Neurologic Complications in Adult and Pediatric Patients with SARS-CoV-2 Infection
Abstract
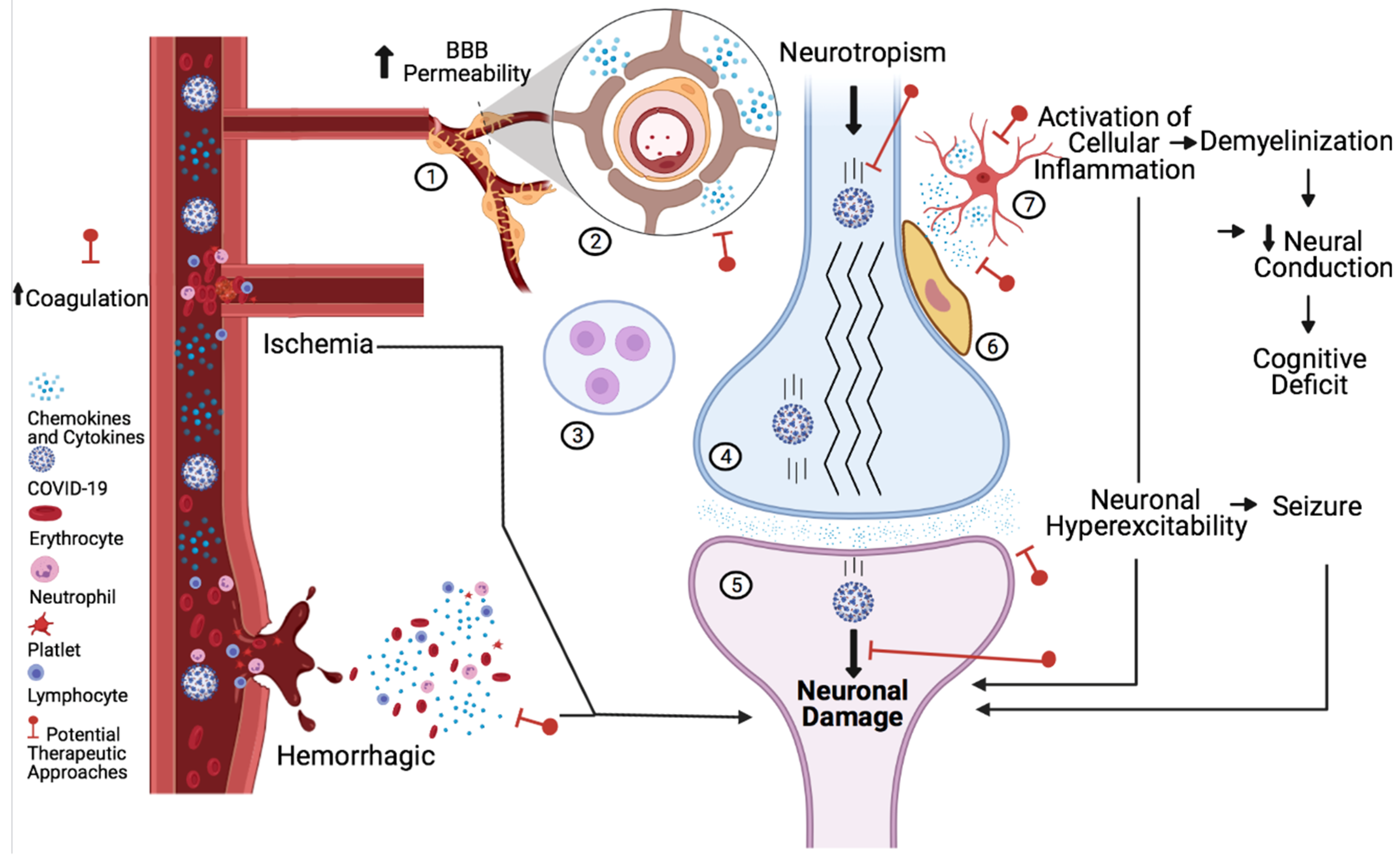
:1. Introduction
2. Neural Tissue
3. Blood Vessels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dafer, R.M.; Osteraas, N.D.; Biller, J. Acute Stroke Care in the Coronavirus Disease 2019 Pandemic. J. Stroke Cerebrovasc. Dis. 2020, 29, 104881. [Google Scholar] [CrossRef] [PubMed]
- Morassi, M.; Bagatto, D.; Cobelli, M.; D’Agostini, S.; Gigli, G.L.; Bnà, C.; Vogrig, A. Stroke in patients with SARS-CoV-2 infection: Case series. J. Neurol. 2020, 267, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Beyrouti, R.; Adams, M.E.; Benjamin, L.; Cohen, H.; Farmer, S.F.; Goh, Y.Y.; Humphries, F.; Jäger, H.R.; Losseff, N.A.; Perry, R.J.; et al. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry 2020, 91, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Nichols, T.; Pike, M.; Subbe, C.; Elghenzai, S. Cerebral Venous Sinus Thrombosis as a Presentation of COVID-19. Eur. J. Case Rep. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Majidi, S.; Fifi, J.T.; Ladner, T.R.; Lara-Reyna, J.; Yaeger, K.A.; Yim, B.; Dangayach, N.; Oxley, T.J.; Shigematsu, T.; Kummer, B.R.; et al. Emergent Large Vessel Occlusion Stroke During New York City’s COVID-19 Outbreak. Stroke 2020, 51, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Farhadian, S.F.; Glick, L.R.; Vogels, C.B.F.; Thomas, J.; Chiarella, J.; Casanovas-Massana, A.; Zhou, J.; Odio, C.; Vijayakumar, P.; Geng, B.; et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020, 20, 248. [Google Scholar] [CrossRef]
- Poyiadji, N.; Shahin, G.; Noujaim, D.; Stone, M.; Patel, S.; Griffith, B. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology 2020, 296, E119–E120. [Google Scholar] [CrossRef] [Green Version]
- Delamarre, L.; Gollion, C.; Grouteau, G.; Rousset, D.; Jimena, G.; Roustan, J.; Gaussiat, F.; Aldigé, E.; Gaffard, C.; Duplantier, J.; et al. COVID-19–associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1004–1006. [Google Scholar] [CrossRef]
- Parauda, S.C.; Gao, V.; Gewirtz, A.N.; Parikh, N.S.; Merkler, A.E.; Lantos, J.; White, H.; Leifer, D.; Navi, B.B.; Segal, A.Z. Posterior reversible encephalopathy syndrome in patients with COVID-19. J. Neurol. Sci. 2020, 416, 117019. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Etemadifar, M.; Salari, M.; Murgai, A.A.; Hajiahmadi, S. Fulminant encephalitis as a sole manifestation of COVID-19. Neurol. Sci. 2020, 41, 3027–3029. [Google Scholar] [CrossRef]
- Hepburn, M.; Mullaguri, N.; George, P.; Hantus, S.; Punia, V.; Bhimraj, A.; Newey, C.R. Acute Symptomatic Seizures in Critically Ill Patients with COVID-19: Is There an Association? Neurocrit. Care 2020, 34, 139–143. [Google Scholar] [CrossRef]
- Sohal, S.; Mansur, M. COVID-19 Presenting with Seizures. IDCases 2020, 20, e00782. [Google Scholar] [CrossRef]
- AlKetbi, R.; AlNuaimi, D.; AlMulla, M.; AlTalai, N.; Samir, M.; Kumar, N.; AlBastaki, U. Acute myelitis as a neurological complication of Covid-19: A case report and MRI findings. Radiol. Case Rep. 2020, 15, 1591–1595. [Google Scholar] [CrossRef]
- Valiuddin, H.; Skwirsk, B.; Paz-Arabo, P. Acute transverse myelitis associated with SARS-CoV-2: A Case-Report. Brain Behav. Immun. -Health 2020, 5, 100091. [Google Scholar] [CrossRef]
- Chakraborty, U.; Chandra, A.; Ray, A.K.; Biswas, P. COVID-19–associated acute transverse myelitis: A rare entity. BMJ Case Rep. 2020, 13, e238668. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Abdelhak, A.; Foschi, M.; Tumani, H.; Otto, M. Guillain–Barré syndrome spectrum associated with COVID-19: An up-to-date systematic review of 73 cases. J. Neurol. 2020, 268, 1133–1170. [Google Scholar] [CrossRef]
- Uncini, A.; Vallat, J.-M.; Jacobs, B.C. Guillain-Barré syndrome in SARS-CoV-2 infection: An instant systematic review of the first six months of pandemic. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1105–1110. [Google Scholar] [CrossRef]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; A Pollak, T.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- LaRovere, K.L.; Riggs, B.J.; Poussaint, T.Y.; Young, C.C.; Newhams, M.M.; Maamari, M.; Walker, T.C.; Singh, A.R.; Dapul, H.; Hobbs, C.V.; et al. Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome. JAMA Neurol. 2021, 78, 536. [Google Scholar] [CrossRef]
- Frontera, J.A.; Sabadia, S.; Lalchan, R.; Fang, T.; Flusty, B.; Millar-Vernetti, P.; Snyder, T.; Berger, S.; Yang, D.; Granger, A.; et al. A Prospective Study of Neurologic Disorders in Hospitalized Patients With COVID-19 in New York City. Neurology 2020, 96, e575–e586. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Bénézit, F.; Le Turnier, P.; Declerck, C.; Paillé, C.; Revest, M.; Dubée, V.; Tattevin, P.; for the RAN COVID Study Group. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020, 20, 1014–1015. [Google Scholar] [CrossRef]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 2020, 24, 368–378. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Huang, S.; Gartung, A.; Cortés-Puch, I.; Sime, P.J.; Phipps, R.P.; Serhan, C.N.; Hammock, B.D. Inflammation resolution: A dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020, 39, 337–340. [Google Scholar] [CrossRef]
- Kennedy, R.H.; Silver, R. Neuroimmune Signaling: Cytokines and the CNS. In Neuroscience in the 21st Century; Pfaff, D.W., Volkow, N.D., Eds.; Springer: New York, NY, USA, 2016; pp. 1–41. [Google Scholar] [CrossRef]
- Qi, X.; Keith, K.A.; Huang, J.H. COVID-19 and stroke: A review. Hemorrhagic Stroke 2020. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhang, Y.; Zhang, S.; Qin, X.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; et al. Antiphospholipid Antibodies in Critically Ill Patients With COVID-19. Arthritis Rheumatol. 2020, 72, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Eldahshan, W.; Rutkowski, E. COVID-19-Related Stroke. Transl. Stroke Res. 2020, 11, 322–325. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Liang, X.; Gao, B.; Liu, M.; Li, W.; Chen, Z.; Wang, Z. COVID-19 Associated Ischemic Stroke and Hemorrhagic Stroke: Incidence, Potential Pathological Mechanism, and Management. Front. Neurol. 2020, 11, 571996. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howard, K.; Williams, T.; Fitch, E.; Ots, H.; Pototskiy, E.; Hawkshead, J.; Ghersin, Z.; Musto, A.E. Neurologic Complications in Adult and Pediatric Patients with SARS-CoV-2 Infection. Clin. Transl. Neurosci. 2022, 6, 1. https://doi.org/10.3390/ctn6010001
Howard K, Williams T, Fitch E, Ots H, Pototskiy E, Hawkshead J, Ghersin Z, Musto AE. Neurologic Complications in Adult and Pediatric Patients with SARS-CoV-2 Infection. Clinical and Translational Neuroscience. 2022; 6(1):1. https://doi.org/10.3390/ctn6010001
Chicago/Turabian StyleHoward, Kendall, Taylor Williams, Elizabeth Fitch, Heather Ots, Esther Pototskiy, Jay Hawkshead, Zelda Ghersin, and Alberto E. Musto. 2022. "Neurologic Complications in Adult and Pediatric Patients with SARS-CoV-2 Infection" Clinical and Translational Neuroscience 6, no. 1: 1. https://doi.org/10.3390/ctn6010001