Narcolepsy Type 1: Should We Only Target Hypocretin Receptor 2?
Abstract
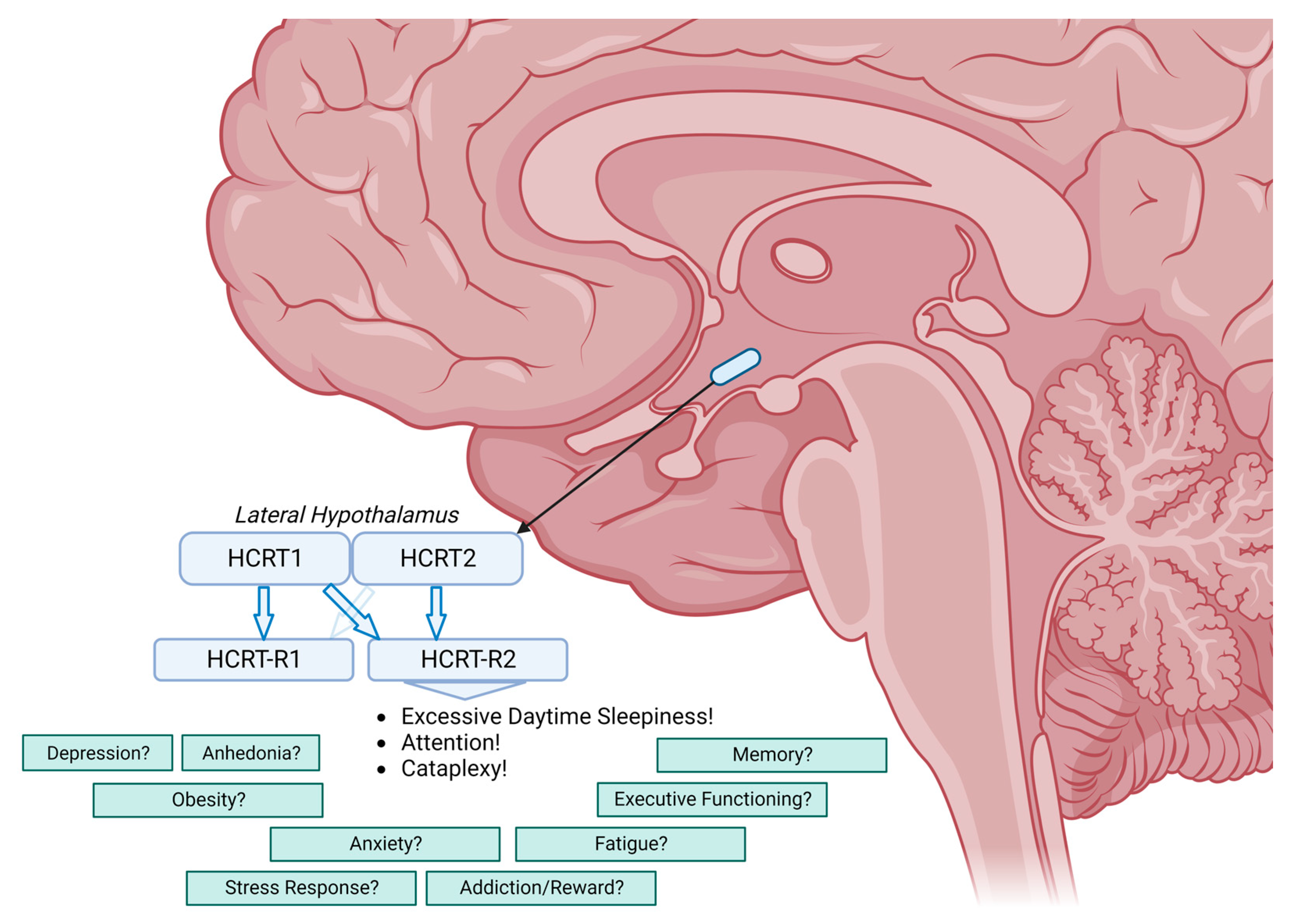
1. Introduction
2. Core Symptoms
2.1. Expressions of Excessive Daytime Sleepiness
2.2. Cataplexy
2.3. Nocturnal Sleep
2.4. Hypnagogic Hallucinations and Sleep Paralysis
3. Beyond the Core Symptoms
3.1. Executive Functioning
3.2. Memory
3.3. Depression and Anhedonia
3.4. Anxiety
3.5. Stress Response
3.6. Fatigue
3.7. Obesity
3.8. Addiction and Reward
3.9. Pain
4. Summary and Conclusions
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, L.; Faraco, J.; Li, R.; Kadotani, H.; Rogers, W.; Lin, X.; Qiu, X.; de Jong, P.J.; Nishino, S.; Mignot, E. The Sleep Disorder Canine Narcolepsy Is Caused by a Mutation in the Hypocretin (Orexin) Receptor 2 Gene. Cell 1999, 98, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Williams, S.C.; Xiong, Y.; Kisanuki, Y.; et al. Narcolepsy in Orexin Knockout Mice Molecular Genetics of Sleep Regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Liblau, R.S.; Latorre, D.; Kornum, B.R.; Dauvilliers, Y.; Mignot, E.J. The Immunopathogenesis of Narcolepsy Type 1. Nat. Rev. Immunol. 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Sakurai, T. The Present and Future of Synthetic Orexin Receptor Agonists. Peptides 2023, 167, 171051. [Google Scholar] [CrossRef]
- Seifinejad, A.; Ramosaj, M.; Shan, L.; Li, S.; Possovre, M.-L.; Pfister, C.; Fronczek, R.; Garrett-Sinha, L.A.; Frieser, D.; Honda, M.; et al. Epigenetic Silencing of Selected Hypothalamic Neuropeptides in Narcolepsy with Cataplexy. Proc. Natl. Acad. Sci. USA 2023, 120, e2220911120. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy—Clinical Spectrum, Aetiopathophysiology, Diagnosis and Treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef]
- Maski, K.; Steinhart, E.; Williams, D.; Scammell, T.; Flygare, J.; McCleary, K.; Gow, M. Listening to the Patient Voice in Narcolepsy: Diagnostic Delay, Disease Burden, and Treatment Efficacy. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2016, 13, 419–425. [Google Scholar] [CrossRef]
- Evans, R.; Kimura, H.; Alexander, R.; Davies, C.H.; Faessel, H.; Hartman, D.S.; Ishikawa, T.; Ratti, E.; Shimizu, K.; Suzuki, M.; et al. Orexin 2 Receptor–Selective Agonist Danavorexton Improves Narcolepsy Phenotype in a Mouse Model and in Human Patients. Proc. Natl. Acad. Sci. USA 2022, 119, e2207531119. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors That Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef] [PubMed]
- De Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.-B.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.F.; Gautvik, V.T.; Bartlett, F.S.; et al. The Hypocretins: Hypothalamus-Specific Peptides with Neuroexcitatory Activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef]
- Lammers, G.J.; Bassetti, C.L.A.; Dolenc-Groselj, L.; Jennum, P.J.; Kallweit, U.; Khatami, R.; Lecendreux, M.; Manconi, M.; Mayer, G.; Partinen, M.; et al. Diagnosis of Central Disorders of Hypersomnolence: A Reappraisal by European Experts. Sleep Med. Rev. 2020, 52, 101306. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, A.; Franken, P. Hypocretin (Orexin) Is Critical in Sustaining Theta/Gamma-Rich Waking Behaviors That Drive Sleep Need. Proc. Natl. Acad. Sci. USA 2017, 114, E5464–E5473. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Liu, H.Y.; Wang, B.Y.; Lin, H.N.; Wang, M.C.; Ren, J.-X. A Review of Physiological Functions of Orexin: From Instinctive Responses to Subjective Cognition. Medicine 2023, 102, e34206. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; Text Revision; American Academy of Sleep Medicine: Darien, IL, USA, 2023. [Google Scholar]
- Lee, M.G.; Hassani, O.K.; Jones, B.E. Discharge of Identified Orexin/Hypocretin Neurons across the Sleep-Waking Cycle. J. Neurosci. 2005, 25, 6716–6720. [Google Scholar] [CrossRef]
- Hasegawa, E.; Yanagisawa, M.; Sakurai, T.; Mieda, M. Orexin Neurons Suppress Narcolepsy via 2 Distinct Efferent Pathways. J. Clin. Investig. 2014, 124, 604–616. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Mignot, E.; del Villegas, R.R.; Du, Y.; Hanson, E.; Inoue, Y.; Kadali, H.; Koundourakis, E.; Meyer, S.; Rogers, R.; et al. Oral Orexin Receptor 2 Agonist in Narcolepsy Type 1. N. Engl. J. Med. 2023, 389, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Executive Functioning. Available online: Merriam-Webster.com (accessed on 1 August 2023).
- Gao, W.-R.; Hu, X.-H.; Yu, K.-Y.; Cai, H.-Y.; Wang, Z.-J.; Wang, L.; Wu, M.-N. Selective Orexin 1 Receptor Antagonist SB-334867 Aggravated Cognitive Dysfunction in 3xTg-AD Mice. Behav. Brain Res. 2023, 438, 114171. [Google Scholar] [CrossRef]
- Quaedackers, L.; Fortuyn, H.D.; Gilst, M.V.; Lappenschaar, M.; Overeem, S. Dissociative Symptoms Are Highly Prevalent in Adults with Narcolepsy Type 1. Behav. Sleep Med. 2022, 20, 63–73. [Google Scholar] [CrossRef]
- Fagan, H.; Jones, E.; Baldwin, D.S. Orexin Receptor Antagonists in the Treatment of Depression: A Leading Article Summarising Pre-Clinical and Clinical Studies. CNS Drugs 2023, 37, 1–12. [Google Scholar] [CrossRef]
- Scott, M.M.; Marcus, J.N.; Pettersen, A.; Birnbaum, S.G.; Mochizuki, T.; Scammell, T.E.; Nestler, E.J.; Elmquist, J.K.; Lutter, M. Hcrtr1 and 2 Signaling Differentially Regulates Depression-like Behaviors. Behav. Brain Res. 2011, 222, 289–294. [Google Scholar] [CrossRef]
- Ruoff, C.M.; Reaven, N.L.; Funk, S.E.; McGaughey, K.J.; Ohayon, M.M.; Guilleminault, C.; Black, J. High Rates of Psychiatric Comorbidity in Narcolepsy: Findings from the Burden of Narcolepsy Disease (BOND) Study of 9,312 Patients in the United States. J. Clin. Psychiatry 2016, 78, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Abenza-Abildua, M.J.; Suárez-Gisbert, E.; Lores-Gutiérrez, V.; Algarra-Lucas, C.; Gómez-Aceña, Á.; Navacerrada-Barrero, F.J.; González-Martín, L.; Pérez-Villena, A.; Pérez-López, C. Anxiety and Depression in Patients with Narcolepsy. J. Sleep Res. 2023, 32, e13812. [Google Scholar] [CrossRef]
- Szakacs, Z.; Dauvilliers, Y.; Mikhaylov, V.; Poverennova, I.; Krylov, S.; Jankovic, S.; Sonka, K.; Lehert, P.; Lecomte, I.; Lecomte, J.-M.; et al. Safety and Efficacy of Pitolisant on Cataplexy in Patients with Narcolepsy: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2017, 16, 200–207. [Google Scholar] [CrossRef]
- Inocente, C.O.; Gustin, M.-P.; Lavault, S.; Guignard-Perret, A.; Raoux, A.; Christol, N.; Gerard, D.; Dauvilliers, Y.; Reimão, R.; Bat-Pitault, F.; et al. Depressive Feelings in Children with Narcolepsy. Sleep Med. 2014, 15, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Linssen, S.; Harteman, Z.; Dekker, F.; Shuker, L.; Balesar, R.; Breesuwsma, N.; Anink, J.; Zhou, J.; Lammers, G.J.; et al. Activated Wake Systems in Narcolepsy Type 1. Ann. Neurol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kok, S.W.; Roelfsema, F.; Overeem, S.; Lammers, G.J.; Strijers, R.L.; Frölich, M.; Meinders, A.E.; Pijl, H. Dynamics of the Pituitary-Adrenal Ensemble in Hypocretin-Deficient Narcoleptic Humans: Blunted Basal Adrenocorticotropin Release and Evidence for Normal Time-Keeping by the Master Pacemaker. J. Clin. Endocrinol. Metab. 2002, 87, 5085–5091. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, E.; Keckeis, M.; Lattová, Z.; Kemlink, D.; Wetter, T.; Schuld, A.; Sonka, K.; Pollmächer, T. Hypothalamo–Pituitary–Adrenal Axis, Glucose Metabolism and TNF-α in Narcolepsy. J. Sleep Res. 2014, 23, 425–431. [Google Scholar] [CrossRef]
- Fortuyn, H.A.D.; Fronczek, R.; Smitshoek, M.; Overeem, S.; Lappenschaar, M.; Kalkman, J.; Renier, W.; Buitelaar, J.; Lammers, G.J.; Bleijenberg, G. Severe Fatigue in Narcolepsy with Cataplexy. J. Sleep Res. 2011, 21, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.; Baumann, C.R.; Scammell, T.E. Narcolepsy; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9781441983893. [Google Scholar]
- Fronczek, R.; Overeem, S.; Reijntjes, R.; Lammers, G.J.; van Dijk, J.G.; Pijl, H. Increased Heart Rate Variability but Normal Resting Metabolic Rate in Hypocretin/Orexin-Deficient Human Narcolepsy. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2008, 4, 248–254. [Google Scholar] [CrossRef]
- Straat, M.E.; Schinkelshoek, M.S.; Fronczek, R.; Lammers, G.J.; Rensen, P.C.N.; Boon, M.R. Role of Brown Adipose Tissue in Adiposity Associated with Narcolepsy Type 1. Front. Endocrinol. 2020, 11, 145. [Google Scholar] [CrossRef]
- Sellayah, D.; Bharaj, P.; Sikder, D. Orexin Is Required for Brown Adipose Tissue Development, Differentiation, and Function. Cell Metab. 2011, 14, 478–490. [Google Scholar] [CrossRef]
- Schinkelshoek, M.S.; Smolders, I.M.; Donjacour, C.E.; Meijden, W.P.; Zwet, E.W.; Fronczek, R.; Lammers, G.J. Decreased Body Mass Index during Treatment with Sodium Oxybate in Narcolepsy Type 1. J. Sleep Res. 2019, 28, e12684. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, M.; Tsuneoka, Y.; Takase, K.; Kim, S.J.; Choi, J.; Ikkyu, A.; Abe, M.; Sakimura, K.; Yanagisawa, M.; Funato, H. Differential Roles of Each Orexin Receptor Signaling in Obesity. iScience 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Peyron, C.; Tighe, D.K.; van den Pol, A.N.; de Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef]
- Harris, G.C.; Wimmer, M.; Aston-Jones, G. A Role for Lateral Hypothalamic Orexin Neurons in Reward Seeking. Nature 2005, 437, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, T.C.; John, J.; Shan, L.; Swaab, D.F.; Wu, M.-F.; Ramanathan, L.; McGregor, R.; Chew, K.-T.; Cornford, M.; Yamanaka, A.; et al. Opiates Increase the Number of Hypocretin-Producing Cells in Human and Mouse Brain and Reverse Cataplexy in a Mouse Model of Narcolepsy. Sci. Transl. Med. 2018, 10, eaao4953. [Google Scholar] [CrossRef] [PubMed]
- Scammell, T.E.; Saper, C.B. Orexins: Looking Forward to Sleep, Back at Addiction. Nat. Med. 2007, 13, 126–128. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, H.; Nagumo, Y.; Ishikawa, Y.; Irukayama-Tomobe, Y.; Namekawa, Y.; Nemoto, T.; Tanaka, H.; Takahashi, G.; Tokuda, A.; Saitoh, T.; et al. OX2R-Selective Orexin Agonism Is Sufficient to Ameliorate Cataplexy and Sleep/Wake Fragmentation without Inducing Drug-Seeking Behavior in Mouse Model of Narcolepsy. PLoS ONE 2022, 17, e0271901. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.; Davey, P.T.; Babbs, A.J.; Irving, E.A.; Sammons, M.J.; Wyles, M.; Jeffrey, P.; Cutler, L.; Riba, I.; Johns, A.; et al. Orexin-A, an Hypothalamic Peptide with Analgesic Properties. Pain 2001, 92, 81–90. [Google Scholar] [CrossRef]
- Asano, H.; Arima, Y.; Yokota, S.; Fujitani, M. New Nociceptive Circuits to the Hypothalamic Perifornical Area from the Spinal Cord and Spinal Trigeminal Nucleus via the Parabrachial Nucleus. Biochem. Biophys. Res. Commun. 2019, 512, 705–711. [Google Scholar] [CrossRef]
- Holland, P.R.; Akerman, S.; Goadsby, P.J. Orexin 1 Receptor Activation Attenuates Neurogenic Dural Vasodilation in an Animal Model of Trigeminovascular Nociception. J. Pharmacol. Exp. Ther. 2005, 315, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Saito, O.; Shono, K.; Aoe, T.; Chiba, T. Anti-Mechanical Allodynic Effect of Intrathecal and Intracerebroventricular Injection of Orexin-A in the Rat Neuropathic Pain Model. Neurosci. Lett. 2003, 347, 183–186. [Google Scholar] [CrossRef]
- Dahmen, N.; Kasten, M.; Wieczorek, S.; Gencik, M.; Epplen, J.; Ullrich, B. Increased Frequency of Migraine in Narcoleptic Patients: A Confirmatory Study. Cephalalgia 2003, 23, 14–19. [Google Scholar] [CrossRef]
- Chabi, A.; Zhang, Y.; Jackson, S.; Cady, R.; Lines, C.; Herring, W.J.; Connor, K.M.; Michelson, D. Randomized Controlled Trial of the Orexin Receptor Antagonist Filorexant for Migraine Prophylaxis. Cephalalgia 2014, 35, 379–388. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fronczek, R.; Lammers, G.J. Narcolepsy Type 1: Should We Only Target Hypocretin Receptor 2? Clin. Transl. Neurosci. 2023, 7, 28. https://doi.org/10.3390/ctn7030028
Fronczek R, Lammers GJ. Narcolepsy Type 1: Should We Only Target Hypocretin Receptor 2? Clinical and Translational Neuroscience. 2023; 7(3):28. https://doi.org/10.3390/ctn7030028
Chicago/Turabian StyleFronczek, Rolf, and Gert Jan Lammers. 2023. "Narcolepsy Type 1: Should We Only Target Hypocretin Receptor 2?" Clinical and Translational Neuroscience 7, no. 3: 28. https://doi.org/10.3390/ctn7030028
APA StyleFronczek, R., & Lammers, G. J. (2023). Narcolepsy Type 1: Should We Only Target Hypocretin Receptor 2? Clinical and Translational Neuroscience, 7(3), 28. https://doi.org/10.3390/ctn7030028





