Synthesis of Quaternary Ammonium Gemini Levelers and Their Action Mechanisms in Microvias Void-Free Copper Filling
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
2.3. Electrochemical Experiments
2.4. Microvias Copper Electroplating
2.5. Calculation of the Adsorption Energies of GL1 and GL2
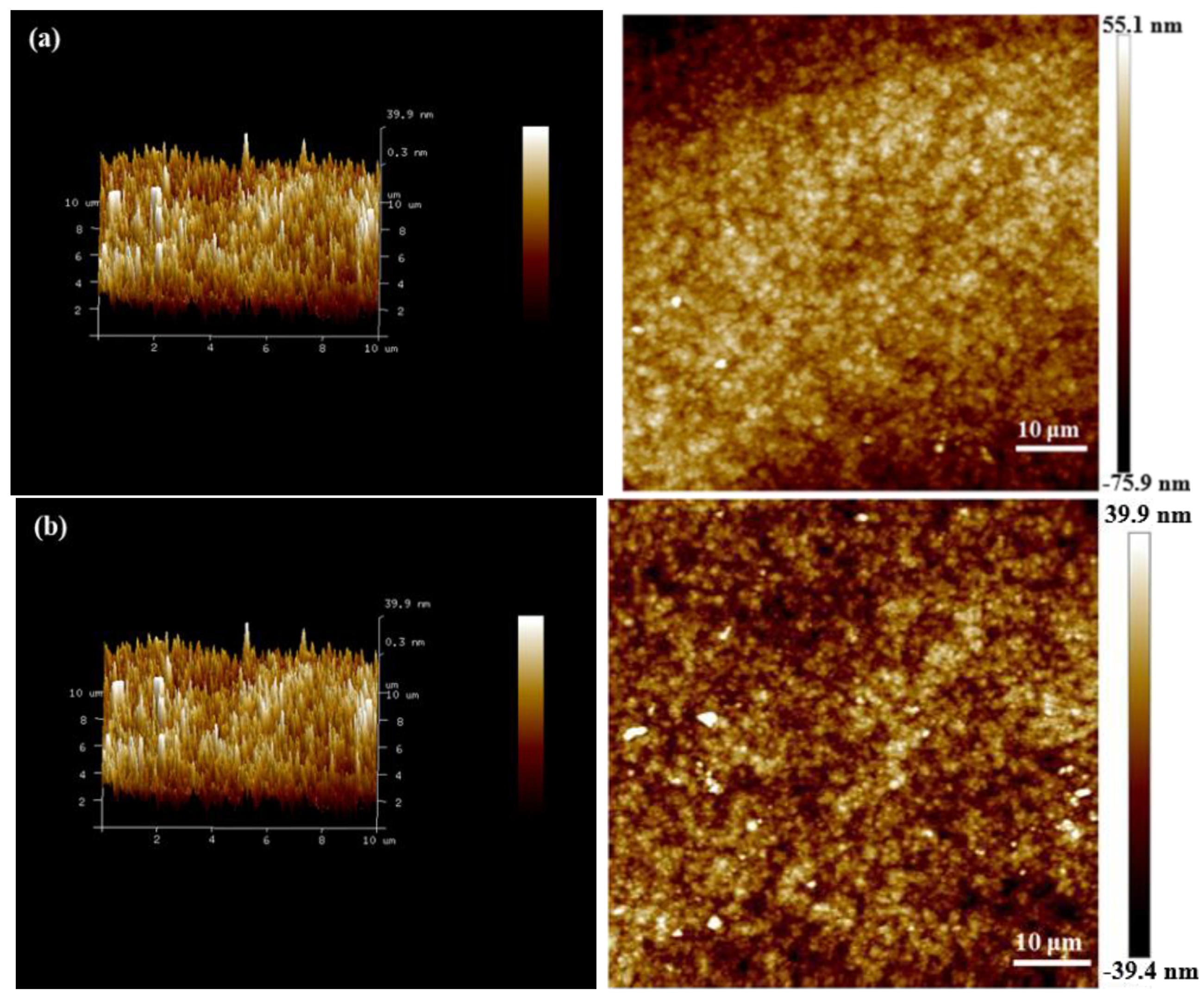
2.6. Surface Morphology and Structure
3. Results and Discussion
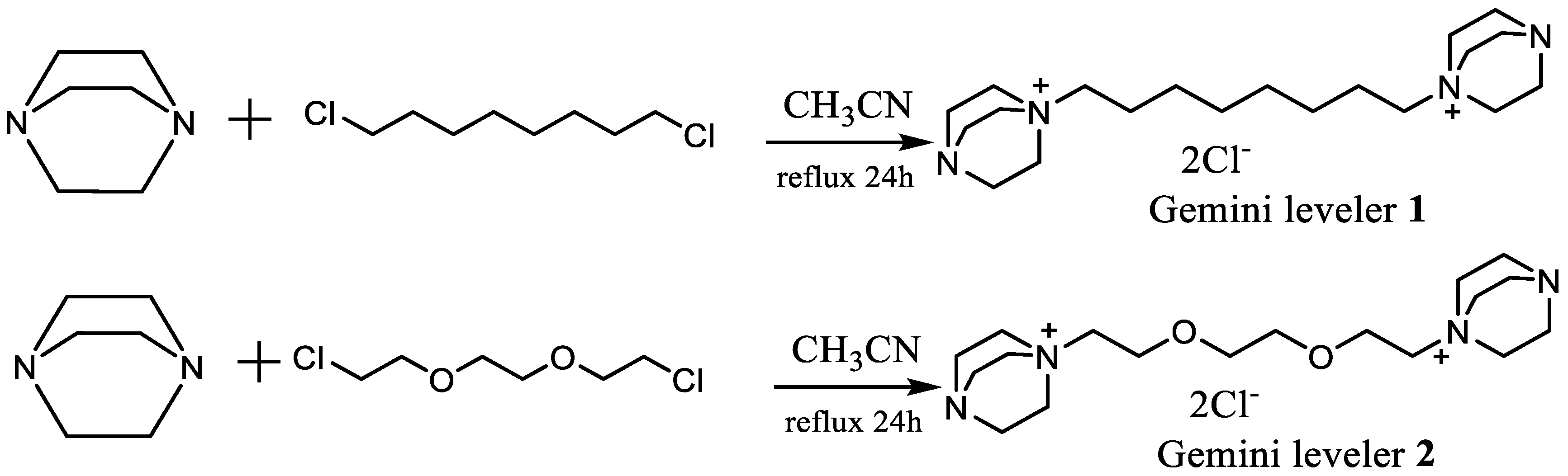
3.1. Synthesis and Characterization of Gemini Levelers
3.2. Galvanostatic Measurements
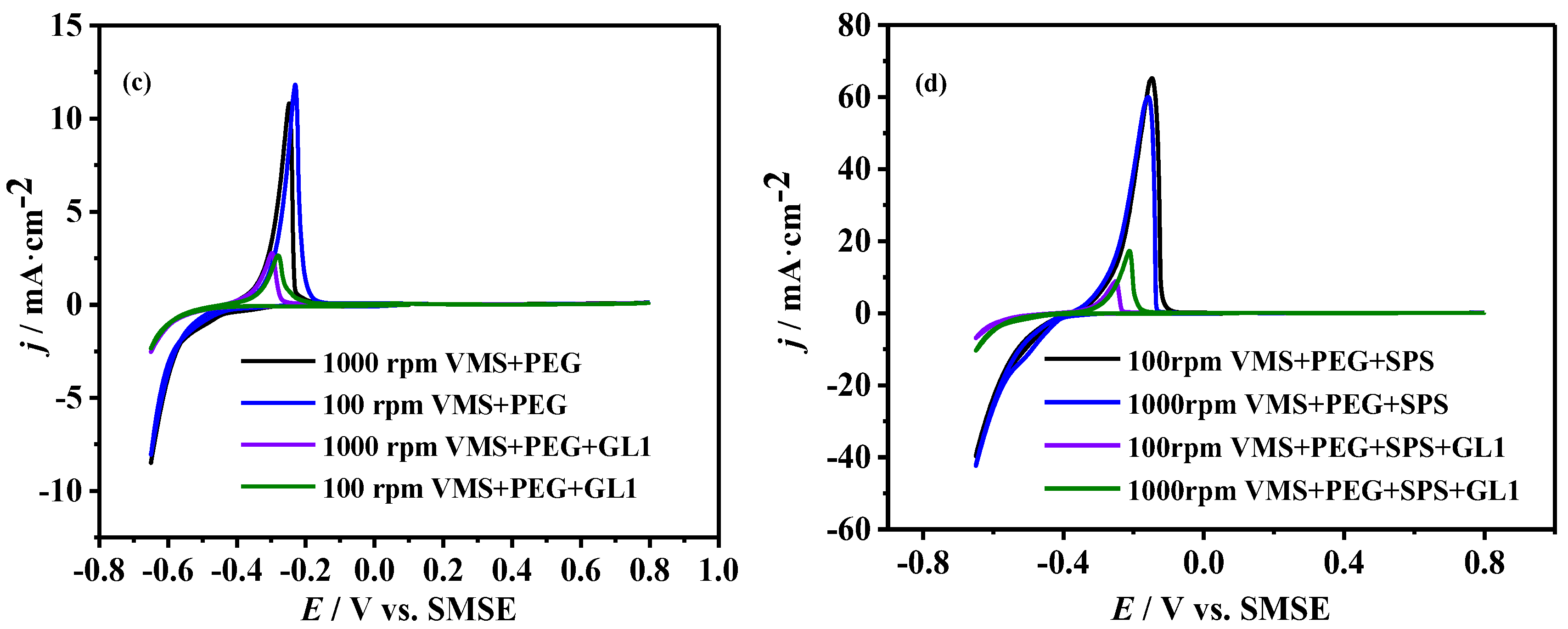
3.3. Cyclic Voltammetry Measurements
3.4. Copper Electroplating for Microvias Filling
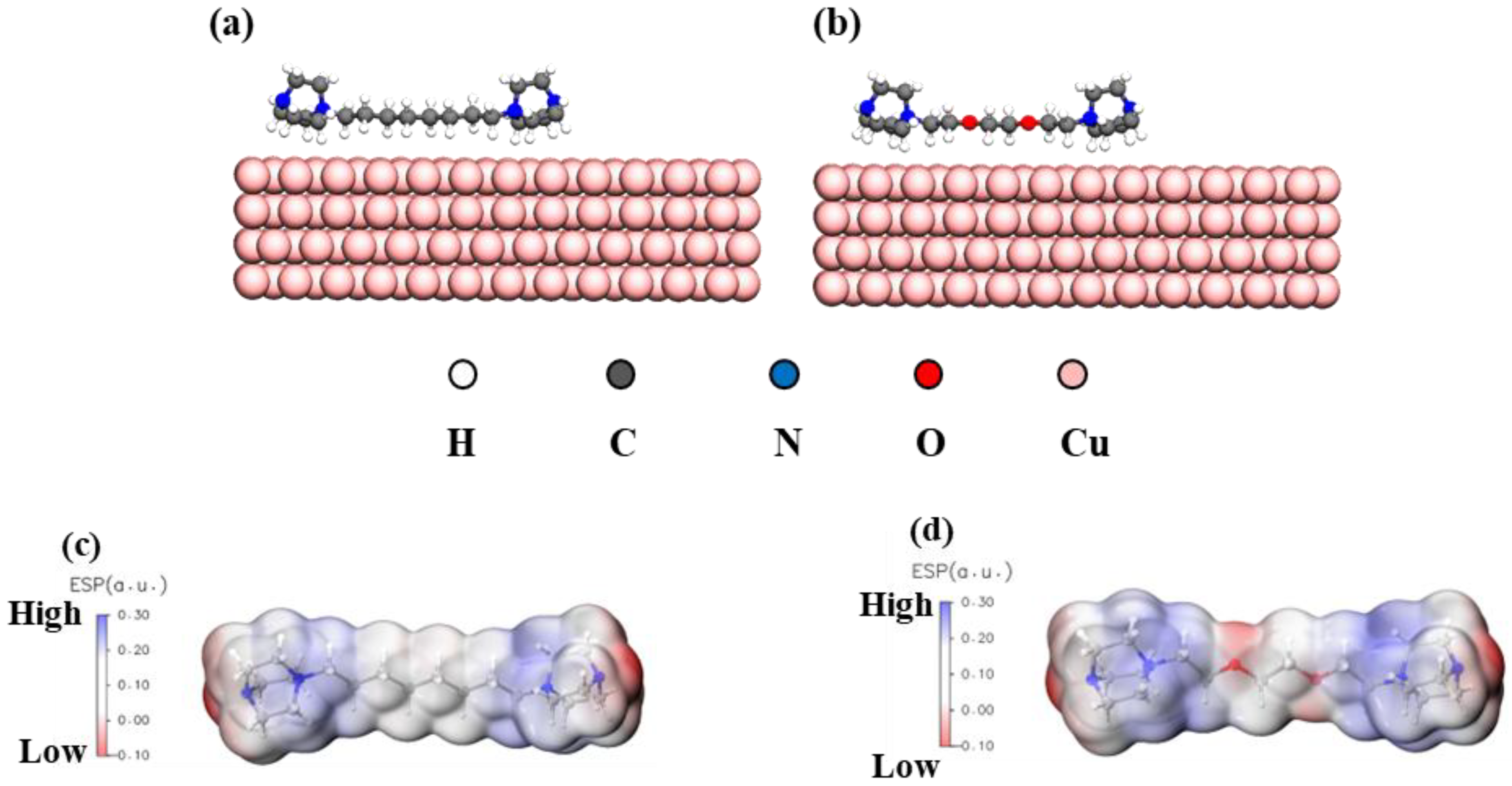
3.5. Adsorption Configurations and Energies of the Gemini Levelers
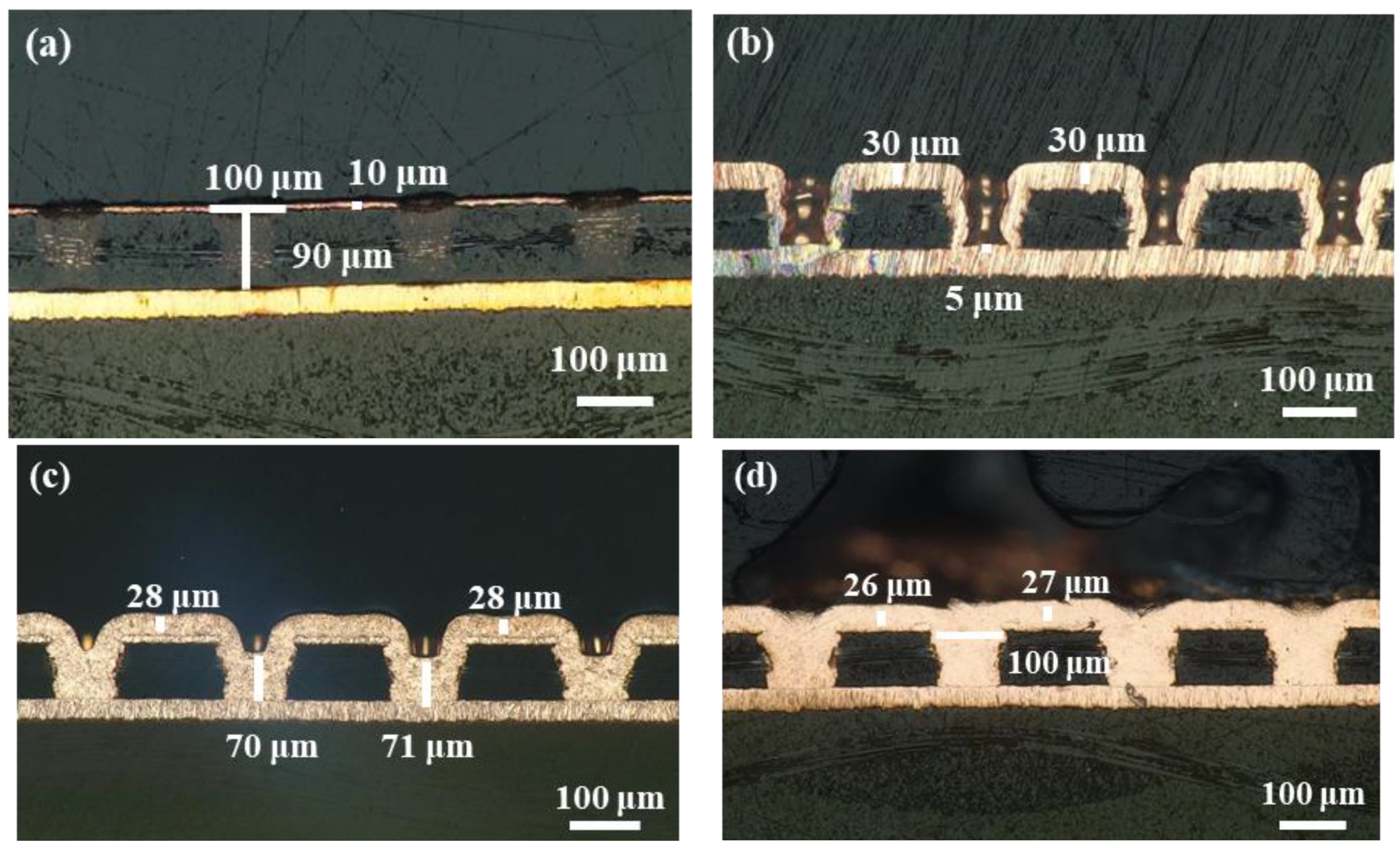
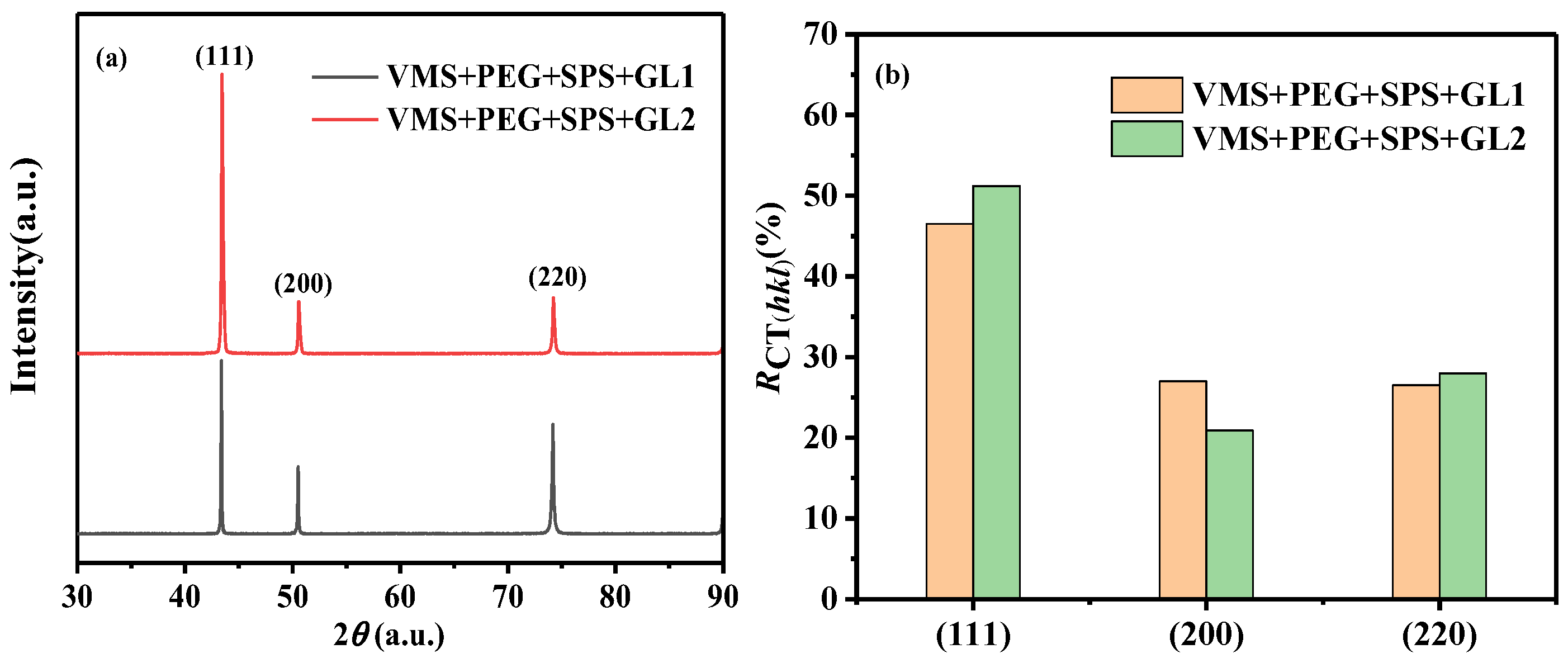
3.6. Surface Morphology and Structure of Copper Layer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Li, Z.; Tan, B.; Cui, C.; Shi, M.; Hao, Z. Communication—Triphenylmethane-Based Leveler for Microvia Filling in Copper Super-Conformal Electroplating. J. Electrochem. Soc. 2019, 166, D603–D605. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Jin, L.; Li, G.; Yang, J.Q.; Li, W.Q.; Zhan, D.; Jiang, Y.X.; Yang, F.Z.; Sun, S.G. Electro-chemical and in Situ FTIR Spectroscopic studies of Gentian Violet as a Novel Leveler in Through-Holes Metal-Lization for Printed Circuit Board Applications. Electrochim. Acta 2022, 410, 140018. [Google Scholar] [CrossRef]
- Lv, J.; Zhao, X.; Jie, X.; Li, J.; Wei, X.; Chen, B.; Hong, G.; Wu, W.; Wang, L. Fatty Acid Quaternary Ammonium Surfactants Based on Renewable Resources as a Leveler for Copper Electroplating. ChemElectroChem 2019, 6, 3254–3263. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.Y.; Jin, L.; Yang, J.Q.; Song, T.; Yang, F.Z.; Zhan, D. 1-Dodecyl-2-methyl-3-benzylimidazolium chloride as a novel leveler: Towards simultaneously both the microvia void-free filling and through hole conformal thickening. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134239. [Google Scholar] [CrossRef]
- Abadias, G.; Chason, E.; Keckes, J.; Sebastiani, M.; Thompson, G.B.; Barthel, E.; Doll, G.L.; Murray, C.E.; Stoessel, C.H.; Martinu, L. Review Article: Stress in thin films and coatings: Current status, challenges, and prospects. J. Vac. Sci. Technol. A 2018, 36, 020801. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, S.; Chen, S.; Qiang, Y.; Liu, G.; Tang, M.; Tan, B.; Fu, D.; Xu, Y. The effect of tricyclazole as a novel leveler for filling electroplated copper microvias. J. Electroanal. Chem. 2018, 827, 151–159. [Google Scholar] [CrossRef]
- Chan, P.F.; Chiu, Y.-D.; Dow, W.P.; Krug, K.; Lee, Y.L.; Yau, S.L. Use of 3,3-Thiobis(1-propanesulfonate) to Accelerate Microvia Filling by Copper Electroplating. J. Electrochem. Soc. 2013, 160, D3271–D3277. [Google Scholar] [CrossRef]
- Ren, X.; Song, Y.; Liu, A.; Zhang, J.; Yang, P.; Zhang, J.; Yuan, G.; An, M.; Osgood, H.; Wu, G. Role of polyethyleneimine as an additive in cyanide-free electrolytes for gold electrodeposition. RSC Adv. 2015, 5, 64806–64813. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Chen, S.; Tan, B.; Guo, H.; Wang, Y.; Qiang, Y.; Fu, S.; Wen, Y. Effects of 2,2-Dithiodipyridine as a Leveler for Through-Holes Filling by Copper Electroplating. J. Electrochem. Soc. 2019, 166, D660–D668. [Google Scholar] [CrossRef]
- Li, Y.; Ren, P.; Zhang, Y.; Li, R.; Zhang, J.; Yang, P.; Liu, A.; Wang, G.; An, M. Investigation of novel leveler Rhodamine B on copper superconformal electrodeposition of microvias by theoretical and experimental studies. Appl. Surf. Sci. 2022, 615, 156266. [Google Scholar] [CrossRef]
- Yang, J.Q.; Qiu, J.P.; Jin, L.; Wang, Z.Y.; Song, T.; Zhao, Y.; Yang, X.-H.; Cheng, J.; Yang, F.-Z.; Zhan, D.P. Molecular structure impacts of tetrazole derivatives on their diffusion and adsorption behaviors for microvia copper void-free filling. Surf. Interfaces 2023, 44, 103679. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.; Luo, J.; Qin, J.; Yang, G.; Cui, C.; Pan, L. Structural influence of nitrogen-containing groups on triphenylmethane-based levelers in super-conformal copper electroplating. Electrochim. Acta 2022, 401, 139445. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, L.; Wang, W.; Wang, Z.; Zhao, C. Tetrazole Derived Levelers for Filling Electroplated Cu Microvias: Electrochemical Behaviors and Quantum Calculations. Electrochim. Acta 2015, 178, 546–554. [Google Scholar] [CrossRef]
- Schmitt, K.G.; Schmidt, R.; Von-Horsten, H.F.; Vazhenin, G.; Gewirth, A.A. 3-Mercapto-1-Propanesulfonate for Cu Electrodeposition Studied by in Situ Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy, Density Functional Theory Calculations, and Cyclic Voltammetry. J. Phys. Chem. C 2015, 119, 23453–23462. [Google Scholar] [CrossRef]
- Yang, J.Q.; Song, T.; Wang, Z.Y.; Jin, L.; Zhao, Y.; Hu, R.; Su, J.-J.; Yang, F.-Z.; Zhan, D.; Han, L. In-operando visualization of the dynamic microvia copper filling process for metal interconnection of integrated circuit. Electrochim. Acta 2025, 535, 146698. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Wang, J.; Hong, Y.; Chen, Y.; He, W.; Zhou, J.; Miao, H.; Chen, Q. Convection-Dependent Competitive Adsorption between SPS and EO/PO on Copper Surface for Accelerating Trench Filling. J. Electrochem. Soc. 2019, 166, D93–D98. [Google Scholar] [CrossRef]
- Feng, Z.V.; Li, X.; Gewirth, A.A. Inhibition Due to the Interaction of Polyethylene Glycol, Chloride, and Copper in Plating Baths: A Surface-Enhanced Raman Study. J. Phys. Chem. B 2003, 107, 9415–9423. [Google Scholar] [CrossRef]
- Li, Y.B.; Wang, W.; Li, Y.L. Adsorption Behavior and Related Mechanism of Janus Green B during Copper Via-Filling Process. J. Electrochem. Soc. 2009, 156, D119–D124. [Google Scholar] [CrossRef]
- Chang, C.; Lu, X.; Lei, Z.; Wang, Z.; Zhao, C. 2-Mercaptopyridine as a new leveler for bottom-up filling of micro-vias in copper electroplating. Electrochim. Acta 2016, 208, 33–38. [Google Scholar] [CrossRef]
- Moffat, T.P.; Wheeler, D.; Huber, W.H.; Josell, D. Superconformal electrodeposition of copper, Electro-chem. Solid State Lett. 2001, 4, C26–C29. [Google Scholar] [CrossRef]
- Moffat, T.P.; Wheeler, D.; Kim, S.K.; Josell, D. Curvature enhanced adsorbate coverage mechanism for bottom-up superfilling and bump control in damascene processing. Electrochim. Acta 2007, 53, 145–154. [Google Scholar] [CrossRef]
- West, A.C.; Mayer, S.; Reid, J. A Superfilling Model that Predicts Bump Formation. Electrochem. Solid-State Lett. 2001, 4, C50–C53. [Google Scholar] [CrossRef]
- West, A.C. Theory of Filling of High-Aspect Ratio Trenches and Vias in Presence of Additives. J. Electrochem. Soc. 2000, 147, 227–232. [Google Scholar] [CrossRef]
- Akolkar, R.; Landau, U. A Time-Dependent Transport-Kinetics Model for Additive Interactions in Copper Interconnect Metallization. J. Electrochem. Soc. 2004, 151, C702–C711. [Google Scholar] [CrossRef]
- Dow, W.P.; Huang, H.S.; Yen, M.Y.; Huang, H.C. Influence of convection-dependent adsorption of addi-tives on microvia filling by copper electroplating. J. Electrochem. Soc. 2005, 152, C425–C434. [Google Scholar] [CrossRef]
- Su, X.; Wang, B.Q.; Lu, Z.Y.; Wei, L.M.; Feng, Y.J. A New Cationic Gemini Surfmer: Synthesis and Sur-face Activities. J. Surfactants Deterg. 2011, 14, 73–76. [Google Scholar] [CrossRef]
- Ghazal, Y.; Najjar, R. Gasoil/sunflower oil blend/water fuel microemulsions prepared with ionic liquids-type Gemini surfactants. Alex. Eng. J. 2024, 90, 161–169. [Google Scholar] [CrossRef]
- Barbieri, C.; Borsotto, P. Essential Oils: Market and Legislation. In Potential of Essential Oils; IntechOpen: London, UK, 2018; pp. 107–127. [Google Scholar]
- Ahmed, T.; O Kamel, A.; Wettig, S.D. Interactions Between DNA and Gemini Surfactant: Impact on Gene Therapy: Part I. Nanomedicine 2016, 11, 289–306. [Google Scholar] [CrossRef]
- Yang, X.; Mao, J.; Zhang, Z.; Zhang, H.; Yang, B.; Zhao, J. Rheology of Quaternary Ammonium Gemini Surfactant Solutions: Effects of Surfactant Concentration and Counterions. J. Surfactants Deterg. 2018, 21, 467–474. [Google Scholar] [CrossRef]
- Numin, M.S.; Hassan, A.; Jumbri, K.; Eng, K.K.; Borhan, N.; Daud, N.M.R.N.M.; A, A.M.N.; Suhor, F.; Wahab, R.A. A recent review on theoretical studies of Gemini surfactant corrosion inhibitors. J. Mol. Liq. 2022, 368, 120649. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic Gemini sur-factant properties, its potential as a promising bioapplication candidate, and strategies for improving its bi-ocompatibility: A review. Adv. Colloid Interfac. 2022, 299, 102581. [Google Scholar] [CrossRef]
- Aguilar Troyano, F.J.; Merkens, K.; Gómez-Suarez, A. Selectfluor® Radical Dication (TE-DA2+)—A Versatile Species in Modern Synthetic Organic Chemistry. Asian J. Org. Chem. 2020, 9, 992–1007. [Google Scholar] [CrossRef]
- Tian, D.; Lai, Z.; Xu, H.; Lin, F.; Jiang, Y.; Huang, Y.; Wang, S.; Cai, Y.; Jin, J.; Xie, R.-J.; et al. Low-Dimensional Organic Lead Halides with Organic–Inorganic Collaborative Luminescence Regulated by Anion in Dimension. Chem. Mater. 2022, 34, 5224–5231. [Google Scholar] [CrossRef]
- He, X.X.; Xi, Y.N.; Lv, C.; He, C.X.; Kang, J.L.; Li, Z.X. Construction of silicone composite with control-lable micro-nano structure via in-situ polymerization on fiber surface and study on SO2 adsorption perfor-mance. Colloid Surf. A 2023, 660, 130815. [Google Scholar] [CrossRef]
- Hu, K.W.; You, X.; Wen, X.A.; Yuan, H.L.; Xu, Q.L.; Lai, Z.W. Synthesis of Functionalized Thiazoli-din-2-imine and Oxazolidin-2-one Derivatives from p-Quinamines via [3+2] Annulation of Isothi-ocyanates and CO2. J. Org. Chem. 2022, 88, 5052–5058. [Google Scholar] [CrossRef] [PubMed]
- Hartwigsen, C.; Goedecker, S.; Hutter, J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B 1998, 58, 3641–3662. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of den-sity functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Bento, A.P.; Hersey, A.; Félix, E.; Landrum, G.; Gaulton, A.; Atkinson, F.; Bellis, L.J.; De Veij, M.; Leach, A.R. An open source chemical structure curation pipeline using RDKit. J. Cheminform. 2021, 12, 51. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, L.; Curtiss, L.; Greeley, J. Effects of van der Waals density functional corrections on trends in furfural adsorption and hydrogenation on close-packed transition metal surfaces. Surf. Sci. 2014, 622, 51–59. [Google Scholar] [CrossRef]
- Song, T.; Wang, Z.Y.; Yang, J.Q.; Zhao, Y.; Yang, F.Z.; Zhan, D. Tributyl(hexyl)phosphonium chloride as a new leveler for microvia copper superconformal electronic plating. J. Electroanal. Chem. 2024, 964, 118340. [Google Scholar] [CrossRef]



| Bath Composition and Plating Parameters | Optimum Condition |
|---|---|
| CuSO4·5H2O (g/L) | 200 |
| H2SO4 (g/L) | 50 |
| Cl− (mg/L) | 60 |
| PEG−8000 (mg/L) | 500 |
| SPS (mg/L) | 2 |
| GL1 or GL2 (mg/L) | 5 |
| Cathode current density (mA/cm2) | 20 |
| Electroplating time (min) | 90 |
| Solution Composition | Q100 rpm (mC/cm2) | Q1000 rpm (mC/cm2) | ΔQ% |
|---|---|---|---|
| VMS | 471.5 | 474.0 | −0.5 |
| VMS + GL1 | 257.0 | 220.0 | 15 |
| VMS + SPS | 515.0 | 523.0 | −1.5 |
| VMS + SPS + GL1 | 230.0 | 162.0 | 30 |
| VMS + PEG | 16.05 | 15.85 | 1.0 |
| VMS + PEG + GL1 | 2.85 | 2.75 | 3.0 |
| VMS + PEG + SPS | 144.5 | 140.0 | 3.0 |
| VMS + PEG + SPS + GL1 | 24.5 | 17.5 | 29 |
| Solution Composition | Q100 rpm (mC/cm2) | Q1000 rpm (mC/cm2) | ΔQ% |
|---|---|---|---|
| VMS | 471.5 | 474.0 | –0.5 |
| VMS + GL2 | 146.5 | 124.5 | 16 |
| VMS + SPS | 515.0 | 523.0 | –1.0 |
| VMS + SPS + GL2 | 162.5 | 85.0 | 48 |
| VMS + PEG | 16.05 | 15.85 | 1.0 |
| VMS + PEG + GL2 | 2.50 | 2.40 | 4.0 |
| VMS + PEG + SPS | 144.5 | 140.0 | –4.0 |
| VMS + PEG + SPS + GL2 | 30.0 | 18.0 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Wang, J.-Y.; Qiu, J.-P.; Yang, J.-Q.; Wang, Z.-Y.; Zhao, Y.; Yang, X.-H.; Hu, R.; Cheng, J.; Yang, F.-Z.; et al. Synthesis of Quaternary Ammonium Gemini Levelers and Their Action Mechanisms in Microvias Void-Free Copper Filling. Colloids Interfaces 2025, 9, 62. https://doi.org/10.3390/colloids9050062
Song T, Wang J-Y, Qiu J-P, Yang J-Q, Wang Z-Y, Zhao Y, Yang X-H, Hu R, Cheng J, Yang F-Z, et al. Synthesis of Quaternary Ammonium Gemini Levelers and Their Action Mechanisms in Microvias Void-Free Copper Filling. Colloids and Interfaces. 2025; 9(5):62. https://doi.org/10.3390/colloids9050062
Chicago/Turabian StyleSong, Tao, Jun-Yi Wang, Jiang-Peng Qiu, Jia-Qiang Yang, Zhao-Yun Wang, Yi Zhao, Xiao-Hui Yang, Ren Hu, Jun Cheng, Fang-Zu Yang, and et al. 2025. "Synthesis of Quaternary Ammonium Gemini Levelers and Their Action Mechanisms in Microvias Void-Free Copper Filling" Colloids and Interfaces 9, no. 5: 62. https://doi.org/10.3390/colloids9050062
APA StyleSong, T., Wang, J.-Y., Qiu, J.-P., Yang, J.-Q., Wang, Z.-Y., Zhao, Y., Yang, X.-H., Hu, R., Cheng, J., Yang, F.-Z., Han, L.-H., & Zhan, D.-P. (2025). Synthesis of Quaternary Ammonium Gemini Levelers and Their Action Mechanisms in Microvias Void-Free Copper Filling. Colloids and Interfaces, 9(5), 62. https://doi.org/10.3390/colloids9050062







