The Walls Are Closing In: Postural Responses to a Virtual Reality Claustrophobic Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
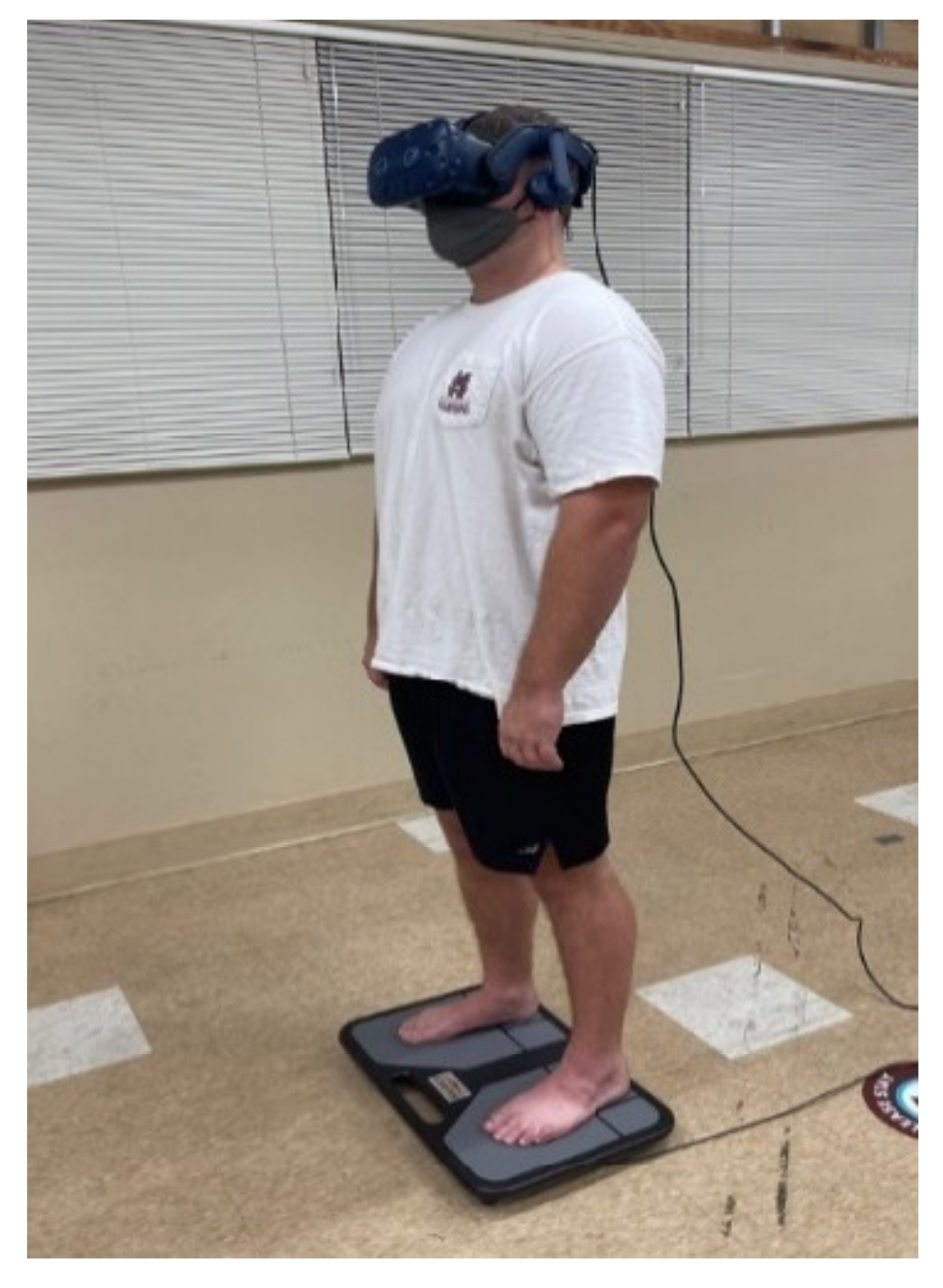
2.2. Instrumentation
2.3. Experimental Procedures
2.4. Data Analyses
2.5. Statistical Analyses
3. Results
4. Discussion
4.1. Findings
4.2. Virtual Reality Environment
4.3. Limitations
4.4. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VR | Virtual Reality |
| VE | Virtual Environment |
| CP | Claustrophobia |
| COP | Center of Pressure |
| COM | Center of Mass |
| No VR | Real Environment (not virtual) |
| VR CP | Virtual Reality Claustrophobia Environment |
| VR CP1, VR CP2, VR CP3 | Virtual Reality Claustrophobia Trials 1, 2, 3 |
| SSQ | Simulator Sickness Questionnaire |
| PAR-Q | Physical Activity Readiness Questionnaire |
| ML | Medial-lateral |
| AP | Anterior-posterior |
| RMS | Root Mean Square |
| 95% EA | 95% Ellipsoid Area |
| CDC | Center for Disease Control |
| IRB | Institutional Review Board |
References
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin. Rehab. 2000, 14, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Massion, J. Postural control system. Curr. Opin. Neurobiol. 1994, 4, 877–887. [Google Scholar] [CrossRef]
- Assländer, L.; Hettich, G.; Mergner, T. Visual contribution to human standing balance during support surface tilts. Hum. Mov. Sci. 2015, 41, 147–164. [Google Scholar] [CrossRef]
- Brink, D.V.D.; Janzen, G. Visual spatial cue use for guiding orientation in two-to-three-year-old children. Front. Psychol. 2013, 4, 904. [Google Scholar] [CrossRef][Green Version]
- Chander, H.; Arachchige, S.N.K.K.; Hill, C.M.; Turner, A.J.; Deb, S.; Shojaei, A.; Hudson, C.; Knight, A.C.; Carruth, D.W. Virtual-Reality-Induced Visual Perturbations Impact Postural Control System Behavior. Behav. Sci. 2019, 9, 113. [Google Scholar] [CrossRef]
- Cleworth, T.W.; Horslen, B.; Carpenter, M.G. Influence of real and virtual heights on standing balance. Gait Posture 2012, 36, 172–176. [Google Scholar] [CrossRef]
- Chander, H.; Shojaei, A.; Deb, S.; Arachchige, S.N.K.K.; Hudson, C.; Knight, A.C.; Carruth, D.W. Impact of Virtual Reality—Generated Construction Environments at Different Heights on Postural Stability and Fall Risk. Work. Health Saf. 2020, 69, 32–40. [Google Scholar] [CrossRef]
- Jacob, R.G.; Redfern, M.S.; Furman, J.M. Space and motion discomfort and abnormal balance control in patients with anxiety disorders. J. Neurol. Neurosurg. Psychiatry 2008, 80, 74–78. [Google Scholar] [CrossRef]
- Balaban, C.D.; Jacob, R.G.; Furman, J.M. Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: Neurotherapeutic implications. Expert Rev. Neurother. 2011, 11, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D.; Furman, J.M.; Staab, J.P. Threat Assessment and Locomotion: Clinical Applications of an Integrated Model of Anxiety and Postural Control. Skull Base 2013, 33, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Raffegeau, T.E.; Fawver, B.; Clark, M.; Engel, B.T.; Young, W.R.; Williams, A.M.; Lohse, K.R.; Fino, P.C. The feasibility of using virtual reality to induce mobility-related anxiety during turning. Gait Posture 2020, 77, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Redfern, M.S.; Furman, J.M.; Jacob, R.G. Visually induced postural sway in anxiety disorders. J. Anxiety Disord. 2007, 21, 704–716. [Google Scholar] [CrossRef]
- Nagano, A.; Yoshioka, S.; Hay, D.C.; Himeno, R.; Fukashiro, S. Influence of vision and static stretch of the calf muscles on postural sway during quiet standing. Hum. Mov. Sci. 2006, 25, 422–434. [Google Scholar] [CrossRef]
- Coelho, C.; Balaban, C. Visuo-vestibular contributions to anxiety and fear. Neurosci. Biobehav. Rev. 2015, 48, 148–159. [Google Scholar] [CrossRef]
- Boffino, C.C.; Sá, C.; Gorenstein, C.; Brown, R.G.; Basile, L.F.H.; Ramos, R.T. Fear of heights: Cognitive performance and postural control. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 259, 114–119. [Google Scholar] [CrossRef]
- Coelho, C.M.; Wallis, G. Deconstructing acrophobia: Physiological and psychological precursors to developing a fear of heights. Depression Anxiety 2010, 27, 864–870. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 1978; p. 405. [Google Scholar]
- Lourenco, S.F.; Longo, M.; Pathman, T. Near space and its relation to claustrophobic fear. Cognition 2011, 119, 448–453. [Google Scholar] [CrossRef]
- Rachman, P.; Taylor, P. Analyses of claustrophobia. J. Anxiety Disord. 1993, 7, 281–291. [Google Scholar] [CrossRef]
- Radomsky, A.; Rachman, S.; Thordarson, D.S.; McIsaac, H.K.; Teachman, B.A. The Claustrophobia Questionnaire. J. Anxiety Disord. 2001, 15, 287–297. [Google Scholar] [CrossRef]
- Lee, D.N.; Aronson, E. Visual proprioceptive control of standing in human infants. Percept. Psychophys. 1974, 15, 529–532. [Google Scholar] [CrossRef]
- Chander, H.; Arachchige, S.N.K.; Turner, A.J.; Knight, A.C. Is it me or the room moving? Recreating the classical “moving room” experiment with virtual reality for postural control adaptation. Adapt. Behav. 2020, 30, 199–204. [Google Scholar] [CrossRef]
- Eseinfeld, S.; Bergström, I.; Epomes, A.; Palacios, J.A.; Evico, F.; Slater, M.; Sanchez-Vives, M. Influence of Music on Anxiety Induced by Fear of Heights in Virtual Reality. Front. Psychol. 2016, 6, 1969. [Google Scholar] [CrossRef]
- Peterson, S.M.; Furuichi, E.; Ferris, D.P. Effects of virtual reality high heights exposure during beam-walking on physiological stress and cognitive loading. PLoS ONE 2018, 13, e0200306. [Google Scholar] [CrossRef]
- Raffegeau, T.E.; Fawver, B.; Young, W.R.; Williams, A.M.; Lohse, K.R.; Fino, P.C. The direction of postural threat alters balance control when standing at virtual elevation. Exp. Brain Res. 2020, 238, 2653–2663. [Google Scholar] [CrossRef]
- Chiarovano, E.; Wang, W.; Rogers, S.J.; MacDougall, H.; Curthoys, I.S.; De Waele, C. Balance in Virtual Reality: Effect of Age and Bilateral Vestibular Loss. Front. Neurol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Vard, A.; Rahani, V.K.; Najafi, M. Claustrophobia game: Design and development of a new virtual reality game for treatment of claustrophobia. J. Med. Signals Sens. 2018, 8, 231–237. [Google Scholar] [CrossRef]
- Wuehr, M.; Breitkopf, K.; Decker, J.; Ibarra, G.; Huppert, D.; Brandt, T. Fear of heights in virtual reality saturates 20 to 40 m above ground. J. Neurol. 2019, 266, 80–87. [Google Scholar] [CrossRef]
- Bruce, M.; Regenbrecht, H. A Virtual Reality Claustrophobia Therapy System—Implementation and Test. In Proceedings of the 2009 IEEE Virtual Reality Conference, Lafayette, LA, USA, 14–18 March 2009; pp. 179–182. [Google Scholar]
- Tsai, C.-F.; Yeh, S.-C.; Huang, Y.; Wu, Z.; Cui, J.; Zheng, L. The Effect of Augmented Reality and Virtual Reality on Inducing Anxiety for Exposure Therapy: A Comparison Using Heart Rate Variability. J. Heal. Eng. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-C.; Li, Y.-Y.; Zhou, C.; Chiu, P.-H.; Chen, J.-W. Effects of Virtual Reality and Augmented Reality on Induced Anxiety. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.M.; Waters, A.; Hine, T.J.; Wallis, G. The use of virtual reality in acrophobia research and treatment. J. Anxiety Disord. 2009, 23, 563–574. [Google Scholar] [CrossRef] [PubMed]
- DeBusk, H.; Hill, C.M.; Chander, H.; Knight, A.C.; Babski-Reeves, K. Influence of military workload and footwear on static and dynamic balance performance. Int. J. Ind. Ergon. 2018, 64, 51–58. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; ISBN 978-1-4698-2666-0. [Google Scholar]
- Nandi, T.; Lewthwaite, R.; Fisher, B.E.; Salem, G.J. Balance confidence scale: Preliminary validity, reliability, and relation to neural excitability in young adults. Psychol. Sport Exerc. 2019, 43, 301–310. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, J.; Choi, Y.; Choe, M. Virtual reality sickness questionnaire (VRSQ): Motion sickness measurement index in a virtual reality environment. Appl. Ergon. 2018, 69, 66–73. [Google Scholar] [CrossRef]
- Horak, F.B.; Henry, S.M.; Shumway-Cook, A. Postural Perturbations: New Insights for Treatment of Balance Disorders. Phys. Ther. 1997, 77, 517–533. [Google Scholar] [CrossRef]
- Horak, F.B.; Shupert, C.L.; Mirka, A. Components of postural dyscontrol in the elderly: A review. Neurobiol. Aging 1989, 10, 727–738. [Google Scholar] [CrossRef]
- O’Connor, S.M.; Baweja, H.S.; Goble, D.J. Validating the BTrackS Balance Plate as a low cost alternative for the measurement of sway-induced center of pressure. J. Biomech. 2016, 49, 4142–4145. [Google Scholar] [CrossRef]
- Shephard, R.J. PAR-Q, Canadian Home Fitness Test and Exercise Screening Alternatives. Sports Med. 1988, 5, 185–195. [Google Scholar] [CrossRef]
- Deb, S.; Carruth, D.W.; Sween, R.; Strawderman, L.; Garrison, T.M. Efficacy of virtual reality in pedestrian safety research. Appl. Ergon. 2017, 65, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Freitas, S.M.S.F. Revision of posturography based on force plate for balance evaluation. Rev. Bras. Fisioter. 2010, 14, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows; Version 27.0.; IBM Corp.: Armonk, NY, USA, 2020. [Google Scholar]
- Wulf, G.; Weigelt, M.; Poulter, D.; McNevin, N. Attentional Focus on Suprapostural Tasks Affects Balance Learning. Q. J. Exp. Psychol. Sect. A 2003, 56, 1191–1211. [Google Scholar] [CrossRef]
- Pline, K.M.; Madigan, M.L.; Nussbaum, M.A. Influence of fatigue time and level on increases in postural sway. Ergonomics 2006, 49, 1639–1648. [Google Scholar] [CrossRef]
- Overstall, P.W.; Exton-Smith, A.N.; Imms, F.J.; Johnson, A.L. Falls in the elderly related to postural imbalance. BMJ 1977, 1, 261–264. [Google Scholar] [CrossRef]
- Warnica, M.J.; Weaver, T.B.; Prentice, S.D.; Laing, A.C. The influence of ankle muscle activation on postural sway during quiet stance. Gait Posture 2014, 39, 1115–1121. [Google Scholar] [CrossRef]
- Santos, M.J.; Kanekar, N.; Aruin, A.S. The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J. Electromyogr. Kinesiol. 2010, 20, 398–405. [Google Scholar] [CrossRef]
- Assländer, L.; Peterka, R.J. Sensory reweighting dynamics in human postural control. J. Neurophysiol. 2014, 111, 1852–1864. [Google Scholar] [CrossRef]
- Hageman, P.; Leibowitz, J.M.; Blanke, D. Age and gender effects on postural control measures. Arch. Phys. Med. Rehab. 1995, 76, 961–965. [Google Scholar] [CrossRef]
- Wolfson, L.; Whipple, R.; Derby, C.A.; Amerman, P.; Nashner, L. Gender Differences in the Balance of Healthy Elderly as Demonstrated by Dynamic Posturography. J. Gerontol. 1994, 49, M160–M167. [Google Scholar] [CrossRef] [PubMed]
- Kollegger, H.; Baumgartner, C.; Wöber, C.; Oder, W.; Deecke, L. Spontaneous Body Sway as a Function of Sex, Age, and Vision: Posturographic Study in 30 Healthy Adults. Eur. Neurol. 1992, 32, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.B.; Willemsen, P.; Gooch, A.A.; Creem-Regehr, S.H.; Loomis, J.M.; Beall, A.C. Does the Quality of the Computer Graphics Matter when Judging Distances in Visually Immersive Environments? Presence Teleoper. Virtual Environ. 2004, 13, 560–571. [Google Scholar] [CrossRef]
- Jones, J.A.; Swan, J.E.; Singh, G.; Ellis, S.R. Peripheral Visual Information and Its Effect on Distance Judgments in Virtual and Augmented Environments. In Proceedings of the ACM SIGGRAPH Symposium on Applied Perception in Graphics and Visualization, Association for Computing Machinery, New York, NY, USA, 3–7 August 2011; pp. 29–36. [Google Scholar]
- Jones, J.A.; Swan, J.E., II; Bolas, M. Peripheral Stimulation and its Effect on Perceived Spatial Scale in Virtual Environments. IEEE Trans. Vis. Comput. Graph. 2013, 19, 701–710. [Google Scholar] [CrossRef]


| Dependent Variables | Main Effects | Pairwise Comparisons |
|---|---|---|
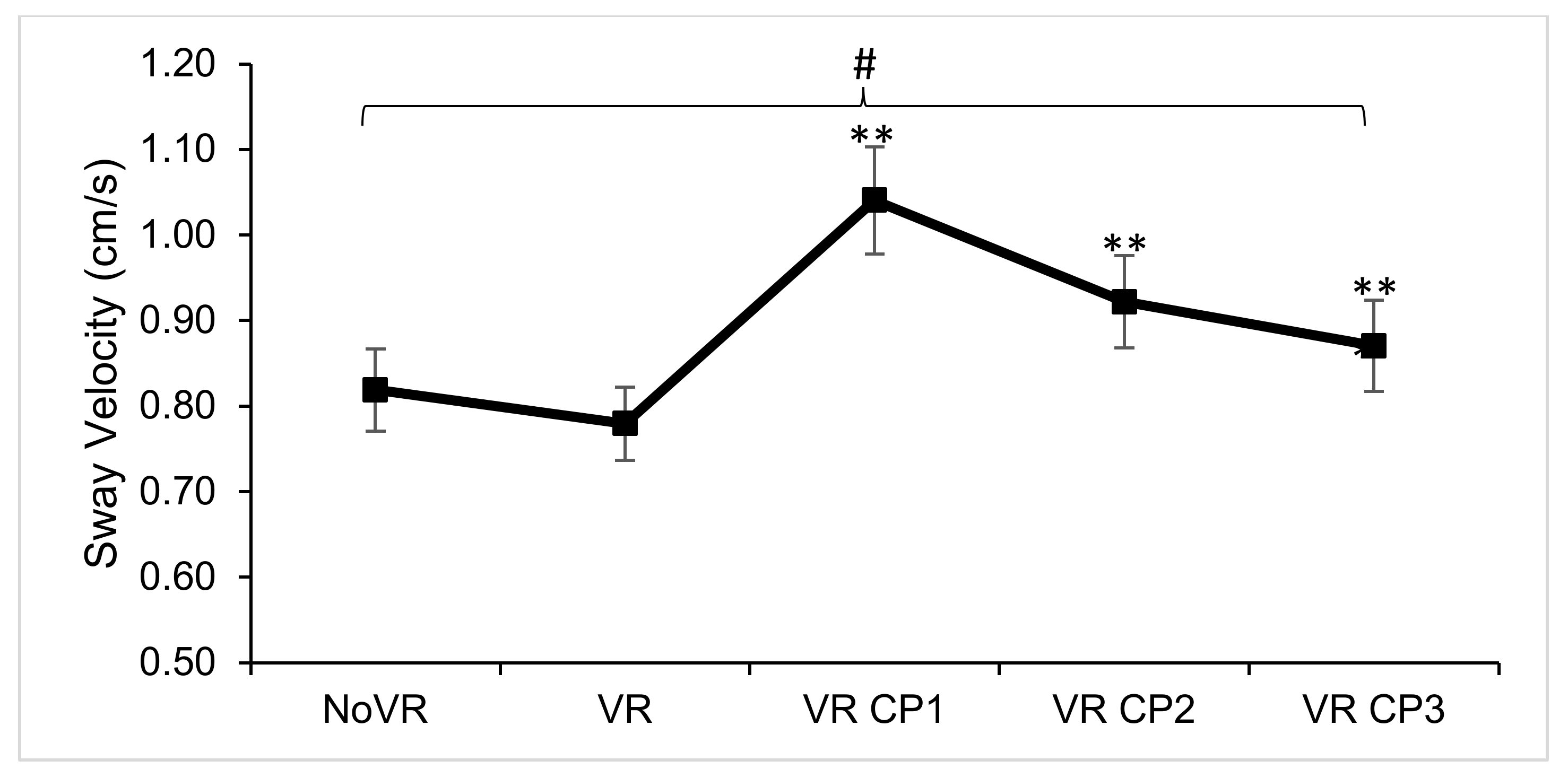
| Sway Velocity (cm/s) | F (4,116) = 14.911, p < 0.001, ƞp2 = 0.340 | NoVR & VR < CP1 & CP2; CP1 > CP3 |
| ML RMS Sway (cm) | F (4,116) = 5.980, p < 0.001, ƞp2 = 0.171 | NoVR > VR & CP2, CP3 |
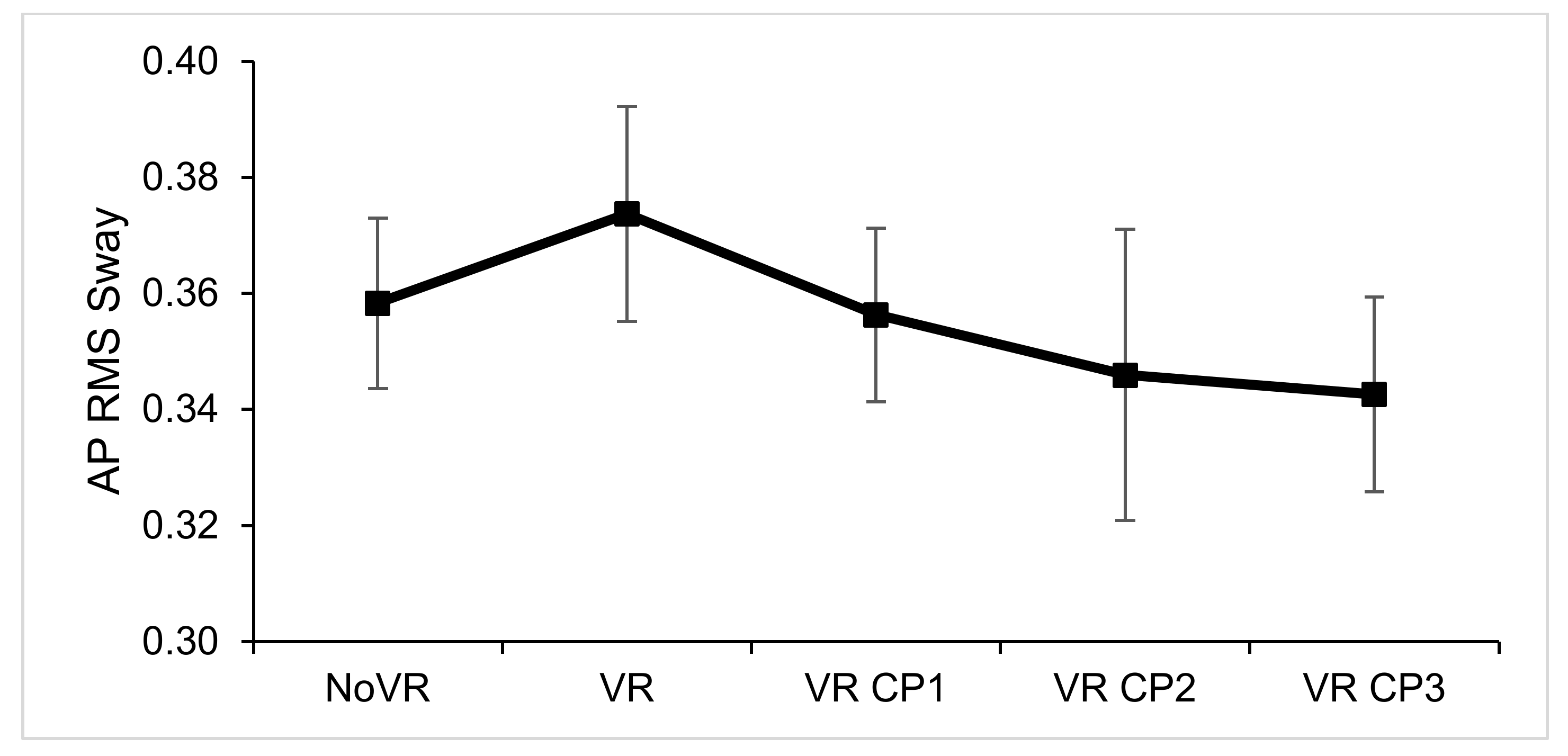
| AP RMS Sway (cm) | F (4,116) = 0.727, p = 0.576, ƞp2 = 0.024 | No significant main effect |
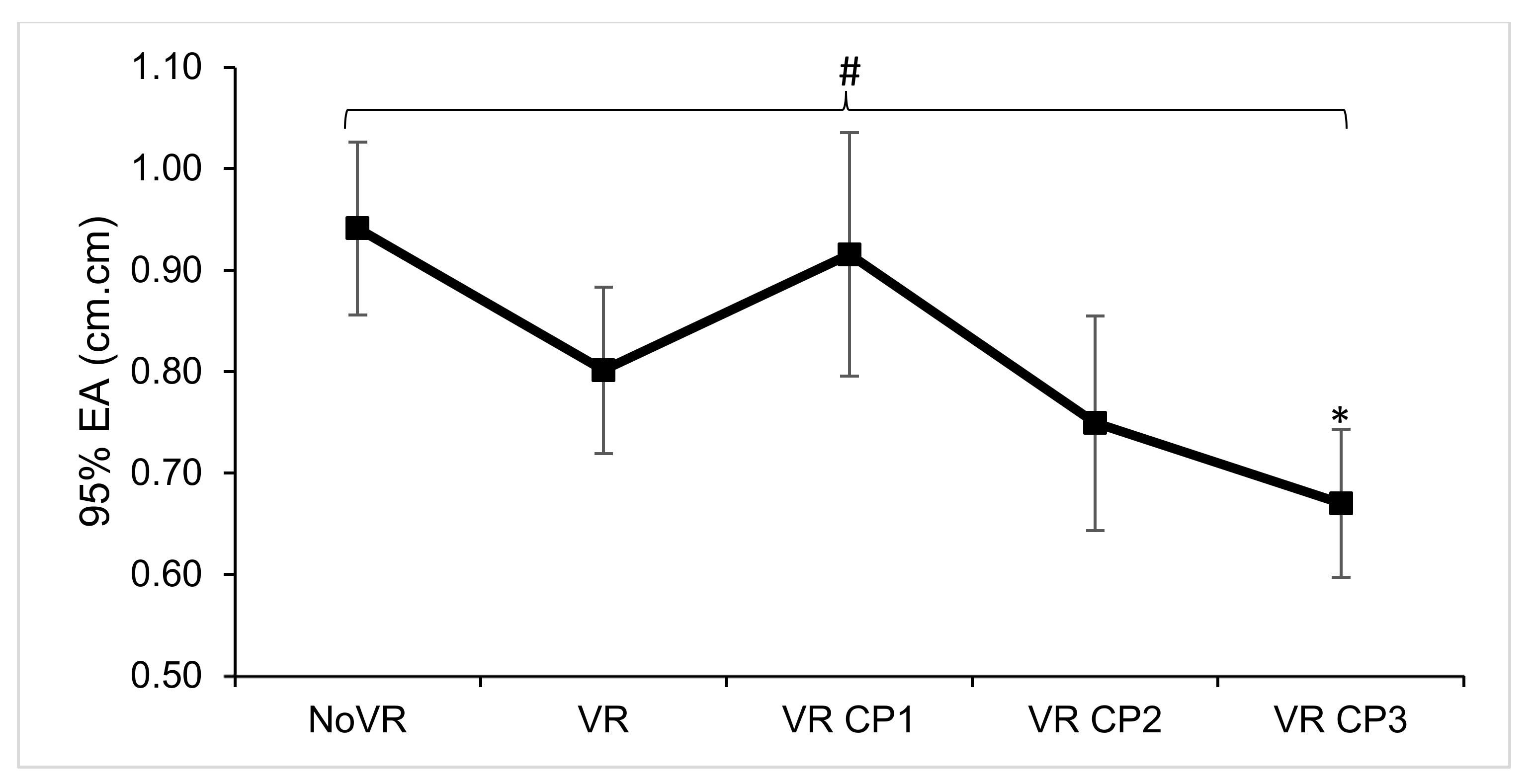
| 95% EA (cm.cm) | F (4,116) = 3.095, p = 0.018, ƞp2 = 0.094 | NoVR > CP3 |
| ML Sway Excursion (cm) | F (4,116) = 5.415, p < 0.001, ƞp2 = 0.157 | NoVR > VR, CP2 & CP3 |
| AP Sway Excursion (cm) | F (4,116) = 5.415, p < 0.001, ƞp2 = 0.157 | No significant pairwise comparisons |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chander, H.; Freeman, H.R.; Hill, C.M.; Hudson, C.R.; Kodithuwakku Arachchige, S.N.K.; Turner, A.J.; Jones, J.A.; Knight, A.C. The Walls Are Closing In: Postural Responses to a Virtual Reality Claustrophobic Simulation. Clin. Transl. Neurosci. 2022, 6, 15. https://doi.org/10.3390/ctn6020015
Chander H, Freeman HR, Hill CM, Hudson CR, Kodithuwakku Arachchige SNK, Turner AJ, Jones JA, Knight AC. The Walls Are Closing In: Postural Responses to a Virtual Reality Claustrophobic Simulation. Clinical and Translational Neuroscience. 2022; 6(2):15. https://doi.org/10.3390/ctn6020015
Chicago/Turabian StyleChander, Harish, Hannah R. Freeman, Christopher M. Hill, Christopher R. Hudson, Sachini N. K. Kodithuwakku Arachchige, Alana J. Turner, J. Adam Jones, and Adam C. Knight. 2022. "The Walls Are Closing In: Postural Responses to a Virtual Reality Claustrophobic Simulation" Clinical and Translational Neuroscience 6, no. 2: 15. https://doi.org/10.3390/ctn6020015
APA StyleChander, H., Freeman, H. R., Hill, C. M., Hudson, C. R., Kodithuwakku Arachchige, S. N. K., Turner, A. J., Jones, J. A., & Knight, A. C. (2022). The Walls Are Closing In: Postural Responses to a Virtual Reality Claustrophobic Simulation. Clinical and Translational Neuroscience, 6(2), 15. https://doi.org/10.3390/ctn6020015









