Influence of the Use of Transepithelial Abutments vs. Titanium Base Abutments on Microgap Formation at the Dental Implant–Abutment Interface: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
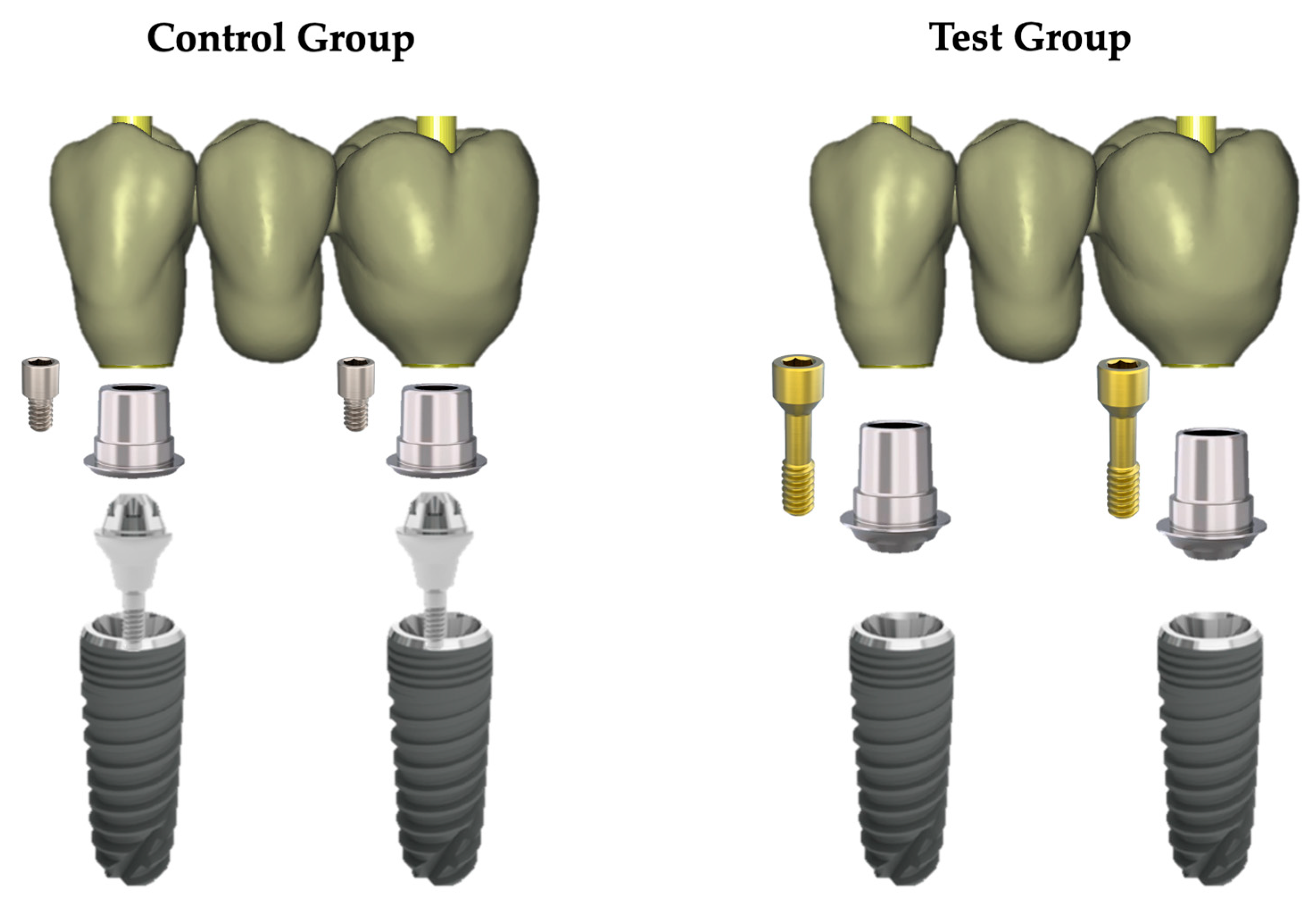
2.1. Study Design and Implant–Abutment System Characteristics
2.2. Specimen Preparation
2.3. Aging Processes
2.4. Scanning Electron Microscope (SEM) Test
2.5. Statistical Analysis
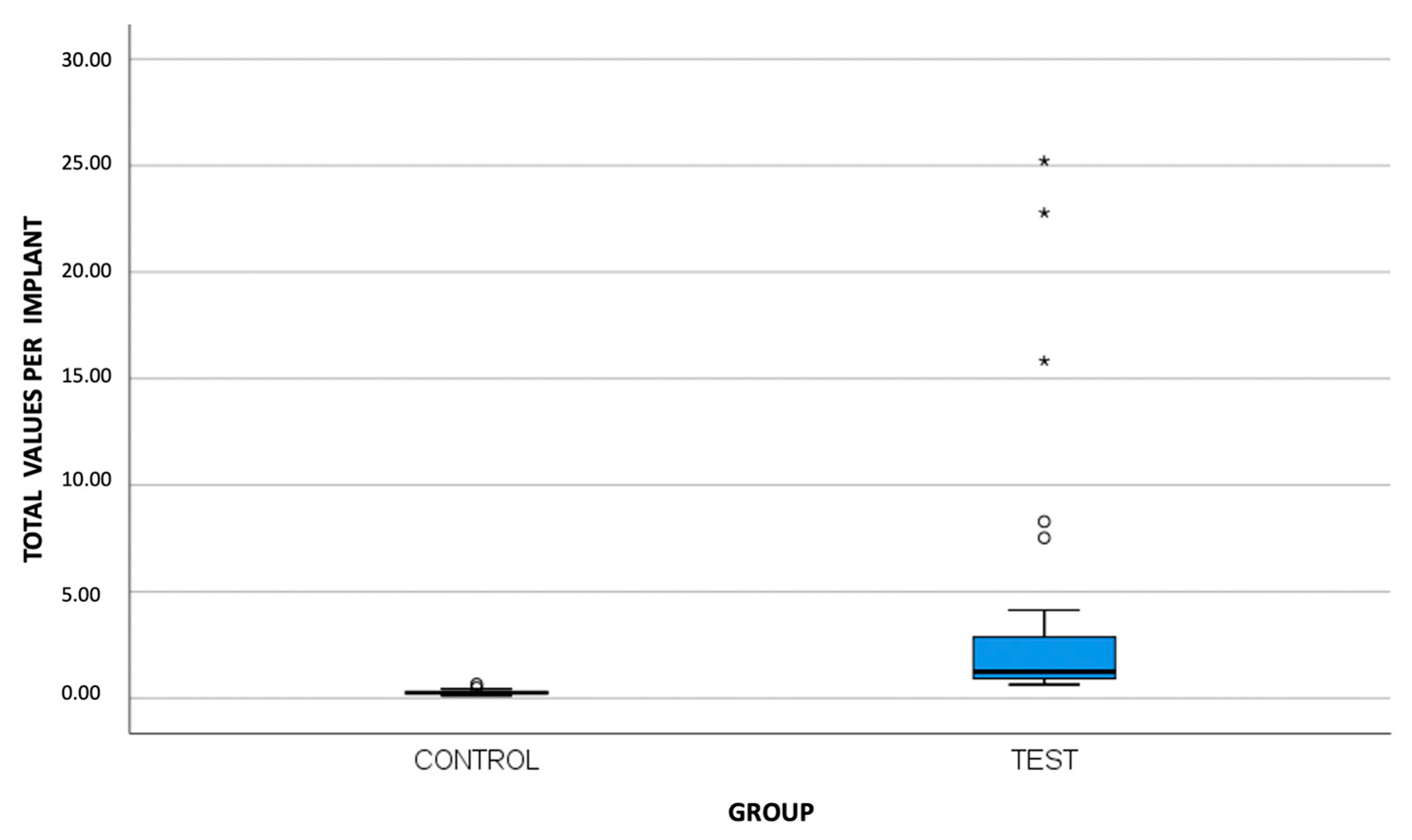
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Janner, S.F.M.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-Year Survival and Success Rates of 511 Titanium Implants with a Sandblasted and Acid-Etched Surface: A Retrospective Study in 303 Partially Edentulous Patients. Clin. Implant. Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarsson, L.; Gheisarifar, M.; Jemt, T. A systematic review of survival of single implants as presented in longitudinal studies with a follow-up of at least 10 years. Eur. J. Oral. Implantol. 2016, 9, S155–S162. [Google Scholar] [PubMed]
- Gómez-Polo, M.; Ortega, R.; Gómez-Polo, C.; Celemin, A.; Del Rio Highsmith, J. Factors Affecting the Decision to Use Cemented or Screw-Retained Fixed Implant-Supported Prostheses: A Critical Review. Int. J. Prosthodont. 2018, 31, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Mühlemann, S.; Zwahlen, M.; Hämmerle, C.H.; Schneider, D. Cemented and Screw-Retained Implant Reconstructions: A Systematic Review of the Survival and Complication Rates. Clin. Oral. Implant. Res. 2012, 23, 163–201. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, C.-Y.; Li, J.; Wang, H.-L.; Yu, H. Influence of Abutment Height on Peri-Implant Marginal Bone Loss: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2019, 122, 14–21. [Google Scholar] [CrossRef]
- Heimes, D.; Becker, P.; Pabst, A.; Smeets, R.; Kraus, A.; Hartmann, A.; Sagheb, K.; Kämmerer, P.W. How Does Dental Implant Macrogeometry Affect Primary Implant Stability? A Narrative Review. Int. J. Implant. Dent. 2023, 9, 20. [Google Scholar] [CrossRef]
- Laleman, I.; Lambert, F. Implant Connection and Abutment Selection as a Predisposing and/or Precipitating Factor for Peri-Implant Diseases: A Review. Clin. Implant. Dent. Relat. Res. 2023, 25, 723–733. [Google Scholar] [CrossRef]
- Sanz-Martín, I.; Sanz-Sánchez, I.; Carrillo de Albornoz, A.; Figuero, E.; Sanz, M. Effects of Modified Abutment Characteristics on Peri-implant Soft Tissue Health: A Systematic Review and Meta-analysis. Clin. Oral. Implant. Res. 2018, 29, 118–129. [Google Scholar] [CrossRef]
- Canullo, L.; Menini, M.; Santori, G.; Rakic, M.; Sculean, A.; Pesce, P. Titanium Abutment Surface Modifications and Peri-Implant Tissue Behavior: A Systematic Review and Meta-Analysis. Clin. Oral. Investig. 2020, 24, 1113–1124. [Google Scholar] [CrossRef]
- Canullo, L.; Annunziata, M.; Pesce, P.; Tommasato, G.; Nastri, L.; Guida, L. Influence of Abutment Material and Modifications on Peri-Implant Soft-Tissue Attachment: A Systematic Review and Meta-Analysis of Histological Animal Studies. J. Prosthet. Dent. 2021, 125, 426–436. [Google Scholar] [CrossRef]
- Tallarico, M.; Fiorellini, J.; Nakajima, Y.; Omori, Y.; Takahisa, I.; Canullo, L. Mechanical Outcomes, Microleakage, and Marginal Accuracy at the Implant-Abutment Interface of Original Versus Nonoriginal Implant Abutments: A Systematic Review of in Vitro Studies. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Del Amo, F.S.-L.; Romero-Bustillos, M.; Catena, A.; Galindo-Moreno, P.; Sánchez-Suárez, J.M.; Garaicoa-Pazmino, C. Effect of Abutment Height on Marginal Bone Loss Around Dental Implants: A Systematic Review. Int. J. Prosthodont. 2022, 20221021. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Cascos-Sanchez, R.; Yilmaz, B.; Lam WY, H.; Pow EH, N.; Del Río Highsmith, J.; Gómez-Polo, M. Effect of Fabrication Technique on the Microgap of Cad/cam Cobalt-Chrome and Zirconia Abutments on a Conical Connection Implant: An in Vitro Study. Materials 2021, 14, 2348. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.C.; Lemos, C.A.A.; de Luna Gomes, J.M.; Verri, F.R.; Pellizzer, E.P. Cad/cam Vs Conventional Technique for Fabrication of Implant-Supported Frameworks: A Systematic Review and Meta-Analysis of in Vitro Studies. Int. J. Prosthodont. 2019, 32, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Jemt, T.; Rubenstein, J.E.; Carlsson, L.; Lang, B.R. Measuring Fit at the Implant Prosthodontic Interface. J. Prosthet. Dent. 1996, 75, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T. Zero Bone Loss Concepts, 1st ed.; Quintessence Publishing: Barcelona, Spain, 2019. [Google Scholar]
- Liu, Y.; Wang, J. Influences of Microgap and Micromotion of Implant-Abutment Interface on Marginal Bone Loss Around Implant Neck. Arch. Oral. Biol. 2017, 83, 153–160. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Roccuzzo, A.; Yilmaz, B.; Lam, W.Y.H.; Pow, E.H.N.; Highsmith, J.D.R.; Gómez-Polo, M. Microleakage Assessment of Cad-Cam Cobalt-Chrome and Zirconia Abutments on a Conical Connection Dental Implant: A comparative in Vitro Study. Clin. Oral. Implant. Res. 2022, 33, 945–952. [Google Scholar] [CrossRef]
- Larrucea, C.; Olivares, D.; Padilla, C.; Barrera, A.; Lobos, O. Bacterial Microleakage at the Abutment-Implant Interface, in Vitro Study. Clin. Implant. Dent. Relat. Res. 2018, 20, 360–367. [Google Scholar] [CrossRef]
- Cortelli, S.C.; Cortelli, J.R.; Romeiro, R.L.; Costa, F.O.; Aquino, D.R.; Orzechowski, P.R.; Araújo, V.C.; Duarte, P.M. Frequency of Periodontal Pathogens in Equivalent Peri-Implant and Periodontal Clinical Statuses. Arch. Oral. Biol. 2013, 58, 67–74. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chowdhary, R.; Kumari, S. Microleakage at the Different Implant Abutment Interface: A Systematic Review. J. Clin. Diagn. Res. 2017, 11, ZE10–ZE15. [Google Scholar] [CrossRef]
- Caricasulo, R.; Malchiodi, L.; Ghensi, P.; Fantozzi, G.; Cucchi, A. The Influence of Implant-Abutment Connection to Peri-Implant Bone Loss: A Systematic Review and Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T. Implant-Abutment Connection as Contributing Factor to Peri-Implant Diseases. Periodontology 2000 2019, 81, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Vetromilla, B.M.; Brondani, L.P.; Pereira-Cenci, T.; Bergoli, C.D. Influence of Different Implant-Abutment Connection Designs on the Mechanical and Biological Behavior of Single-Tooth Implants in the Maxillary Esthetic Zone: A Systematic Review. J. Prosthet. Dent. 2019, 121, 398–403. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Lucchese, A.; Viganoni, C.; Limongelli, L.; Carinci, F. The Impact of Implant-Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review. Materials 2020, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Asvanund, P.; Morgano, S.M. Photoelastic Stress Analysis of External Versus Internal Implant-Abutment Connections. J. Prosthet. Dent. 2011, 106, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Bosshardt, D.D.; Lang, N.P.; Abrahamsson, I.; Berglundh, T.; Lindhe, J.; Ivanovski, S.; Donos, N. Temporal Sequence of Hard and Soft Tissue Healing Around Titanium Dental Implants. Periodontology 2000 2015, 68, 135–152. [Google Scholar] [CrossRef]
- Moráguez, O.D.; Belser, U.C. The Use of Polytetrafluoroethylene Tape for the Management of Screw Access Channels in Implant-Supported Prostheses. J. Prosthet. Dent. 2010, 103, 189–191. [Google Scholar] [CrossRef]
- ISO/TS 11405:2015; Dentistry—Testing of Adhesion to Tooth Structure. International Organization for Standardization (ISO): Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/62898.html (accessed on 27 April 2020).
- ISO 14801:2016; Dentistry-Implants–Dynamic Loading Test for Endosseous Dental Implants. International Organization for Standardization (ISO): Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/61997.html (accessed on 27 April 2020).
- Gonzalo, E.; Vizoso, B.; Lopez-Suarez, C.; Diaz, P.; Pelaez, J.; Suarez, M.J. Evaluation of Milled Titanium Versus Laser Sintered Co-Cr Abutments on the Marginal Misfit in Internal Implant-Abutment Connection. Materials 2020, 13, 4873. [Google Scholar] [CrossRef]
- Baldassarri, M.; Hjerppe, J.; Romeo, D.; Fickl, S.; Thompson, V.P.; Stappert, C.F.J. Marginal Accuracy of Three Implant-Ceramic Abutment Configurations. Int. J. Oral. Maxillofac. Implant. 2012, 27, 537–543. [Google Scholar]
- de-Azevedo-Vaz, S.L.; Araujo-Siqueira, C.; Carneiro, V.C.; Oliveira, M.L.; Azeredo, R.A. Misfit Detection in Implant-Supported Prostheses of Different Compositions by Periapical Radiography and Cone Beam Computed Tomography: An in vitro Study. J. Prosthet. Dent. 2021, 126, 205–213. [Google Scholar] [CrossRef]
- Gassino, G.; Barone Monfrin, S.; Scanu, M.; Spina, G.; Preti, G. Marginal Adaptation of Fixed Prosthodontics: A New in Vitro 360-Degree External Examination Procedure. Int. J. Prosthodont. 2004, 17, 218–223. [Google Scholar] [PubMed]
- Vandeweghe, S.; Coelho, P.G.; Vanhove, C.; Wennerberg, A.; Jimbo, R. Utilizing Micro-Computed Tomography to Evaluate Bone Structure Surrounding Dental Implants: A Comparison with Histomorphometry. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2013, 101, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, I.; Witek, L.; Coelho, P.G.; Bergamo, E.; Pegoraro, L.F.; Bonfante, E.A. Influence of Abutment Fabrication Method on 3d Fit at the Implant-Abutment Connection. Int. J. Prosthodont. 2020, 33, 641–647. [Google Scholar] [CrossRef]
- Prasad, R.; Al-Kheraif, A.A. Three-Dimensional Accuracy of Cad/cam Titanium and Ceramic Superstructures for Implant Abutments Using Spiral Scan Microtomography. Int. J. Prosthodont. 2013, 26, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Narra, N.; Antalainen, A.-K.; Zipprich, H.; Sándor, G.K.; Wolff, J. Microcomputed Tomography-Based Assessment of Retrieved Dental Implants. Int. J. Oral. Maxillofac. Implant. 2015, 30, 308–314. [Google Scholar] [CrossRef]
- Bagegni, A.; Zabler, S.; Nelson, K.; Rack, A.; Spies, B.C.; Vach, K.; Kohal, R. Synchrotron-Based Micro Computed Tomography Investigation of the Implant-Abutment Fatigue-Induced Microgap Changes. J. Mech. Behav. Biomed. Mater. 2021, 116, 104330. [Google Scholar] [CrossRef]
- Pan, Y.; Tsoi, J.K.H.; Lam, W.Y.H.; Pow, E.H.N. Implant Framework Misfit: A Systematic Review on Assessment Methods and Clinical Complications. Clin. Implant. Dent. Relat. Res. 2021, 23, 244–258. [Google Scholar] [CrossRef]
- Queiroz, D.A.; Hagee, N.; Lee, D.J.; Zheng, F. The behavior of a zirconia or metal abutment on the implant-abutment interface during cyclic loading. J. Prosthet. Dent. 2020, 124, 211–216. [Google Scholar] [CrossRef]
- Block, M.S. Evidence-Based Criteria for an Ideal Abutment Implant Connection-A Narrative Review. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2022, 80, 1670–1675. [Google Scholar] [CrossRef]
- Katsoulis, J.; Takeichi, T.; Gaviria, A.S.; Peter, L.; Katsoulis, K. Misfit of implant prostheses and its impact on clinical outcomes. Definition, assessment and a systematic review of the literature. Eur. J. Oral. Implant. 2017, 10, 121–138. [Google Scholar]
- Jemt, T. Failures and complications in 391 consecutively inserted fixed prostheses supported by Brånemark implants in edentulous jaws: A study of treatment from the time of prosthesis placement to the first annual checkup. Int. J. Oral. Maxillofac. Implant. 1991, 3, 270–276. [Google Scholar]
- de França, D.G.B.; Morais, M.H.S.T.; das Neves, F.D.; Barbosa, G.A.S. Influence of Cad/cam on the Fit Accuracy of Implant-Supported Zirconia and Cobalt-Chromium Fixed Dental Prostheses. J. Prosthet. Dent. 2015, 113, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.R.; Alotaibi, A.; Al Hazza, N.; Allam, Y.; AlGhazi, M. Two-body wear behavior of human enamel versus monolithic zirconia, lithium disilicate, ceramometal and composite resin. J. Adv. Prosthodont. 2019, 11, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Jonasch, M. Effect of Cyclic Fatigue Tests on Aging and Their Translational Implications for Survival of All-Ceramic Tooth-Borne Single Crowns and Fixed Dental Prostheses. J. Prosthodont. 2018, 27, 364–375. [Google Scholar] [CrossRef] [PubMed]



| Group | Area | Mean | Standard Deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
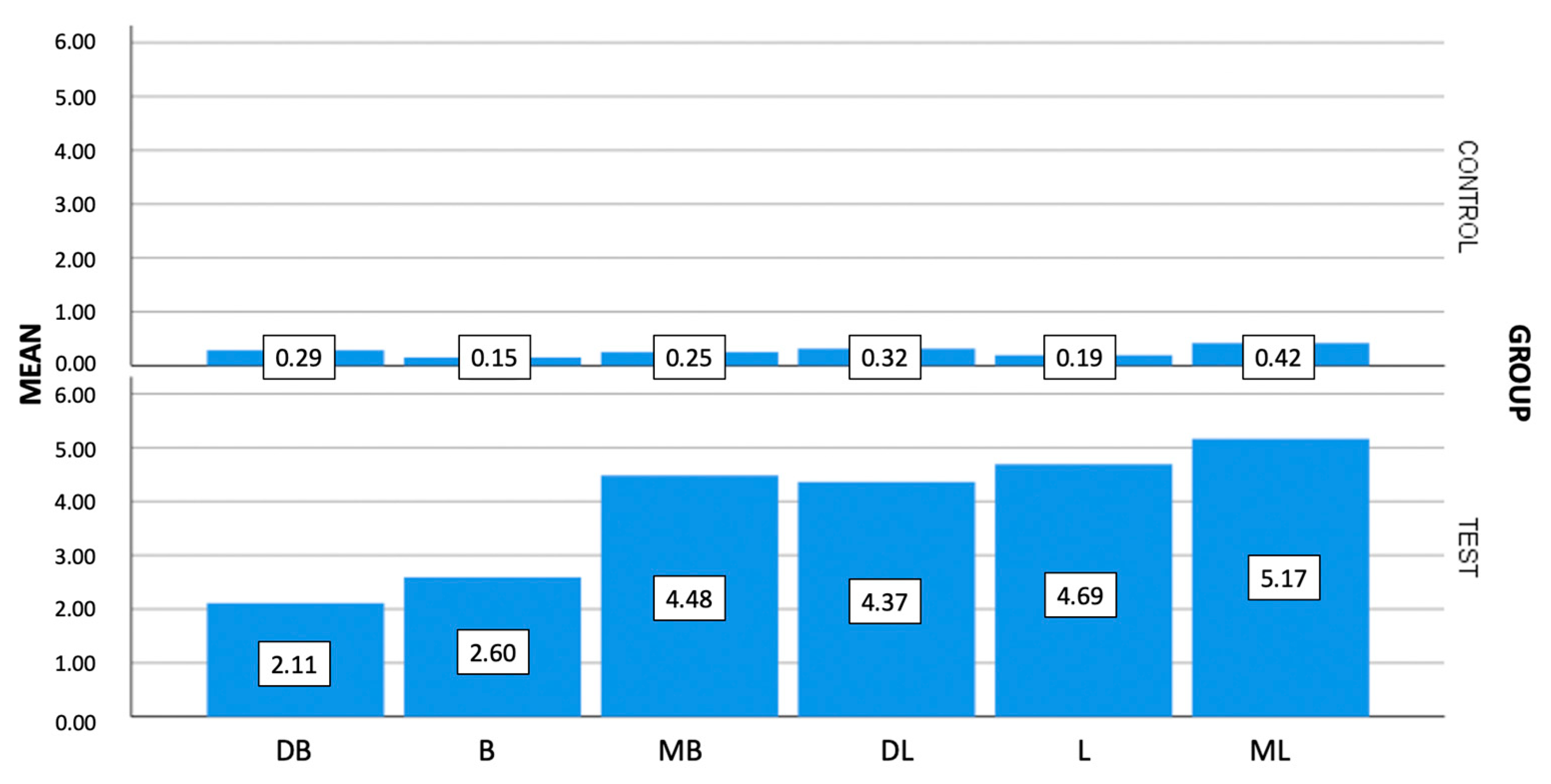
| Control group (transmucosal abutment) (N = 30) | MB * | 0.25 | 0.17 | 0.22 | 0.11 | 0.78 |
| B * | 0.15 | 0.10 | 0.11 | 0.11 | 0.45 | |
| DB * | 0.29 | 0.28 | 0.23 | 0.11 | 1.52 | |
| ML * | 0.42 | 0.31 | 0.34 | 0.11 | 1.20 | |
| L * | 0.19 | 0.19 | 0.11 | 0.11 | 0.99 | |
| DL * | 0.32 | 0.18 | 0.29 | 0.11 | 0.74 | |
| T * | 0.27 | 0.12 | 0.25 | 0.11 | 0.65 | |
| Test Group (Ti-base) (N = 30) | MB * | 4.48 | 8.70 | 0.77 | 0.11 | 37.08 |
| B * | 2.60 | 4.17 | 0.94 | 0.11 | 17.77 | |
| DB * | 2.11 | 2.36 | 1.12 | 0.11 | 10.41 | |
| ML * | 5.17 | 11.24 | 0.98 | 0.33 | 49.50 | |
| L * | 4.69 | 9.18 | 1.11 | 0.11 | 39.91 | |
| DL * | 4.37 | 9.09 | 1.26 | 0.11 | 46.92 | |
| T * | 3.90 | 6.30 | 1.25 | 0.64 | 25.22 |
| Area | |||||||
|---|---|---|---|---|---|---|---|
| MB Gap | B Gap | DB Gap | ML Gap | L Gap | DL Gap | T Gap | |
| Mann–Whitney U | 54.50 | 26.50 | 56.50 | 109.00 | 37.00 | 67.00 | 1.00 |
| Z | −5.87 | −6.47 | −5.84 | −5.05 | −6.24 | −5.67 | −6.64 |
| Significance * | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascos, R.; Celemín-Viñuela, A.; Mory-Rubiños, N.; Gómez-Polo, C.; Ortega, R.; Agustín-Panadero, R.; Gómez-Polo, M. Influence of the Use of Transepithelial Abutments vs. Titanium Base Abutments on Microgap Formation at the Dental Implant–Abutment Interface: An In Vitro Study. Materials 2023, 16, 6532. https://doi.org/10.3390/ma16196532
Cascos R, Celemín-Viñuela A, Mory-Rubiños N, Gómez-Polo C, Ortega R, Agustín-Panadero R, Gómez-Polo M. Influence of the Use of Transepithelial Abutments vs. Titanium Base Abutments on Microgap Formation at the Dental Implant–Abutment Interface: An In Vitro Study. Materials. 2023; 16(19):6532. https://doi.org/10.3390/ma16196532
Chicago/Turabian StyleCascos, Rocío, Alicia Celemín-Viñuela, Nataly Mory-Rubiños, Cristina Gómez-Polo, Rocío Ortega, Rubén Agustín-Panadero, and Miguel Gómez-Polo. 2023. "Influence of the Use of Transepithelial Abutments vs. Titanium Base Abutments on Microgap Formation at the Dental Implant–Abutment Interface: An In Vitro Study" Materials 16, no. 19: 6532. https://doi.org/10.3390/ma16196532
APA StyleCascos, R., Celemín-Viñuela, A., Mory-Rubiños, N., Gómez-Polo, C., Ortega, R., Agustín-Panadero, R., & Gómez-Polo, M. (2023). Influence of the Use of Transepithelial Abutments vs. Titanium Base Abutments on Microgap Formation at the Dental Implant–Abutment Interface: An In Vitro Study. Materials, 16(19), 6532. https://doi.org/10.3390/ma16196532







