The Swiss Narcolepsy Network (SNaNe)
Abstract
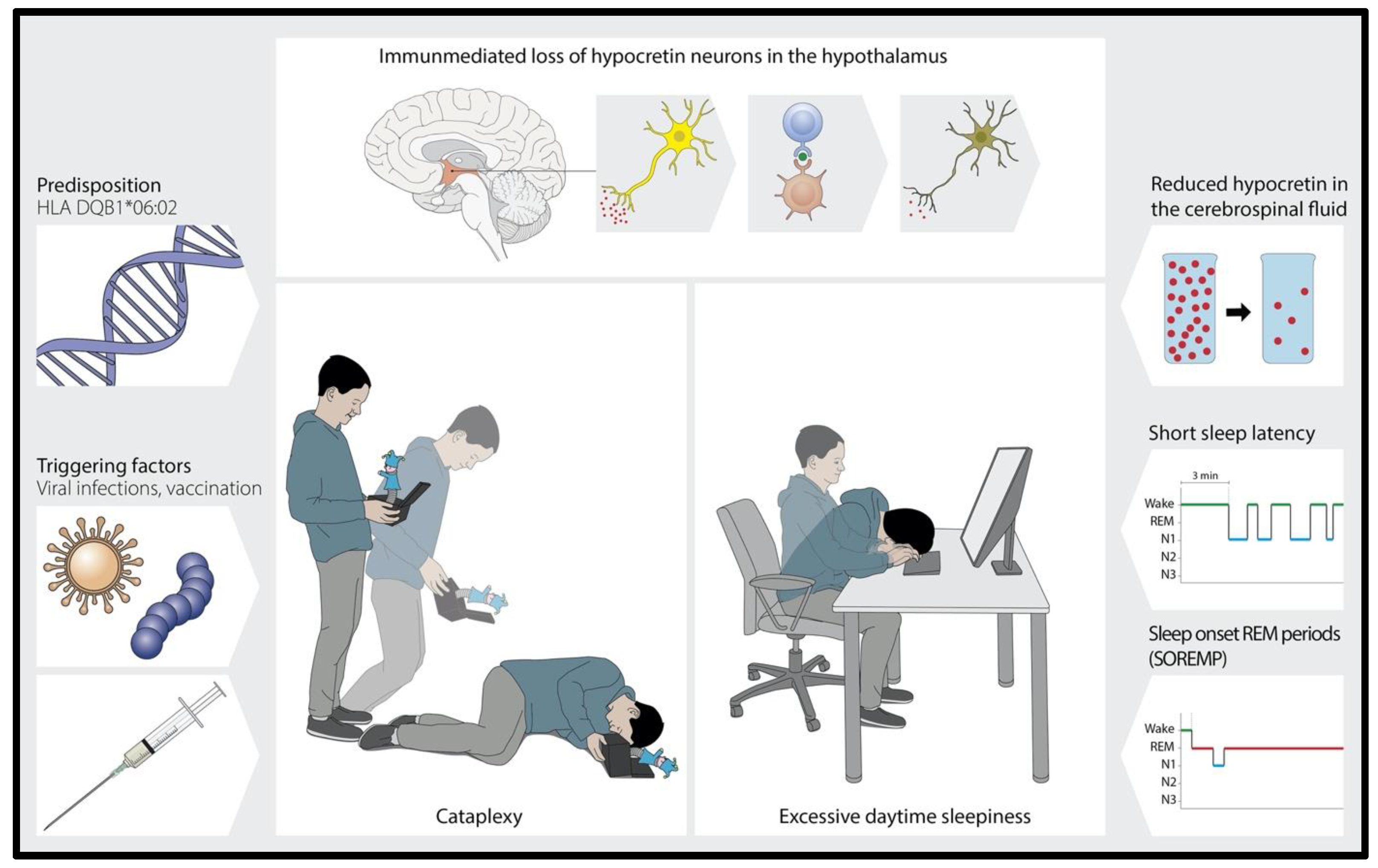
:1. Introduction
- Primary central disorders of hypersomnolence (CDH), including narcolepsy, are often diagnosed late (to date, the diagnostic latency for these disorders in Switzerland is still >5 years [1]).
- There is, overall, little expertise for the management of CDH in Switzerland.
- The «Burden of disease» of CDH, which often begins early in life, is high (comparable to that of patients with epilepsy, for example) [3].
- The therapy is neither well established, nor reimbursed for most CDH.
- The need for supportive and psychosocial therapies for CDH is not met in Switzerland.
2. Narcolepsy and Primary Central Disorders of Hypersomnolence
3. Narcolepsy Medicine and Research in Switzerland: A Long-Standing Tradition
4. The Swiss Narcolepsy Network (SNaNe): Foundation and Mission
5. The Swiss Narcolepsy Network (SNaNe): The First 6 Years
- The virtual presentation and discussion on complex patient cases in collaboration with the European Narcolepsy Network (EU-NN; https://www.snane.ch/eventi/snane-case-discussion-webinar-3, accessed on 1 September 2023).
- The organization of the Swiss Narcolepsy Days (last meeting held in Basel 2023) in collaboration with the SNaG patient organization (https://www.snane.ch/eventi/8th-swiss-narcolepsy-day, accessed on 1 September 2023).
- The coordination of multicenter research projects, such as the SPHYNCS study, was supported by the SNSF (Swiss National Science Foundation) for the period of 2023-6 [15]. This study (Figure 2) aims to identify new biomarkers for primary central nervous hypersomnia. An international extension of SPHYNCS (so-called iSPHYNCS) was approved by the SNSF for the period of 2023-6. This new study will involve three further international centers, including Bologna (Italy), Witten-Herdecke (Germany), and Leiden (Holland). In addition to the search of new biomarkers, this new study will investigate both patients’ treatment adherence as well as “patient-related outcomes” (PROMs).
- The establishment of an anonymous Swiss registry for patients with primary CDH (SNaNe Data Registry). The registry is operated by the Clinical Trial Unit (CTU) in Bern according to the latest safety standards and contains information on symptoms, sleep studies, and molecular disease markers. Currently, as of September 2023, more than 170 patients from five different centers have been included in the registry.
- The exchange and collaboration with the Swiss (SNAG) Narcolepsy society, as well as with the European patient organization (European Narcolepsy alliance for patients, eNAP);
- The exchange and collaboration at the national and international level with the Swiss Society of Sleep Research, Sleep Medicine, and Chronobiology (SSSSC); Swiss Society of Neurology (SSN); Swiss Society of Neuropediatrics (SSNP); and the European Narcolepsy Network (EU-NN)
6. The Swiss Narcolepsy Network (SNaNe): Outlook and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Dauvilliers, Y.; Plazzi, G.; Mayer, G.; Jan Lammers, G.; Santamaria, J.; Partinen, M.; Overeem, S.; Del Rio Villegas, R.; Sonka, K.; et al. Idling for decades: A Europen study on risk factors associated with the delay before a narcolepsy diagnosis. Nat. Sci. Sleep. 2022, 14, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Amstad, H.; Neinhaus, A. Bessere Versorgungsstruktur für Menschen mit seltenen Krankheiten. Schweiz. Aerztezeitung 2016, 97, 49–50. [Google Scholar]
- Bassetti, C.L.A.; Adamantidis, A.R.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Jan Lammers, G.; et al. Narcolepsy. Clinical features, etio-pathophysiology, diagnosis and management of a hypothalamic, immune-mediated disease. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed.; (ICSD-3); Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2023. [Google Scholar]
- Fregolente, L.G.; Wenz, E.; Wulf, M.A.; Warncke, J.D. Narkolepsie: Eine chronische, immun-vermittelte hypothalamsiche Erkrankung. Swiss Med. Forum 2023, 23, 914–917. [Google Scholar]
- Latorre, D.; Kallweit, U.; Armentani, E.; Foglierini, M.; Mele, F.; Cassotta, A.; Jovic, S.; Jarrossay, D.; Mathis, J.; Zellini, F.; et al. Autoreactive T cells in narcolepsy patients target antigens of hypocretin-producing neurons. Nature 2018, 562, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.; Kallweit, U.; Vignatelli, L. European guideline and expert statements on the management of narcolepsy in adults and children. Eur. J. Neurol. 2021, 28, 2815–2830. [Google Scholar] [CrossRef] [PubMed]
- Lammers, G.J.; Bassetti, C.L.A.; Dolenc-Groselj, L.; Jennum, P.J.; Kallweit, U.; Khatami, R.; Lecendreux, M.; Manconi, M.; Mayer, G.; Partinen, M.; et al. Diagnosis of central disorders of hypersomnolence: A reappraisal by European experts. Sleep. Med. Rev. 2020, 52, 101306. [Google Scholar] [CrossRef] [PubMed]
- Heyck, H.; Hess, R. Some results of clinical studies on narcolepsy. Schweiz. Arch. Neurol. Psychiatr. 1955, 75, 401–403. [Google Scholar] [PubMed]
- Hess, R. Narkolepsie und Epilepsie. Praxis 1965, 54, 96–102. [Google Scholar]
- Hess, C.W. Narkoleptische Syndrome. Schweiz. Med. Wochenschr. 1984, 114, 1664–1670. [Google Scholar] [PubMed]
- Luca, G.; Haba-Rubio, J.; Dauvilliers, Y.; Lammers, G.-J.; Overeem, S.; Donjacour, C.E.; Mayer, G.; Javidi, S.; Iranzo, A.; Santamaria, J.; et al. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: A European Narcolepsy Network study. J. Sleep. Res. 2013, 22, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Khatami, R.; Luca, G.; Baumann, C.R.; Bassetti, C.L.; Bruni, O.; Canellas, F.; Dauvilliers, Y.; Del Rio-Villegas, R.; Feketeova, E.; Ferri, R.; et al. The European Narcolepsy Network (EU-NN) database. JSR 2016, 25, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mayer, G.; Dauvilliers, Y.; Plazzi, G.; Pizza, F.; Fronczek, R.; Santamaria, J.; Partinen, M.; Overeem, S.; Peraita-Adrados, R.; et al. Exploring the clinical features of narcolepsy type 1 versus narcolepsy type 2 from European Narcolepsy Network database with machine learning. Sci. Rep. 2018, 8, 10628. [Google Scholar] [CrossRef] [PubMed]
- Dietmann, A.; Wenz, E.; van der Meer, J.; Ringli, M.; Warncke, J.D.; Edwards, E.; Schmidt, M.H.; Bernasconi, C.A.; Nirkko, A.; Strub, M.; et al. The Swiss Primary Hypersomnolence and Narcolepsy Cohort study (SPHYNCS): Study protocol for a prospective, multicentre cohort observational study. J. Sleep. Res. 2021, 30, e13296. [Google Scholar] [CrossRef] [PubMed]

| Narcolepsy with cataplexy (NT1) |
| Narcolepsy without cataplexy (NT2) |
| Idiopathic hypersomnia |
| Kleine–Levin syndrome |
| Insufficient sleep syndrome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassetti, C.L.A.; Khatami, R.; Miano, S.; Wenz, E.; Werth, E. The Swiss Narcolepsy Network (SNaNe). Clin. Transl. Neurosci. 2023, 7, 31. https://doi.org/10.3390/ctn7040031
Bassetti CLA, Khatami R, Miano S, Wenz E, Werth E. The Swiss Narcolepsy Network (SNaNe). Clinical and Translational Neuroscience. 2023; 7(4):31. https://doi.org/10.3390/ctn7040031
Chicago/Turabian StyleBassetti, Claudio L. A., Ramin Khatami, Silvia Miano, Elena Wenz, and Esther Werth. 2023. "The Swiss Narcolepsy Network (SNaNe)" Clinical and Translational Neuroscience 7, no. 4: 31. https://doi.org/10.3390/ctn7040031
APA StyleBassetti, C. L. A., Khatami, R., Miano, S., Wenz, E., & Werth, E. (2023). The Swiss Narcolepsy Network (SNaNe). Clinical and Translational Neuroscience, 7(4), 31. https://doi.org/10.3390/ctn7040031








