1. Introduction
Primary progressive aphasia (PPA) is a neurodegenerative disease whose prominent clinical feature is language impairment. We can further distinguish between non-fluent or agrammatic, logopenic, and semantic variants, which cover different neuropathological diagnostic categories (Alzheimer’s disease; frontotemporal dementia; and, more rarely, cortico-basal degeneration, progressive supranuclear palsy, and Pick’s disease) [
1]. Non-fluent variant PPA (nfvPPA), in particular, is characterized [
1] by (i) agrammatism and/or apraxia of speech; (ii) impaired comprehension of complex sentences and/or preserved comprehension of single words and/or preserved object knowledge; and (iii) left posterior frontal and insular atrophy or hypoperfusion/hypometabolism in the same region.
It has a progressive course of up to 14 years, with agrammatism as a prominent feature. However, in some cases, it can evolve with a dominant presence of apraxia of speech (DAoS) compared to agrammatism, or even solely with the presence of Progressive Primary Apraxia of Speech (PPAoS) in the absence of agrammatism [
2]. PPAoS is characterized by a slow speech rate, sound distortions, segmentation of syllables, and increased difficulty with long words [
3]. Particularly, naming capacities often show a marked decline (for example 20% decline over 2 years in naming tasks) [
4].
Many therapies for PPA are based on word retrieval, using both phonological and semantic treatment, or sometimes both [
5,
6]. A classical therapy for nfvPPA is script training. Working on personalized scripts allows the patient to target the two main symptoms of nfvPPA, namely agrammatism and apraxia of speech [
7]. Lastly, implementing an assistive augmentative communication (AAC) device is essential to ensure functional communication as the disease is progressing [
8,
9].
Several tDCS therapy strategies have been proposed to support communication and slow the diseases’ progression, which classically involves the left prefrontal region, including the inferior frontal gyrus. Besides the established focused speech therapy [
7], several non-invasive brain stimulation techniques (NIBS) have been proposed, giving some interesting, although inconsistent, results in post-stroke aphasia [
10]. Two of the most common NIBS that are being investigated in post-stroke aphasia research are TMS and tDCS. The rationale behind their application in aphasia rehabilitation is that both methods employ an electric field to influence neurons’ activity, with the first inducing neuron action potentials and the latter producing significant cortical network excitability [
11]. Repetitive TMS applied to the left inferior and superior frontal gyrus has been shown to improve language production and spontaneous speech, together with increasing left parietotemporal and left frontal areas metabolism [
12]. Moreover, tDCS has been essentially used in vascular aphasia, but degenerative nfvPPA has also been proposed for neuromodulation. Written naming [
13] and verb comprehension were the only tasks that improved significantly after right parietal tDCS for 5 days, and improvement lasted 2 weeks. Furthermore, tDCS over the left IFG, along with written naming and spelling therapy for 2 weeks, gave rise to a greater improvement compared to the sham coupled with the same therapy. The generalization of tDCS gains persisted for 2 months. In another study, tDCS over the left IFG during 10–14 days improved written verb naming compared to sham [
14,
15]. Interestingly, a lower resting-state fMRI connectivity in the left IFG and temporal areas following tDCS stimulation compared to sham condition [
16] has been observed, and the changes correlate with language outcomes’ improvement. However, most of the studies are of rather short therapy [
13]. In their systematic review, Coemans et al. (2021) [
17] reported that 13 of the 17 studies focused stimulation sites on the main locus of atrophy in nfvPPA patients either with an anode placed in the left inferior frontal, corresponding to the F7 electrode in the EEG 10–20 electrode position system or in the left temporoparietal areas, and the cathode placed on the right fronto-orbital area, forehead, or right shoulder. Interestingly, a longitudinal study showed improved sentence processing and neurocognitive process changes after 18 months of speech therapy in a patient with nfvPPA [
18]. Moreover, these studies mainly focused on written and oral lexical access rather than measures of speech or language fluency. A systematic review [
19] included 16 studies and analyzed the factors that predicted the maintenance of language performance over time. Continuous practice following therapy, length of the treatment, and frequency of the sessions were the different factors found.
A meta-analysis focusing on tDCS and TMS effects added to speech therapy showed interesting results concerning the use of tDCS for PPA [
20]. A significant effect was found on language performance in patients who received tDCS or TMS compared with those who received the sham in addition to speech therapy. However, future research needs to be conducted, particularly to assess which variables can generate positive gains for persons with PPA.
Thus, there are a certain number of encouraging results in the tDCS stimulation of patients with nfvPPA. However, these data reflected rather short interventions [
21], considering that nfvPPA develops over more than 6 years. For this reason, we were interested in assessing the effects of a longer therapeutic approach. In particular, our aim was to assess if our therapy is sustainable, that is, to be correctly followed if it lasts more than 6 months and if the initial improvement persists with longer speech training. Given this configuration, we adapted an initial hospital training followed by home training for the patient with alternation of 3 weeks with and without tDCS therapy. Some studies showed that patients with PPA may exhibit mood and/or behavioral symptoms, even in the early stages of the disease [
22,
23,
24]. The study by Tarun et al. [
22] found that nfvPPA patients are more likely to present with appetite changes. On the other hand, there were some studies indicating that tDCS can improve mood in healthy individuals [
25,
26,
27]. To the best of our knowledge, no study on the effects of tDCS on mood in patients with PPA was reported. Our main interest was to confirm a good adherence to therapy, a language improvement, or at least non-deterioration, over 8 months, and to analyze mood and behavioral questionnaires with the prediction that such changes in subjective markers would be long lasting. Additionally, the imaging characteristic of nfvPPA is asymmetrical atrophy in cerebral regions involved in language processing, including the inferior frontal gyrus and the insula cortex, with extension to the superior temporal gyrus [
28]. A study on longitudinal imaging changes in PPA indicated that the severest atrophic progression within a 1-year follow-up occurred in the basal ganglia in nfvPPA patients [
29]. Hence, we were also interested in whether this patient presented nfvPPA-specific atrophy patterns in structural MRI and comparing brain morphometry before and after the program.
Case Report: Medical History and Clinical Examination
Mr. LA is a 75-years-old native English speaker and former right-handed security professional born in the USA and currently living in Switzerland. He complained of frustrating speech impediments, which started in 2016, for which he had speech therapy in Switzerland and in Hong Kong. In 2019, language had become more effortful and less accessible. During his first evaluation, language had become less spontaneous, and he spoke in shorter sentences, without agrammatism. The volume of his voice was weaker. He had the impression of having a frozen face and jaw without dysphagia. His wife reported difficulty in finding the words, sometimes with a stammer, stumbling on the first syllable. Writing was less spontaneous. Speaking French had become almost impossible. There were neither personality changes nor delusional symptoms. He did not mention memory or planning disorders, and the activities of daily living were preserved. He complained of some motor slowing, but neither muscle stiffness nor a history of cramps or myoclonus cramps was present. He had no difficulty with the fine motility of his hand to open boxes, cut meat, and shave. The axial motility to get up from a chair, turn over in bed, and put on shirts with sleeves or trousers was normal.
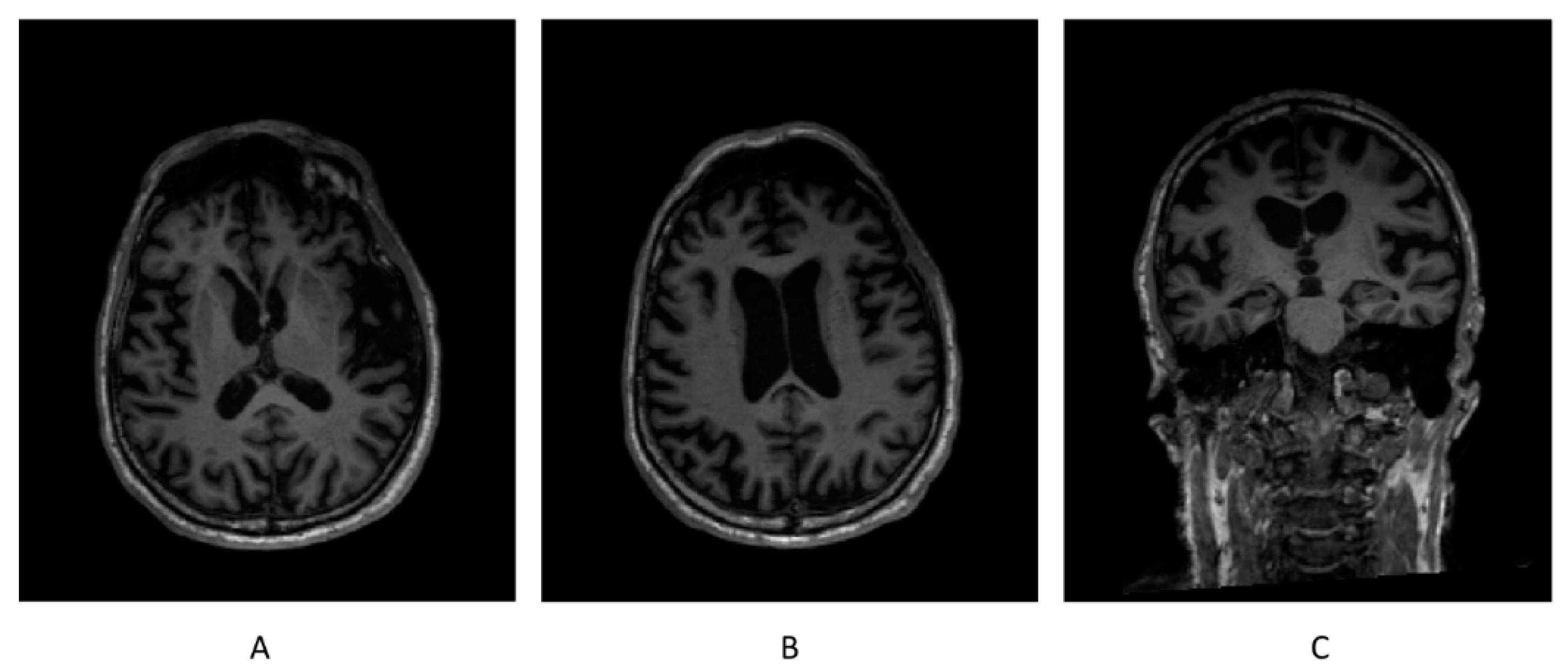
Neurological examination in September 2020 disclosed a well-oriented patient in time and space. His score on the Montreal Cognitive Assessment was 29/30, and 16/18 on the Frontal Assessment Battery. His working memory, recent verbal, and episodic memory were also well preserved. No difficulty in descriptions or semantic associations were observed. He recognized famous faces well. He had neither perceptual nor associative difficulties with visual gnosis. Meaningless gestures were at 4/6; pantomimes, and meaningful gestures were well executed by both hands. Mathematical calculation was rapid. There was no emotional lability. The rest of the neurological examination was normal. His brain MRI showed moderate bi-frontal atrophy.
The subject’s language capacity was tested in September 2020 and was deemed to be non-fluent, with short sentences and a tendency toward breathlessness, and he had a moderate apraxia of speech (AoS). Spontaneous and elicited speech were non-fluent yet informative, marked by latencies before speaking, with the need to close his eyes before speaking. Word naming did not show any paraphasia or semantic disorder. Comprehension, repetition, and written language were normal. Writing was a bit slow but not micrographic. Speech was characterized by a slowed speech rate (83 words/minute) and syntactically simple utterances (MLU of 9.4, with a mean of 9.2 [
30]). There were also rare phonetic distortions. In a picture description test, iterations at the beginning of sentences were present. Noun and action naming were quantitatively preserved. Sentence and text reading was below standard, characterized by substitutions of visually related words (e.g., “then” for “the”), self-corrections, and disfluencies. The rest of language evaluation is summarized in
Table 1.
2. Materials and Methods
2.1. Therapy Program—Design
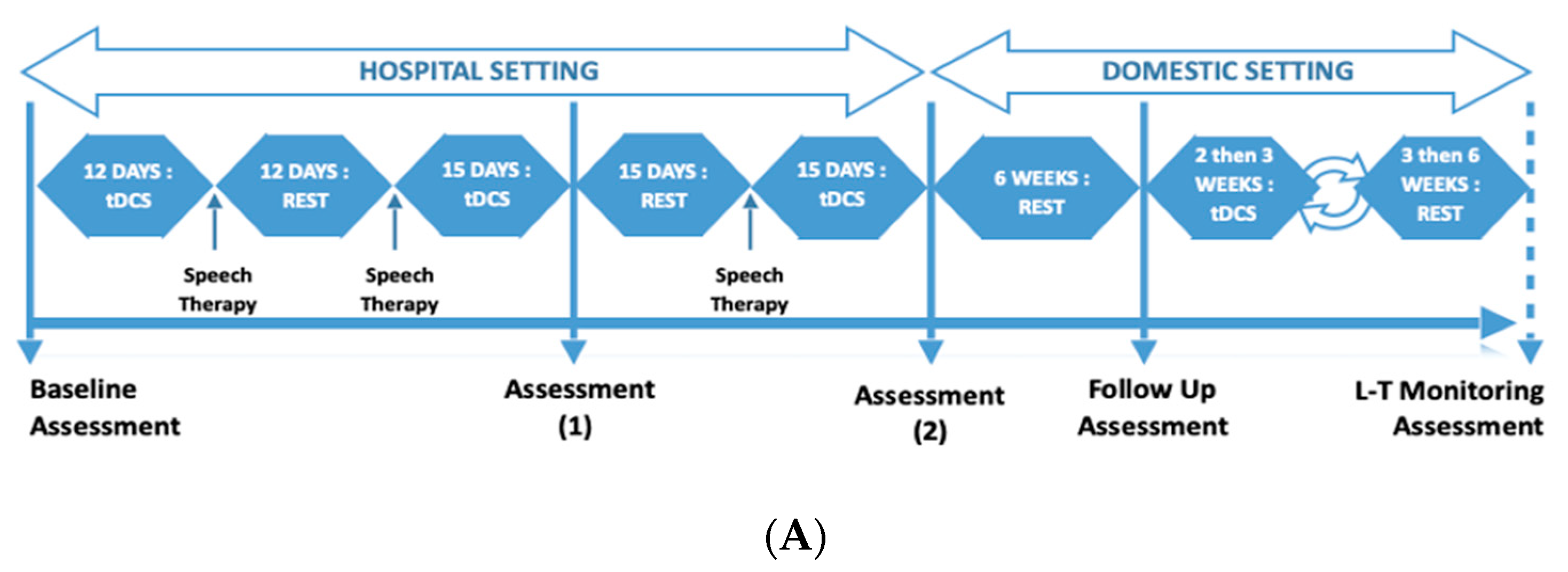
This was a repeated measures design where LA underwent three rounds of alternating tDCS and rest phases (
Figure 1A). The tDCS phase comprised tDCS treatment accompanied with intense language exercises (15 days, 5 days per week), followed by the rest phase comprising minimal language exercises (15 days, alternative days) (see
Figure 1B for measured key endpoints). This was over a total period of 3 months in a hospital setting. After the third round, there was a 6-week rest phase, which was followed by the second part of the program. This followed a similar design (alternating tDCS phase and rest phase) in a domestic setting and using a commercially available tDCS stimulator.
2.2. tDCS Treatment
During the tDCS phases, fixed at 5:00 p.m. in order to be consistent and control the influence of fatigue, LA received a twenty-minute tDCS stimulation while performing multiple types of language exercises. The rest phases consisted of 15 consecutive days of rest (or up to 6 weeks in the domestic setting part), with 30–45 min of language therapy every two days.
In the second part of the program, the procedures were similar, with the exception that the exercises and the stimulation were performed at LA’s home with occasional clinical contact (once per round) and the overall support of his partner. LA received anodal tDCS stimulation over the left IFG paired with language therapy, and tDCS was administered with the DC-Stimulator Plus
®, NeuroConn. Two relatively small anodes and cathodes (5 × 5 cm
2) were used to increase focality [
31]. The electrodes were inserted in saline-soaked sponges (0.17 mL/cm
2) before being positioned. The anode was placed over the left Broca Area (F7), individually located with the Beam A8 system [
32]. The cathode was positioned over the right inferior frontolateral gyrus [
23] (AF8). We decided to place the returning electrode (cathode) around AF8 in order to have a better control of the electric current flow over the surface of the left inferior frontal gyrus (
Figure 2). In addition, the tDCS from NeuroConn is designed to place electrodes on brain and not on the shoulder because of the length of cable [
33]. A standard 1.5 mA stimulation was first planned, but during the pilot trial, the patient asked to decrease the intensity. Thus, 1 mA intensity was chosen in order to compromise between the literature and the patient’s feelings [
17]. The current was ramped up for 30 s at the beginning and ramped down for 15 s at the end of the stimulation.
2.3. Exercises during tDCS Phase
The tDCS phase consisted of 3 exercises during 20 min at each tDCS session: a tongue twister, text reading (300–500 words), and the creation of 30 sentences based on an image presented on a screen for 5 s. Directly after the tDCS phase, when the stimulation was over, LA continued the language exercises for another twenty-five minutes [
15] and performed one of the 3 following exercises: (a) construction of 15 sentences based on 3 words one of which was a conjunction, (b) construction of 3 sentences based on an image for 15 different images, and (c) description of action words based on an image and construction of a sentence by using the same word for 20 images. The procedure for subsequent tDCS phases was identical to the first, except for the rest phase, which lasted 15 days. During the third tDCS phase, a diadochokinetic exercise was added as an alternative to the tongue-twister exercise. In addition, the length of text reading exercise was increased to 400–600 words. All other instructions were identical.
The difference between the tasks used during and after stimulation resides in LA’s main language impairment. Indeed, LA’s productions contained sound distortions because of his apraxia of speech. Plus, his speech rate was slow. The three tasks used during stimulation target those symptoms and allows him to work on coarticulation, phonetic accuracy, and fluency in response to a stimulus. The remaining tasks target syntactic processing, which is, at this point, slightly impaired (mild difficulties in sentence repetition and a grammatical error in a written picture description).
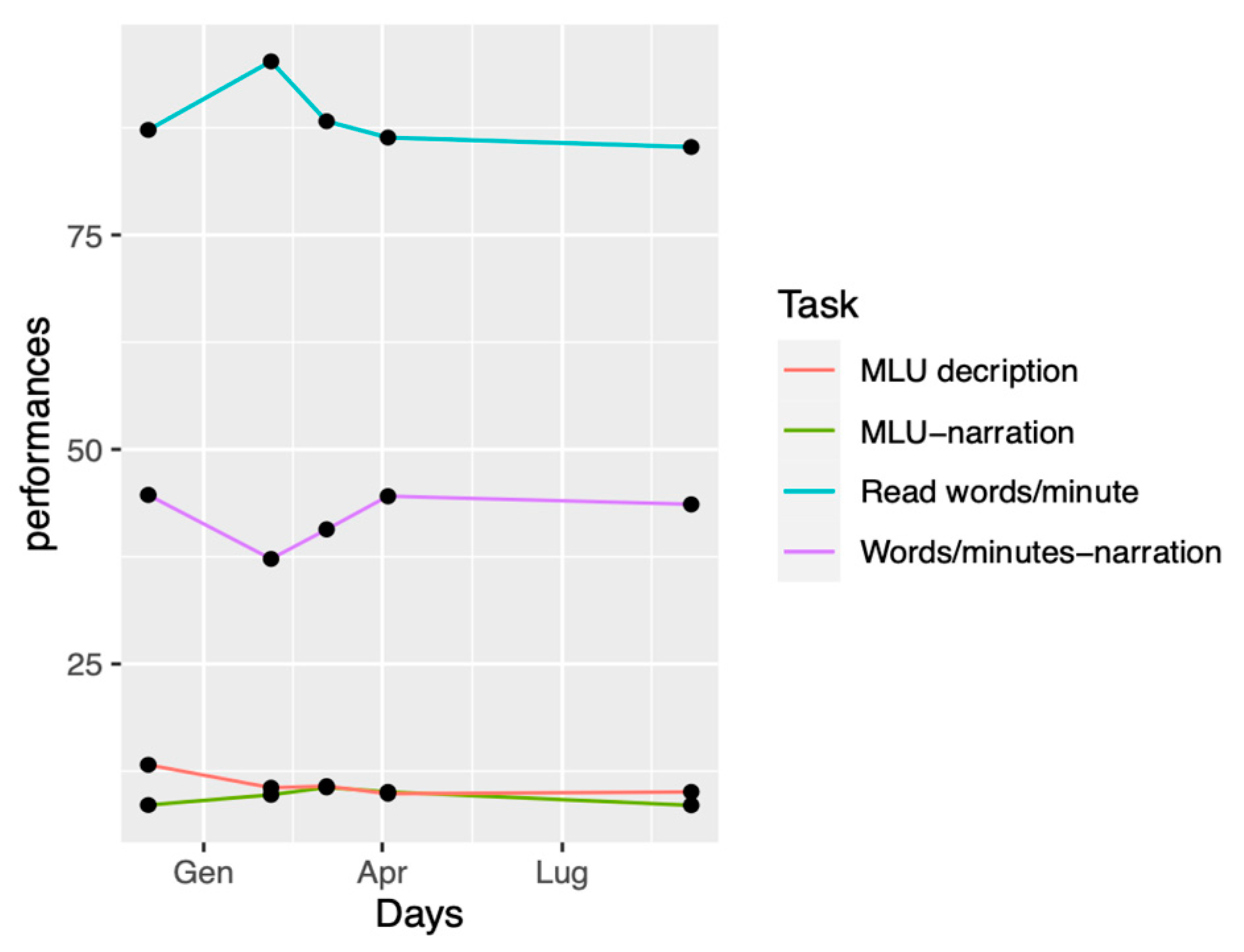
After each round, language and speech were assessed by using different tasks from those used during training. Baseline language assessment comprised elicit spontaneous speech samples and a text reading. To collect elicit spontaneous speech, we used a narration task (Frog, Where Are You?) and a picture description (Birthday Cake by Brookshire). For text reading, we used the text from YAA-R battery.
Using different tasks between training phases and baseline assessment allowed us to see to which point those demanding language exercises could generalize to a more natural context, meaning elicited speech. This is the reason why we chose a narrative task and a picture description one. This way, we were able to collect measures such as MLU and number of words per minute, measures being interesting for syntactic complexity and fluency. In addition, we chose a reading text to assess the number of mistakes made and fluency.
2.4. Exercises during Rest Phase
Every two days, LA performed one of the following exercises: construction of a sentence based on 3 words, one of which is a conjunction; creation of 3 sentences based on an image; and description of action words based on an image and construction of a sentence. He then performed the 3 following exercises: creation of a sentence based on image which was shown for 5–6 s, reading tongue twisters, and text reading of 300–500 words.
2.5. Image Acquisition, Processing, and Data Analysis
Whole-brain images were acquired on a 3T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) at 15-month intervals, 3 months before the beginning and two months after the end of the study, respectively. The acquisition protocol included high-resolution 3D T1-weighted sequence. MorphoBox prototype was used to automatically segment brain regions and measure regional brain volumes [
34]. The total intracranial volume normalized volume (TIV-normalized volume) was adopted as the measurement of the volumes of different brain regions and compared with the normative ranges adjusted for head size, age, and sex. Brain regions with volume deviations from the normative ranges were considered to be abnormal [
35].
2.6. Outcomes
2.6.1. Adherence to Treatment and Subjective Feeling
Considering the duration and the discipline required for the therapy, one of our objectives was to assess the patient’s motivation, his punctuality, his regularity of attendance in the sessions. It is also interesting to identify times when motivation may have fluctuated. We also added descriptions from both of the experimenters’ points of view and the neurologists, and the patient’s subjective feelings and expectations, as well as his partner’s.
2.6.2. Language Assessment
An exhaustive aphasiological evaluation was conducted in September 2020 before the therapy started, and one month after the end of the 8 months of intermittent training. The Psycholinguistic Assessment of Language Processing in Aphasia (PALPA) was used to evaluate word and sentence repetition, spoken words, and spoken sentence–picture matching, as well as logatom, word, and sentence reading [
36]. Word and action-word retrieving abilities were assessed by using the Grémots battery. A task of picture description was employed to evaluate the mean length utterance. Picture matching (Pyramid and Palm Trees Test, 1992) and text reading performances (YAA-R/5) were evaluated [
37]. Non-verbal semantic language (PPTT) was not assessed at the end of the intervention [
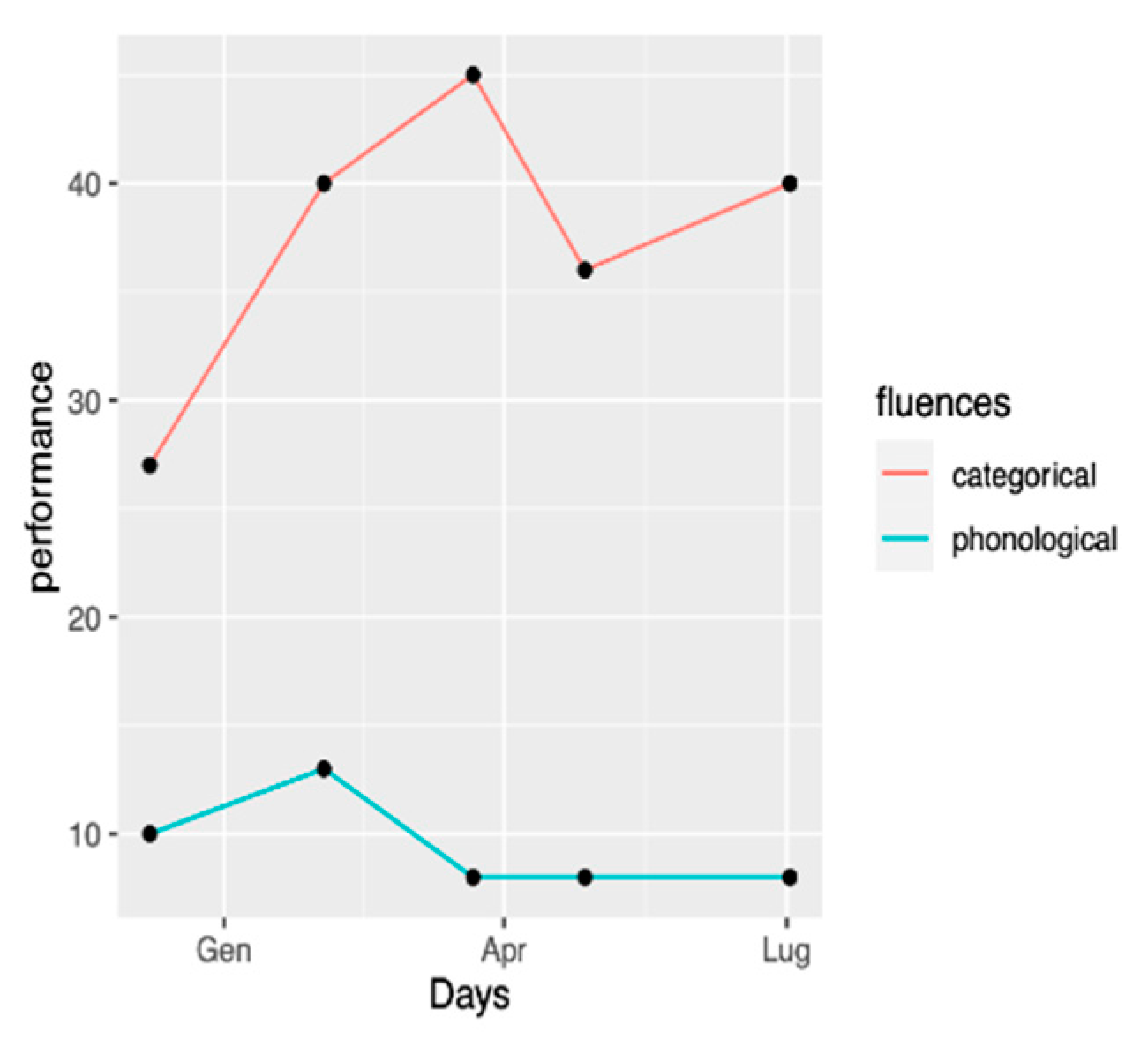
38]. During the tDCS intervention, some additional follow-ups were conducted in order to assess the evolution of performances during the therapy. Categorical and phonological fluencies were assessed four times with Isaac’s test [
39] and “P” letter, respectively [
40], during clinical performed between December 2020 and September 2021. The number of words per minute and MLU were assessed by means of a picture narration task (Frog where are you?), as well as a picture description task (Birthday Cake by Nicholas & Brookshire, 1993). Text-reading performances (YAA-R/5) also were assessed [
37] during the language assessments. Assessment Timepoints and Table of measured endpoints are shown in
Figure 1.
2.6.3. Multidimensional Mood State Questionnaire (MDBF)
We used the English version of the MDBF, originally devised by Steyer et al. (1997) [
41]. The questionnaire was adapted to the patients’ needs, and the scoring scale was augmented. Three scales are provided: good–bad, awake–tired, and calm–nervous. This questionnaire was measured daily.
2.6.4. Brain Atrophy
The atrophy rates of different brain regions between 2020 and 2021 were measured by calculating the % volume change with the following formula: (1-Normalized Volume_2021/Normalized Volume_2020) × 100. The brain regions found to be possibly atrophic in nfvPPA in the previous literature [
29,
42] and detected to be abnormally atrophic in this patient by MorphoBox prototype were taken as the regions of interest (ROIs). An atrophy rate of >4% (following data from Lombardi et al., 2021) was identified as abnormal.
2.6.5. Statistical Analysis
Statistical analyses were conducted with RStudio (2009–2019) version 1.2.5033. Language effects were evaluated by comparing September 2020 and September 2021 logopedic assessment. Language analyses on syntactic complexity were conducted by using TAASC [
43,
44]. Moreover, to have an idea of the effect size of the intervention, we conducted a Non-Overlap of All Pairs (NAP) analysis [
45]. The NAP method allows us to compare the degree of measures overlap between two phases, with the assumption that the more the performances for the two phases overlap, the less likely the intervention had an effect [
46]. The influence of tDCS stimulation on mood was investigated by the mean of
t-tests in a repeated measures paradigm.
2.7. Written Informed Consent
The participant’s written consent, expressing his willingness to participate in the study, was obtained, and the study was approved by the Swiss Vaud cantonal human research ethics committee, Switzerland. See the Institutional Review Board Statement section.