Implementation of an International Vessel Wall MR Plaque Imaging Research Network: Experience with the ChAMPION Study
Abstract
:1. Introduction
2. Methods
2.1. Overview of Project
2.2. Development of PUMC as a Central Coordinating Center
2.3. Selection of Sites in China
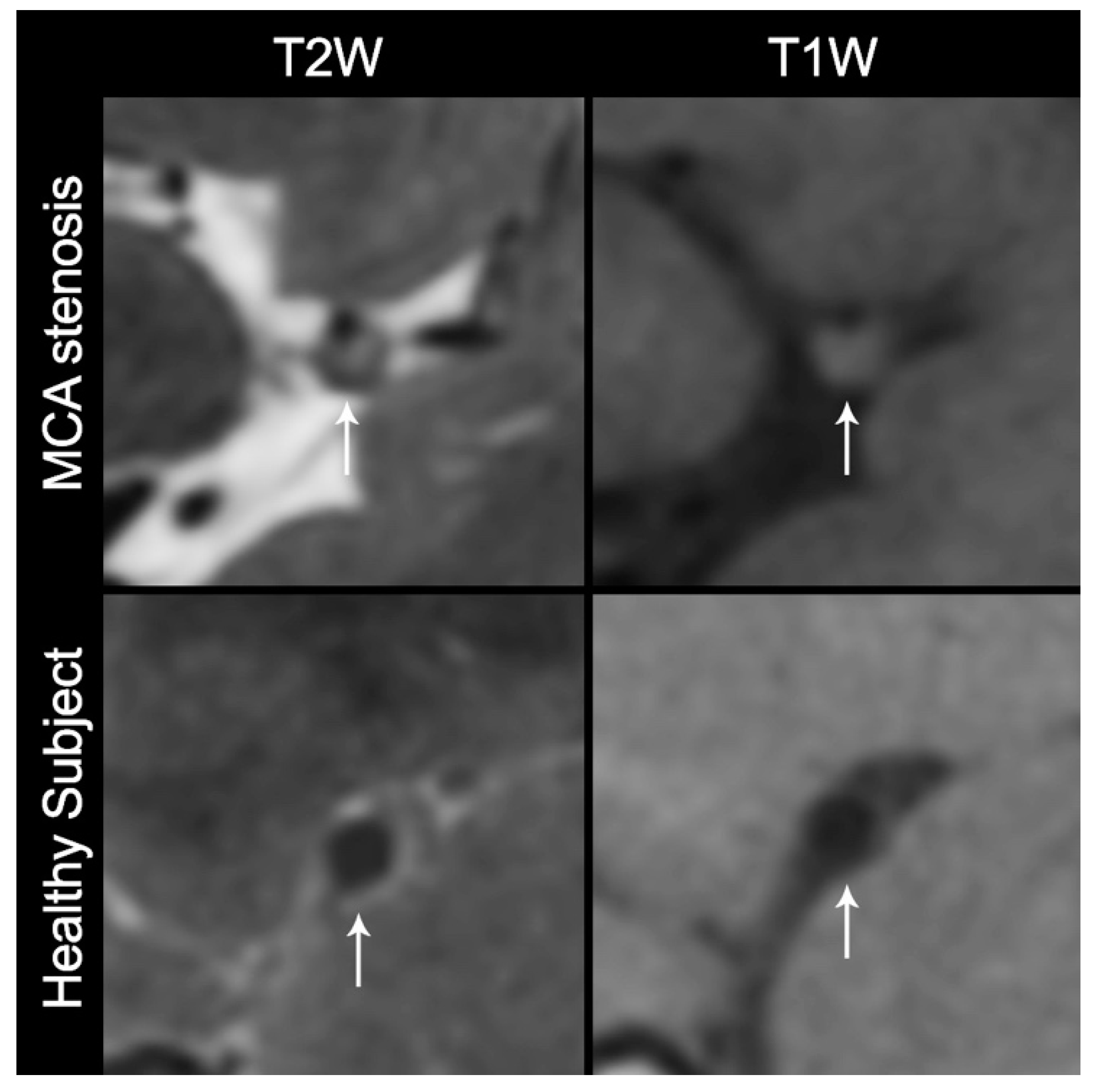
2.4. Development of VW-MR Research Infrastructure
2.4.1. Selection of Imaging Sequences
2.4.2. Image Quality Assessment and Refinement
2.5. Collaboration and Interaction
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorelick, P.B.; Wong, K.S.; Bae, H.J.; Pandey, D.K. Large artery intracranial occlusive disease—A large worldwide burden but a relatively neglected frontier. Stroke A J. Cereb. Circ. 2008, 39, 2396–2399. [Google Scholar] [CrossRef] [Green Version]
- Johnston, S.C.; Mendis, S.; Mathers, C.D. Global variation in stroke burden and mortality: Estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009, 8, 345–354. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Liu, L.; Soo, Y.O.; Pu, Y.; Pan, Y.; Wang, Y.; Zou, X.; Leung, T.W.; Cai, Y.; et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: The Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014, 45, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhou, Y.; Zhang, Y.; Gao, X.; Zhang, Q.; Wang, A.; Jia, Z.; Wu, S.; Zhao, X. Prevalence and risk factors of asymptomatic intracranial arterial stenosis in a community-based population of Chinese adults. Eur. J. Neurol. 2013, 20, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Li, M.L.; Gao, S.; Ni, J.; Yao, M.; Zhou, L.X.; Peng, B.; Feng, F.; Jin, Z.Y.; Cui, L.Y. Middle cerebral artery intraplaque hemorrhage: Prevalence and Clinical Relevance. Ann. Neurol. 2012, 71, 195–198. [Google Scholar] [CrossRef]
- Turan, T.N.; Rumboldt, Z.; Granholm, A.C.; Columbo, L.; Welsh, C.T.; Lopes-Virella, M.F.; Spampinato, M.V.; Brown, T.R. Intracranial atherosclerosis: Correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis 2014, 237, 460–463. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.L.; Zhu, C.C.; Peng, W.J.; Degnan, A.J.; Chen, L.G.; Wang, X.R.; Liu, Q.; Wang, Y.; Xiang, Z.Z.; Teng, Z.Z.; et al. Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI. Atherosclerosis 2016, 249, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.H.; Li, M.L.; Gao, S.; Ni, J.; Zhou, L.X.; Yao, M.; Peng, B.; Feng, F.; Jin, Z.Y.; Cui, L.Y. Plaque distribution of stenotic middle cerebral artery and its clinical relevance. Stroke A J. Cereb. Circ. 2011, 42, 2957–2959. [Google Scholar] [CrossRef] [Green Version]
- Dieleman, N.; van der Kolk, A.G.; Zwanenburg, J.J.; Harteveld, A.A.; Biessels, G.J.; Luijten, P.R.; Hendrikse, J. Imaging intracranial vessel wall pathology with magnetic resonance imaging: Current prospects and future directions. Circulation 2014, 130, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.; Lee, D.H.; Kang, D.W.; Kwon, S.U.; Kim, J.S. Single Subcortical Infarction and Atherosclerotic Plaques in the Middle Cerebral Artery High-Resolution Magnetic Resonance Imaging Findings. Stroke A J. Cereb. Circ. 2013, 44, 2462–2467. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.N.; Liu, M.W.; Villablanca, J.P.; Li, M.L.; Xu, Y.Y.; Gao, S.; Feng, F.; Liebeskind, D.S.; Scalzo, F.; Xu, W.H. Middle Cerebral Artery Plaque Hyperintensity on T2-Weighted Vessel Wall Imaging Is Associated with Ischemic Stroke. AJNR Am. J. Neuroradiol. 2019, 40, 1886–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandell, D.M.; Mossa-Basha, M.; Qiao, Y.; Hess, C.P.; Hui, F.; Matouk, C.; Johnson, M.H.; Daemen, M.J.A.P.; Vossough, A.; Edjlali, M.; et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. Am. J. Neuroradiol. 2017, 38, 218–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chueh, J.Y.; van der Marel, K.; Gounis, M.J.; LeMatty, T.; Brown, T.R.; Ansari, S.A.; Carroll, T.J.; Buck, A.K.; Zhou, X.J.; Chatterjee, A.R.; et al. Development of a high resolution MRI intracranial atherosclerosis imaging phantom. J. Neurointerv. Surg. 2018, 10, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Turan, T.N.; Rumboldt, Z.; Brown, T.R. High-resolution MRI of basilar atherosclerosis: Three-dimensional acquisition and FLAIR sequences. Brain Behav. 2013, 3, 1–3. [Google Scholar] [CrossRef]
- Turan, T.N.; Bonilha, L.; Morgan, P.S.; Adams, R.J.; Chimowitz, M.I. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2011, 21, e159–e161. [Google Scholar] [CrossRef]
- Bodle, J.D.; Feldmann, E.; Swartz, R.H.; Rumboldt, Z.; Brown, T.; Turan, T.N. High-resolution magnetic resonance imaging: An emerging tool for evaluating intracranial arterial disease. Stroke A J. Cereb. Circ. 2013, 44, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.H.; Li, M.L.; Gao, S.; Ni, J.; Zhou, L.X.; Yao, M.; Peng, B.; Wang, J.M.; Cui, L.Y. Middle Cerebral Artery Plaque in Patients with A Single Infarct in the Territory of Deep Penetrating Arteries: A High-resolution MRI Study. Stroke A J. Cereb. Circ. 2011, 42, E125. [Google Scholar]
- Xu, Y.Y.; Li, M.L.; Gao, S.; Hou, B.; Sun, Z.Y.; Zhou, H.L.; Feng, F.; Xu, W.H. Non-moyamoya vessel network formation along steno-occlusive middle cerebral artery. Neurology 2016, 86, 1957–1963. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.N.; Li, M.L.; Xu, Y.Y.; Meng, Y.; Trieu, H.; Villablanca, J.P.; Gao, S.; Feng, F.; Liebeskind, D.S.; Xu, W.H. Middle cerebral artery geometric features are associated with plaque distribution and stroke. Neurology 2018, 91, e1760–e1769. [Google Scholar] [CrossRef]
- Xu, W.H.; Li, M.L.; Gao, S.; Ni, J.; Wang, H.; Liu, C.Y.; Peng, B.; Cui, L.Y. Different Cross-sectional Images Between Symptomatic And Asymptomatic Atherosclerotic Middle Cerebral Artery Stenosis: A 3-T MRI Study In Vivo. Stroke A J. Cereb. Circ. 2009, 40, E119. [Google Scholar]
- Lin, Q.; Liu, X.; Chen, B.; Tian, D.; Liu, C.; Du, A.; Lu, B.; Liu, G.; Wu, B.; Li, L.; et al. Design of stroke imaging package study of intracranial atherosclerosis: A multicenter, prospective, cohort study. Ann. Transl. Med. 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Minisman, G.; Bhanushali, M.; Conwit, R.; Wolfe, G.I.; Aban, I.; Kaminski, H.J.; Cutter, G. Implementing clinical trials on an international platform: Challenges and perspectives. J. Neurol. Sci. 2012, 313, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndebele, P.; Blanchard-Horan, C.; Shahkolahi, A.; Sanne, I. Regulatory challenges associated with conducting multicountry clinical trials in resource-limited settings. J. Acquir. Immune Defic. Syndr. 2014, 65 (Suppl. 1), S29–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Joensuu, H.; Simes, R.J.; Price, T.J.; Yip, S.; Hague, W.; Sjoquist, K.M.; Zalcberg, J. Challenges of international oncology trial collaboration-a call to action. Br. J. Cancer 2019, 121, 515–521. [Google Scholar] [CrossRef] [PubMed]

| Site Name | How Many Patients Who Have 50–99% Stenosis | How Was the Answer Determined? | MRI Scanner Magnet | Make and Model | Software | Head Coil | Available for Research |
|---|---|---|---|---|---|---|---|
| Fujian Medical University Union Hospital | 160–180 | Registry and estimation | 3 | GE Discovery MR750 3.0T | 2.6.27 | 8 | Yes, patients |
| Peking Union Medical College Hospital | 100 | registry | 3 | Siemens Skyra, GE750 | VA20, DV24 | 32 | Yes, patients |
| Second Affiliated Hospital of Zhejiang University | 100 | estimation | 1.5 and 3 | GE 750W | DV 23.1_1317c | 8 | Yes, do not use it |
| Northern Jiangsu People’s Hospital | 275 | registry | 1.5 and 3 | GE MR750; GE MR 750W: GE MR360 | GE DV 24 | 8, 16, 32 | Yes, patients |
| Daping Hospital, Third Military Medical University | 2000+ | estimation | 1.5 and 3 | Siemens Verio 3T | VB17 | 8 | Yes, Research Only |
| Chinese People’s Armed Police General Hospital | 66 | estimation | 1.5 and 3 | Siemens Trio Tim | Syngo MR B17 | 8 | Yes, patients |
| The First Hospital of Jilin University | 100’s | estimation | 1.5 and 3 | Siemens Trio | Siemens VB15 | 12 | Yes, patients |
| Tangshan Gongren Hospital | 40 | estimation | 1.5 and 3 | GE Signa HDst: Philips Achieva | GE Release HD16.0_1131a; Philips Release 3.2.3.2 | 8, 16 | Yes, do not use it |
| The First Affiliated Hospital of University of South China | 800 | Registry and estimation | 1.5 and 3 | Philips Achieva TX 3.0T | Achieva Release 2.6.3.6 | 8, 16 | Yes, patients |
| Peking University First Hospital | 500 | estimation | 3 | GE MR750 | DV22 | 32 | Yes, patients |
| General Hospital of Shenyang Military Region | 800–1000 | estimation | 1.5 and 3 | GE Discovery MR750 | V03.20 | 8 | Yes, patients |
| Site Name | MRI Scanner Type | Number of Patient Scans | Number of Phantom Scans | Number of Incomplete Patient Scans | Total Scans |
|---|---|---|---|---|---|
| Fujian Medical University Union Hospital | GE | 11 | 1 | 0 | 12 |
| Peking Union Medical College Hospital | GE, Siemens | 13 | 2 | 1 | 16 |
| Second Affiliated Hospital of Zhejiang University | GE | 2 | 1 | 1 | 4 |
| Northern Jiangsu People’s Hospital | GE | 1 | 1 | 1 | 3 |
| Daping Hospital of Third Military Medical University | Siemens | 3 | 1 | 0 | 4 |
| Chinese People’s Armed Police General Hospital | Siemens | 6 | 1 | 1 | 8 |
| First Hospital Jilin University | Siemens | unsuccessful | 0 | ||
| Tangshan Gongren Hospital | GE, Philips | unsuccessful | 0 | ||
| The First Affiliated Hospital of University of South China | Philips | unsuccessful | 0 | ||
| Peking University First Hospital | GE | unsuccessful | 0 | ||
| General Hospital of Shenyang Military Region | GE | unsuccessful | 0 | ||
| Total | 36 | 7 | 4 | 47 |
| T1-Weighted | T2-Weighted | |||||||
|---|---|---|---|---|---|---|---|---|
| Site Name | MRI Model | Slice Thickness, mm | Resolution | Field of View | TR, ms | TE, ms | TR, ms | TE, ms |
| Fujian Medical University Union Hospital | GE Discovery MR750 | 1.2 | 0.468 × 0.468 | 256 × 256 | 440 | 14 | 1500 | 114 |
| Peking Union Medical College Hospital | GE Discovery MR750 | 1.2 | 0.468 × 0.468 | 256 × 256 | 440 | 14 | 1500 | 109 |
| Second Affiliated Hospital of Zhejiang University | GE Discovery MR750 | 1.2 | 0.234 × 0.234 | 512 × 512 | 440 | 14 | 1500 | 114 |
| Northern Jiangsu People’s Hospital | GE Discovery MR750 | 1.2 | 0.468 × 0.468 | 256 × 256 | 440 | 15 | 1500 | 107 |
| Daping Hospital of Third Military Medical University | Siemens Verio | 1.2 | 0.4 × 0.4 | 320 × 320 | 458 | 16 | 1500 | 64 |
| Chinese People’s Armed Police General Hospital | Siemens Trio Tim | 1.2 | 0.4 × 0.4 | 320 × 320 | 458 | 16 | 1500 | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Xu, W.-H.; Chatterjee, A.R.; LeMatty, T.; Yao, M.; Li, M.-L.; Brown, T.; Spampinato, M.V.; Martin, R.; Chimowitz, M.I.; et al. Implementation of an International Vessel Wall MR Plaque Imaging Research Network: Experience with the ChAMPION Study. Clin. Transl. Neurosci. 2022, 6, 16. https://doi.org/10.3390/ctn6030016
Yu Y, Xu W-H, Chatterjee AR, LeMatty T, Yao M, Li M-L, Brown T, Spampinato MV, Martin R, Chimowitz MI, et al. Implementation of an International Vessel Wall MR Plaque Imaging Research Network: Experience with the ChAMPION Study. Clinical and Translational Neuroscience. 2022; 6(3):16. https://doi.org/10.3390/ctn6030016
Chicago/Turabian StyleYu, Yannan, Wei-Hai Xu, Arindam Rano Chatterjee, Todd LeMatty, Meng Yao, Ming-Li Li, Truman Brown, Maria Vittoria Spampinato, Renee Martin, Marc I. Chimowitz, and et al. 2022. "Implementation of an International Vessel Wall MR Plaque Imaging Research Network: Experience with the ChAMPION Study" Clinical and Translational Neuroscience 6, no. 3: 16. https://doi.org/10.3390/ctn6030016
APA StyleYu, Y., Xu, W.-H., Chatterjee, A. R., LeMatty, T., Yao, M., Li, M.-L., Brown, T., Spampinato, M. V., Martin, R., Chimowitz, M. I., Derdeyn, C., & Turan, T. N., on behalf of ChAMPION Investigators. (2022). Implementation of an International Vessel Wall MR Plaque Imaging Research Network: Experience with the ChAMPION Study. Clinical and Translational Neuroscience, 6(3), 16. https://doi.org/10.3390/ctn6030016






