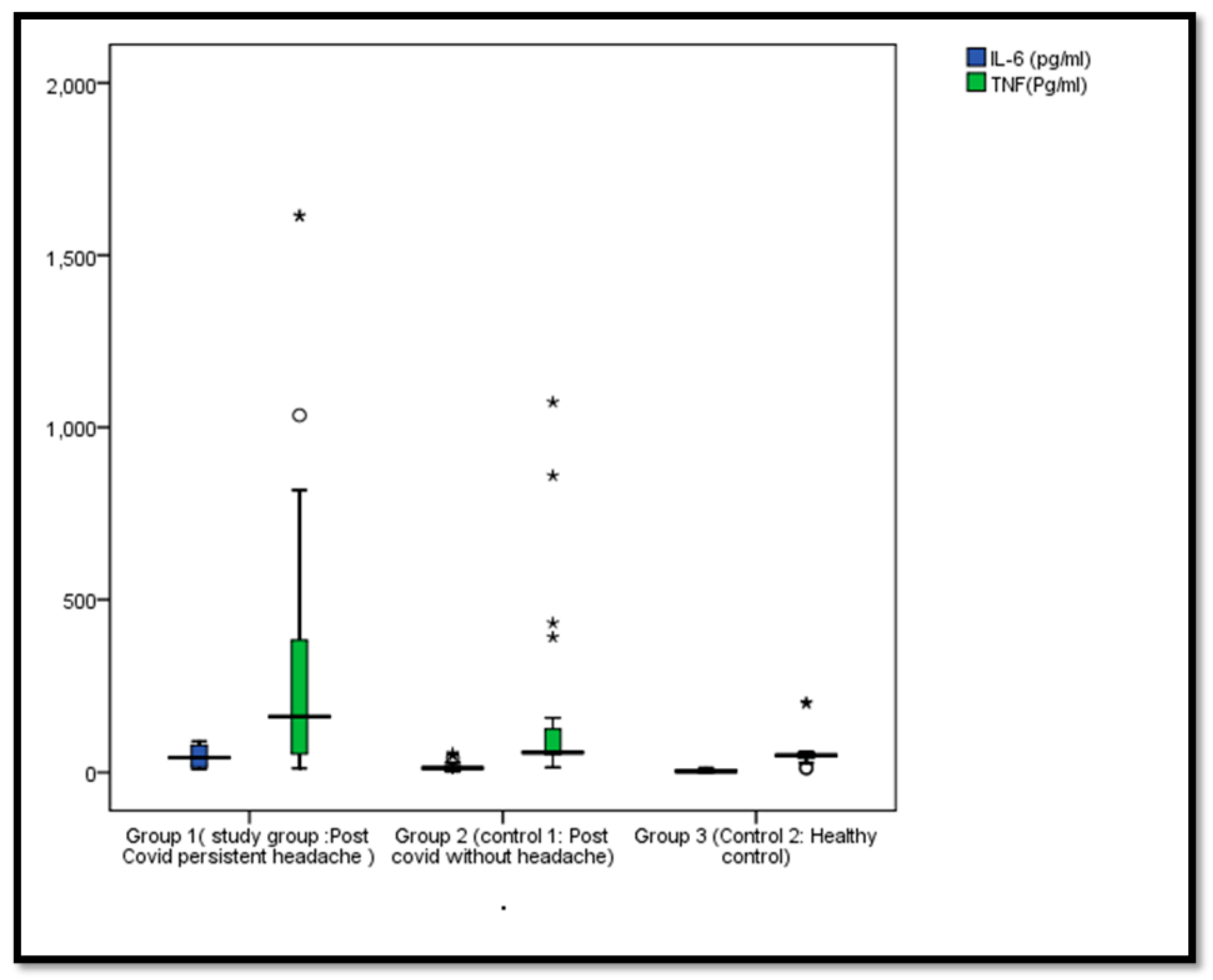
3.3. Comparison Between Our Study Groups Regarding IL-6, and TNF-α Levels
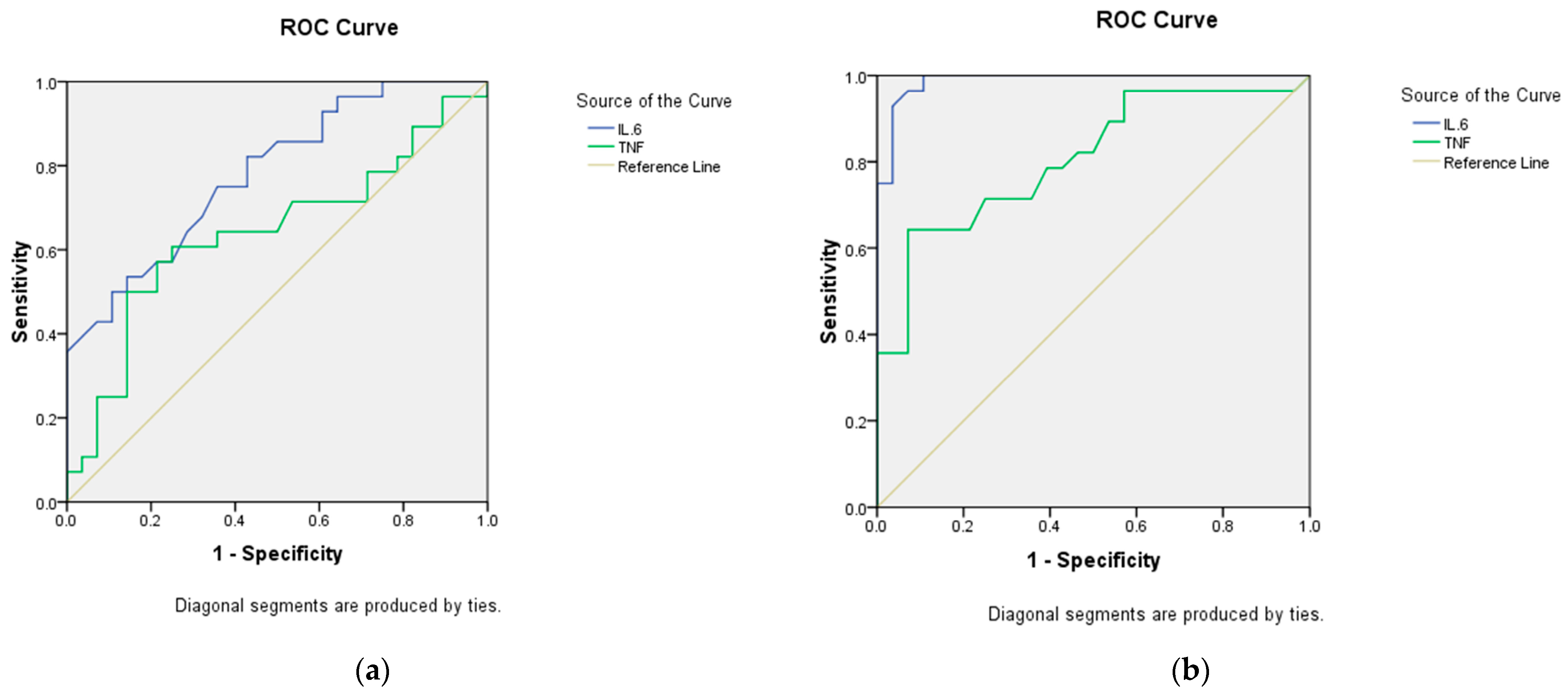
3.4. Validity of IL-6, and TNF-α Levels in Differentiating Between Our Study Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; ElHafeez, S.A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Jameie, M.; Togha, M.; Looha, M.A.; Jafari, E.; Panah, M.Y.; Hemmati, N.; Nasergivehchi, S. Characteristics of headaches attributed to SARS-CoV-2 vaccination and factors associated with its frequency and prolongation: A cross-sectional cohort study. Front. Neurol. 2023, 14, 1214501. [Google Scholar] [CrossRef]
- Chang, Y.; Song, T.J. COVID-19 Infection-related Headache: A Narrative Review. Headache Pain Res. 2024, 25, 24–33. [Google Scholar] [CrossRef]
- Tana, C.; Bentivegna, E.; Cho, S.; Harriott, A.M.; García-Azorín, D.; Labastida-Ramirez, A.; Ornello, R.; Raffaelli, B.; Beltrán, E.R.; Ruscheweyh, R.; et al. Long COVID headache. J. Headache Pain 2022, 23, 93. [Google Scholar] [CrossRef]
- Ceccardi, G.; Di Cola, F.S.; Di Cesare, M.; Liberini, P.; Magoni, M.; Perani, C.; Gasparotti, R.; Rao, R.; Padovani, A. Post COVID-19 vaccination headache: A clinical and epidemiological evaluation. Front. Pain Res. 2022, 3, 994140. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, M.P. Headaches as part of post-COVID syndrome: A review of epidemiology, mechanisms, anmanagement. Front. Neurol. 2021, 12, 715254. [Google Scholar]
- Chou, R. Post-COVID syndrome and headache in young adults: Epidemiology, pathophysiology, and management. J. Headache Pain 2023, 24, 12. [Google Scholar]
- Luo, R.; Zhang, X.; Zhu, Y. Sex differences in the severity of COVID-19 and post-infectious conditions: A multicenter retrospective study. Front. Public Health 2023, 11, 123456. [Google Scholar]
- Membrilla, J.; Caronna, E.; Trigo-López, J.; González-Martínez, A.; Layos-Romero, A.; Pozo-Rosich, P.; Guerrero-Peral, Á.; Gago-Veiga, A.; Andrés-López, A.; De Terán, J.D. Persistent headache after COVID-19: Pathophysioloy, clinic and treatment. Neurol. Perspect. 2021, 1, S31–S36. [Google Scholar] [CrossRef]
- Toptan, T.; Aktan, Ç.; Başarı, A.; Bolay, H. Case Series of Headache Characteristics in COVID-19: Headache Can Be an Isolated Symptom. Headache 2020, 60, 1788–1792. [Google Scholar] [CrossRef]
- World Health Organization. Global Surveillance for Human Infection with Novel Coronavirus (2019-nCoV): Interim Guidance. 31 January 2020. Available online: https://iris.who.int/handle/10665/330857 (accessed on 7 October 2022).
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef] [PubMed]
- Dhiman, N.R.; Joshi, D.; Singh, R.; Gyanpuri, V.; Kumar, A. Post-COVID-19 headache- NDPH phenotype: A systematic review of case reports. Front. Pain Res. 2024, 5, 1376506. [Google Scholar] [CrossRef] [PubMed]
- Perini, F.; D’Andrea, G.; Galloni, E.; Pignatelli, F.; Billo, G.; Alba, S.; Bussone, G.; Toso, V. Plasma Cytokine Levels in Migraineurs and Controls. Headache 2005, 45, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Palacios-Ceña, D.; Florencio, L.L.; Guerrero, A.L.; García-Azorín, D.; Hernández-Barrera, V.; Arendt-Nielsen, L. The presence of headache at onset in SARS-CoV-2 infection is associated with long-term post-COVID headache and fatigue: A case-control study. Cephalalgia 2021, 41, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Nessi, F.S.; Ascanio, L.C.; Pineda-Arapé, A.G.; Omaña-Ávila, Ó.D.; Mendoza-Millán, D.L.; Romero, S.R.; Almao-Rivero, A.B.; Camejo-Ávila, N.A.; Gebran-Chedid, K.J.; Rodriguez-Saavedra, C.M.; et al. New daily persistent headache after SARS-CoV-2 infection in Latin America: A cross-sectional study. BMC Infect. Dis. 2023, 23, 877. [Google Scholar] [CrossRef] [PubMed]
- Caronna, E.; Pozo-Rosich, P. Headache as a Symptom of COVID-19: Narrative Review of 1-Year Research. Curr. Pain Headache Rep. 2021, 25, 73. [Google Scholar] [CrossRef] [PubMed]
- Bolay, H.; Karadas, Ö.; Oztürk, B.; Sonkaya, R.; Tasdelen, B.; Bulut, T.D.S.; Gülbahar, Ö.; Özge, A.; Baykan, B. HMGB1, NLRP3, IL-6, and ACE2 levels are elevated in COVID-19 with headache: A window to the infection-related headache mechanism. J. Headache Pain 2021, 22, 94–120. [Google Scholar] [CrossRef]
- Bolay, H.; Gül, A.; Baykan, B. COVID-19 is a Real Headache! Headache 2020, 60, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, L.; Zheng, L. Elevated serum interleukin-6 levels are associated with severe clinical outcomes in COVID-19 patients: A meta-analysis. J. Infect. 2021, 82, 293–303. [Google Scholar]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Möller, M.; Möller, H.E. Cytokine storm and neuroinflammation in COVID-19: A focus on IL-6. Front. Immunol. 2021, 12, 613032. [Google Scholar]
| Characteristic | Post-COVID with Headache (n = 28) | Post-COVID Without Headache (n = 28) | Healthy Controls (n = 28) | Test of Significance | Within Group Significance |
|---|---|---|---|---|---|
| Age (years), Mean ± SD | 39.82 ± 14.49 | 43.46 ± 14.05 | 40.68 ± 11.06 | F = 0.575 P = 0.565 | P1 = 0.308 P2 = 0.810 P3 = 0.435 |
| Male, no. (%) | 5 (17.9) | 9 (32.1) | 10 (35.7) | χ2 = 2.45 p = 0.294 | P1 = 0.217 P2 = 0.131 P3 = 0.777 |
| Female, no. (%) | 23 (82.1) | 19 (67.9) | 18 (64.3) |
| Headache Characteristics | Post-COVID with Headache Group (n = 28) | |
|---|---|---|
| Site | Bilateral, no. (%) | 15 (53.6) |
| Occipital, no. (%) | 3 (10.7) | |
| Unilateral, no. (%) | 10 (35.7) | |
| Character | Throbbing-like, no. (%) | 17 (60.7) |
| Pressure-like, no. (%) | 11 (39.3) | |
| Headache days per month, median (IQR) | 12.0 (10.0–20.0) | |
| Visual Analog Scale, median (IQR) | 80 (70–100) | |
| Family history of migraine, no. (%) | 10 (35.7) | |
| Previous history of migraine, no. (%) | 11 (39.3) | |
| Associated symptoms and signs | Nausea, no. (%) | 17 (60.7) |
| Photophobia, no. (%) | 21 (75) | |
| Phonophobia, no. (%) | 24 (85.7) | |
| Dizziness, no. (%) | 20 (71.4) | |
| Fatigue, no. (%) | 22 (78.6) | |
| Anorexia, no. (%) | 14 (50) | |
| Food craving, no. (%) | 7 (25) | |
| Analgesics used | Paracetamol, no. (%) | 21 (75) |
| NSAIDs, no. (%) | 15 (53.6) | |
| Triptans, no. (%) | 6 (21.4) | |
| Response to analgesics | Not satisfactory, no. (%) | 10 (35.7) |
| Partial, no. (%) | 4 (14.3) | |
| Satisfactory, no. (%) | 14 (50) | |
| Prophylactic treatment | Topiramate, no. (%) | 6 (21.4) |
| Amitriptyline, no. (%) | 10 (35.7) | |
| Post-COVID with Headache (n = 28) | Post-COVID Without Headache (n = 28) | Healthy Controls (n = 28) | Test of Significance | Within Group Significance | |
|---|---|---|---|---|---|
| IL-6 (pg/mL), Median (IQR) | 42.9 (14.25–80.4) | 13.05 (7.33–16.93) | 3.6 (0.325–7.43) | KW = 50.35 p < 0.001 | P1 < 0.001 P2 < 0.001 P3 < 0.001 |
| TNF-α (Pg/mL), Median (IQR) | 161.8 (53.6–463.98) | 57.85 (52.4–128.38) | 49.9 (41.4–57.4) | KW = 18.11 p < 0.001 | P1 < 0.001 P2 < 0.001 P3 < 0.001 |
| AUC (95% CI) | p Value | Cut Off Point | Sensitivity % | Specificity % | |
|---|---|---|---|---|---|
| Differentiating between post-COVID with headache group and post-COVID without headache group | |||||
| IL6 | 0.783 (0.665–0.900) | <0.0001 * | 13.7 | 82.1 | 57.1 |
| TNF-α | 0.642 (0.492–0.791) | 0.069 | 103.55 | 60.7 | 71.4 |
| Differentiating between post-COVID with headache group and healthy controls | |||||
| IL6 | 0.988 (0.967–1.0) | <0.001 * | 9.8 | 96.4 | 92.9 |
| TNF-α | 0.807 (0.693–0.922) | <0.001 * | 50.6 | 82.1 | 53.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abualhasan, A.; Fathi, S.; Gabr, H.; Mahmoud, A.; Khedr, D. Unclosing Clinical Criteria and the Role of Cytokines in the Pathogenesis of Persistent Post-COVID-19 Headaches: A Pilot Case-Control Study from Egypt. Clin. Transl. Neurosci. 2025, 9, 5. https://doi.org/10.3390/ctn9010005
Abualhasan A, Fathi S, Gabr H, Mahmoud A, Khedr D. Unclosing Clinical Criteria and the Role of Cytokines in the Pathogenesis of Persistent Post-COVID-19 Headaches: A Pilot Case-Control Study from Egypt. Clinical and Translational Neuroscience. 2025; 9(1):5. https://doi.org/10.3390/ctn9010005
Chicago/Turabian StyleAbualhasan, Ahmed, Shereen Fathi, Hala Gabr, Abeer Mahmoud, and Diana Khedr. 2025. "Unclosing Clinical Criteria and the Role of Cytokines in the Pathogenesis of Persistent Post-COVID-19 Headaches: A Pilot Case-Control Study from Egypt" Clinical and Translational Neuroscience 9, no. 1: 5. https://doi.org/10.3390/ctn9010005
APA StyleAbualhasan, A., Fathi, S., Gabr, H., Mahmoud, A., & Khedr, D. (2025). Unclosing Clinical Criteria and the Role of Cytokines in the Pathogenesis of Persistent Post-COVID-19 Headaches: A Pilot Case-Control Study from Egypt. Clinical and Translational Neuroscience, 9(1), 5. https://doi.org/10.3390/ctn9010005






