Removal of Copper (II) from Aqueous Solutions Using Silica Xerogel as Sorbent: Adsorption Properties and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of SiO2 Xerogel
2.2. Characterization of SiO2 Xerogel
2.3. Adsorption Properties
3. Results
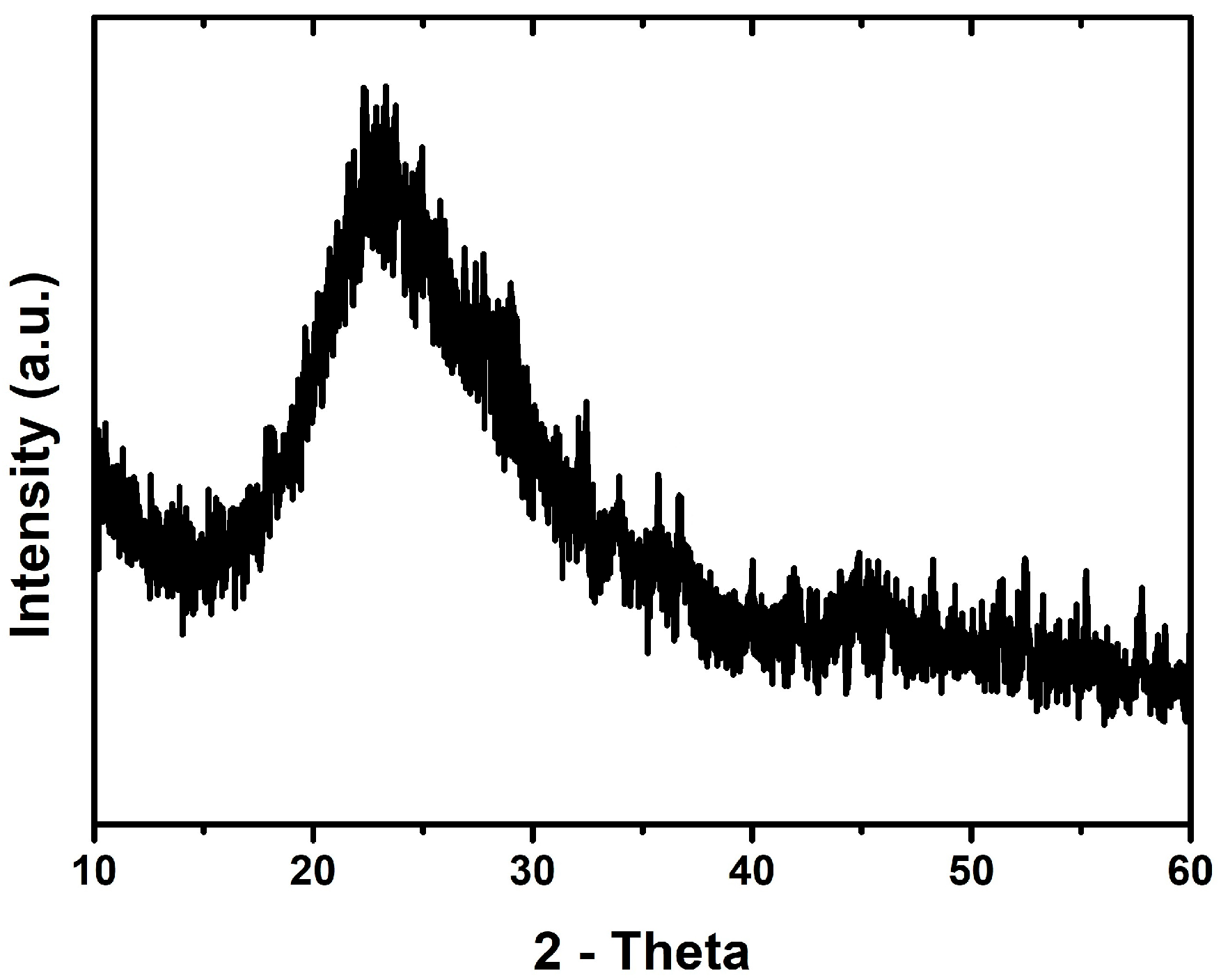
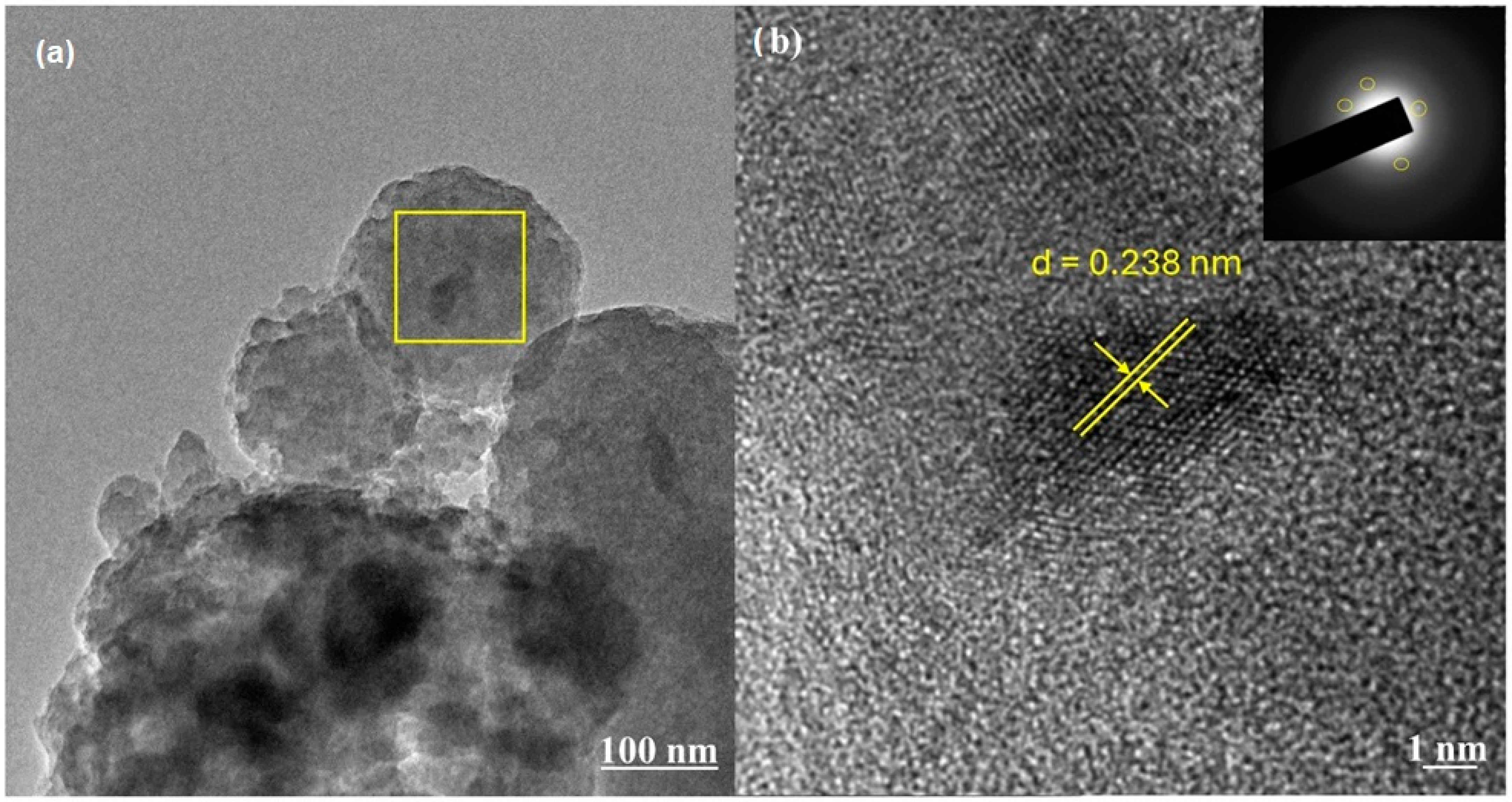
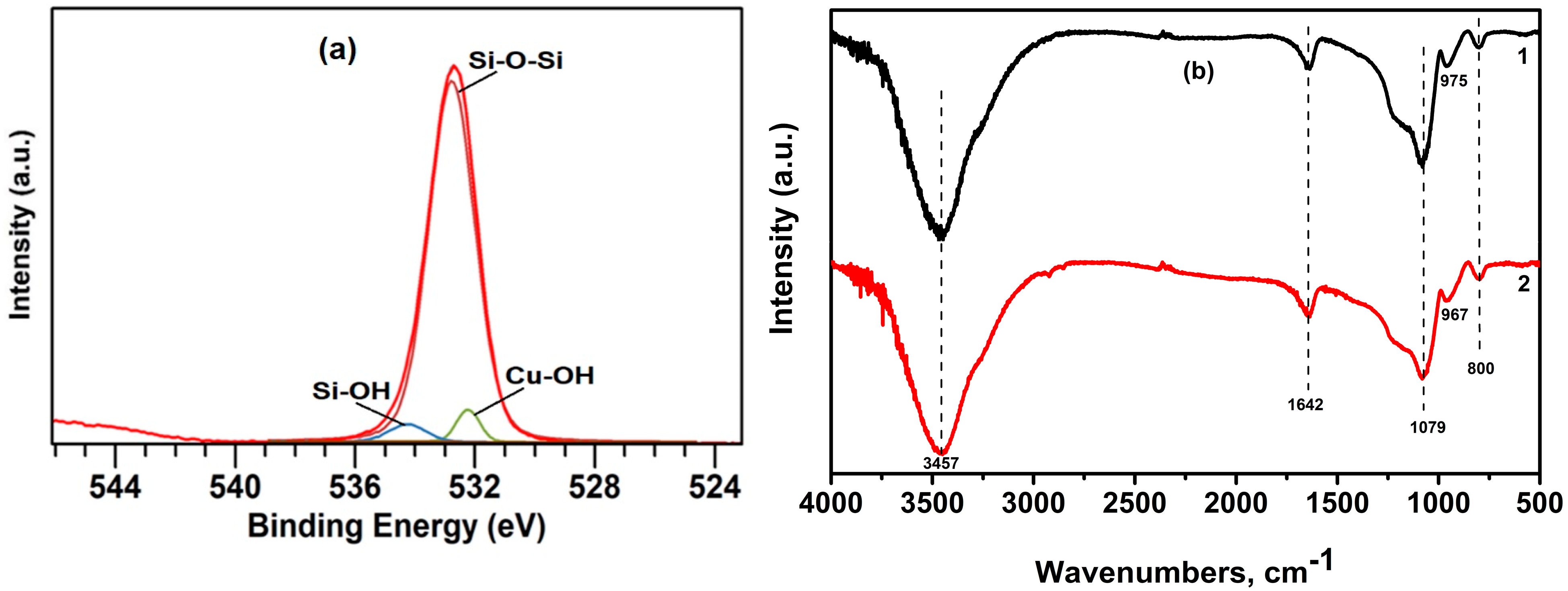
3.1. Characterization of the Adsorbent
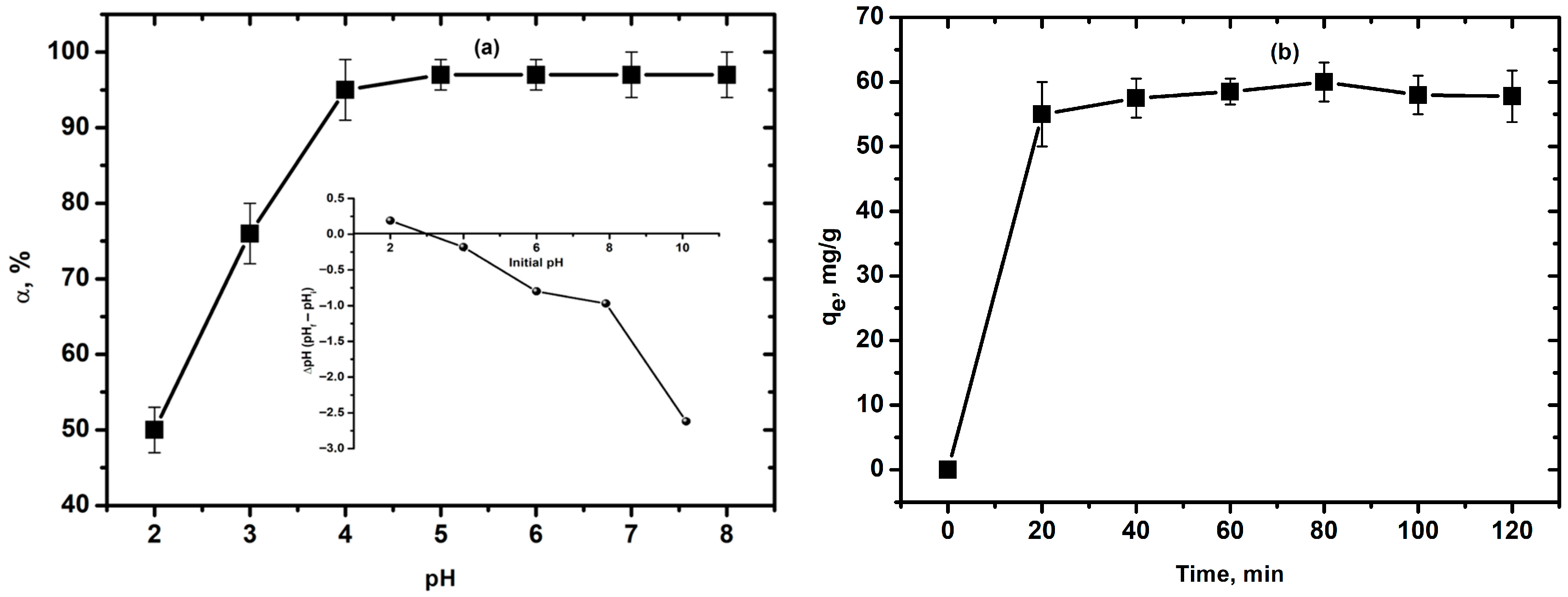
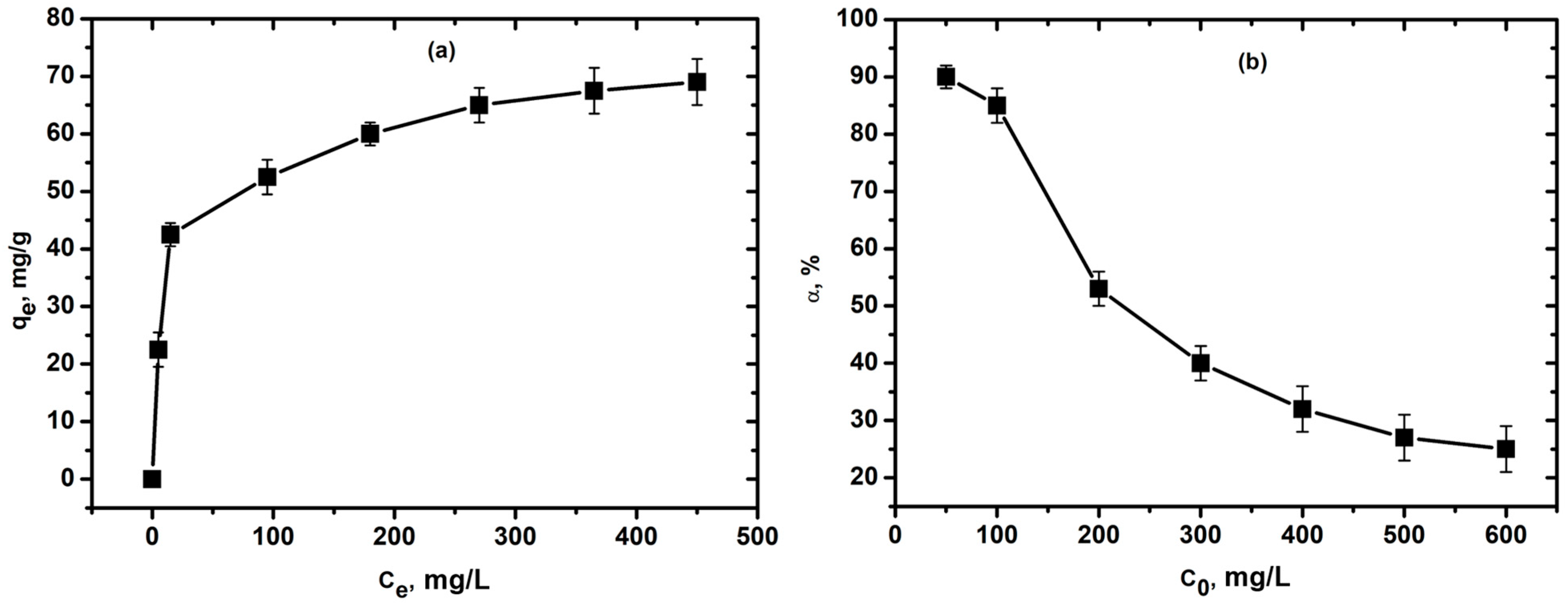
3.2. Adsorption of Cu2+
3.3. Competitive Ions
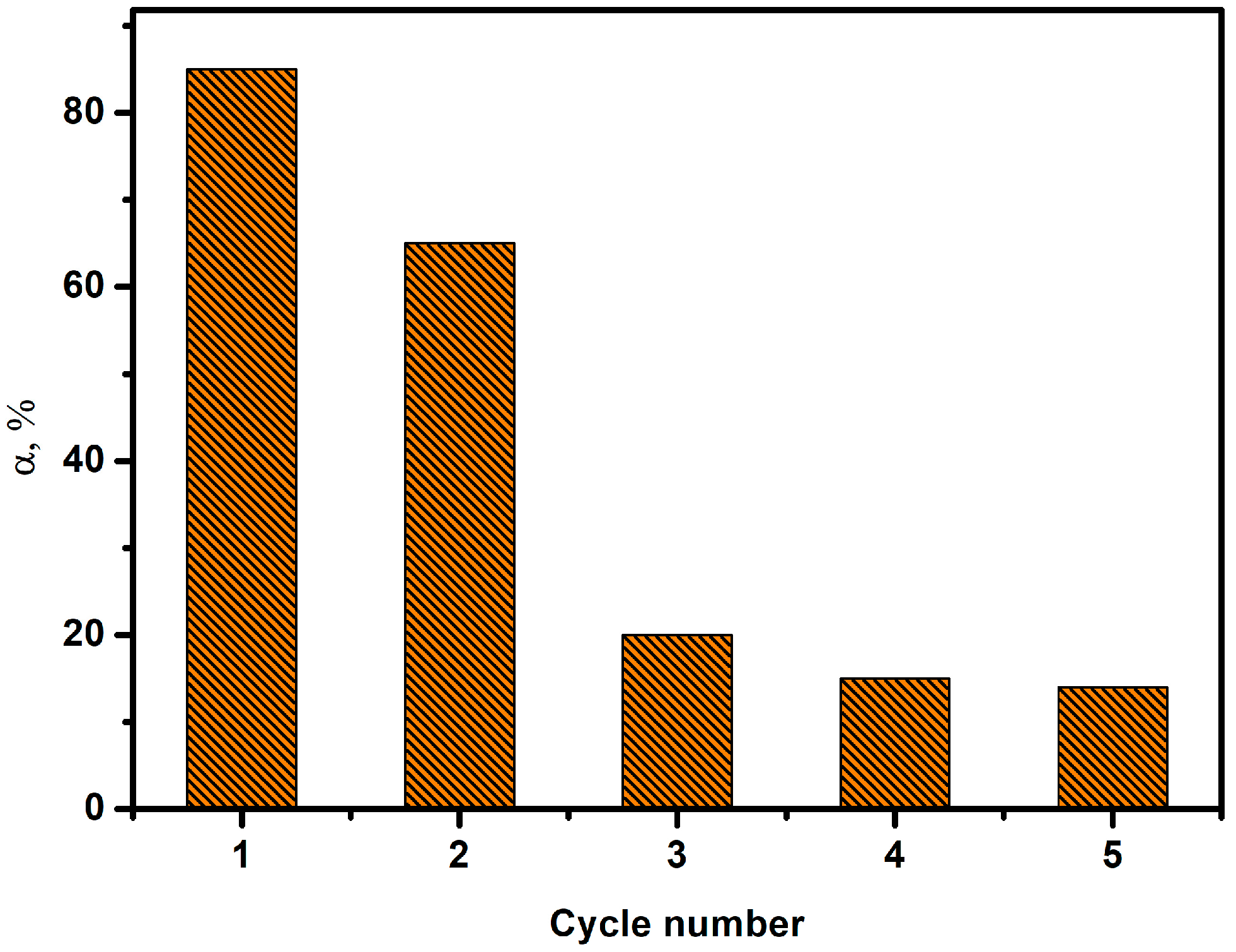
3.4. Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.; Arjunan, A.; Baroutaji, A.; Zakharova, J. A Review of Physicochemical and Biological Contaminants in Drinking Water and Their Impacts on Human Health. Water Sci. Eng. 2023, 16, 333–344. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; Shebl, A.; Abo El-Dahab, S.A. Drinking Water Treatment Sludge as an Efficient Adsorbent for Heavy Metals Removal. Appl. Clay Sci. 2017, 146, 343–349. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy Metals in Drinking Water: Occurrences, Implications, and Future Needs in Developing Countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Isaev, A.B.; Nabi, S.; Omarov, G.; Gulov, R.; Isaeva, M.A.; Nidheesh, P.V.; Oturan, M.A. Recent Progress in the Removal of Arsenic Using Iron Oxide and Oxyhydroxide Based Sorbents. Sep. Purif. Technol. 2025, 360, 131220. [Google Scholar] [CrossRef]
- Bora, A.J.; Dutta, R.K. Removal of Metals (Pb, Cd, Cu, Cr, Ni, and Co) from Drinking Water by Oxidation-Coagulation-Absorption at Optimized PH. J. Water Process Eng. 2019, 31, 100839. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of Three Dimensional Porous Aerogels as Adsorbent for Removal of Heavy Metal Ions from Water/Wastewater: A Review Study. Adv. Colloid. Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of Heavy Metals from Water Sources in the Developing World Using Low-Cost Materials: A Review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global Profile of Heavy Metals and Semimetals Adsorption Using Drinking Water Treatment Residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Senanu, L.D.; Kranjac-Berisavljevic, G.; Cobbina, S.J. The Use of Local Materials to Remove Heavy Metals for Household-Scale Drinking Water Treatment: A Review. Environ. Technol. Innov. 2023, 29, 103005. [Google Scholar] [CrossRef]
- Ismail, U.M.; Vohra, M.S.; Onaizi, S.A. Adsorptive Removal of Heavy Metals from Aqueous Solutions: Progress of Adsorbents Development and Their Effectiveness. Environ. Res. 2024, 251, 118562. [Google Scholar] [CrossRef]
- Li, B.; Li, K.; Li, X. Fabrication of Abundantly Functionalized Dendritic Biochar Composites as Adsorbents for the High-Efficiency Removal of Heavy Metal Ions and Dyes. Sep. Purif. Technol. 2024, 337, 126368. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy Metal Removal from Aqueous Solution by Advanced Carbon Nanotubes: Critical Review of Adsorption Applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Du, X.; Cui, S.; Fang, X.; Wang, Q.; Liu, G. Adsorption of Cd(II), Cu(II), and Zn(II) by Granules Prepared Using Sludge from a Drinking Water Purification Plant. J. Environ. Chem. Eng. 2020, 8, 104530. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul, A.K.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent Advances in Activated Carbon Modification Techniques for Enhanced Heavy Metal Adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Simate, G.S.; Maledi, N.; Ochieng, A.; Ndlovu, S.; Zhang, J.; Walubita, L.F. Coal-Based Adsorbents for Water and Wastewater Treatment. J. Environ. Chem. Eng. 2016, 4, 2291–2312. [Google Scholar] [CrossRef]
- Ugwu, E.I.; Othmani, A.; Nnaji, C.C. A Review on Zeolites as Cost-Effective Adsorbents for Removal of Heavy Metals from Aqueous Environment. Int. J. Environ. Sci. Technol. 2021, 19, 8061–8084. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkova, P.; Nickolov, R. Modified and unmodified silica gel used for heavy metal ions removal from aqueous solutions. J. Univ. Chem. Technol. Metall. 2012, 47, 498–504. [Google Scholar]
- Najafi, M.; Yousefi, Y.; Rafati, A.A. Synthesis, Characterization and Adsorption Studies of Several Heavy Metal Ions on Amino-Functionalized Silica Nano Hollow Sphere and Silica Gel. Sep. Purif. Technol. 2012, 85, 193–205. [Google Scholar] [CrossRef]
- Xia, K.; Ferguson, R.Z.; Losier, M.; Tchoukanova, N.; Brüning, R.; Djaoued, Y. Synthesis of Hybrid Silica Materials with Tunable Pore Structures and Morphology and Their Application for Heavy Metal Removal from Drinking Water. J. Hazard. Mater. 2010, 183, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Ezati, F.; Sepehr, E.; Ahmadi, F. The Efficiency of Nano-TiO2 and γ-Al2O3 in Copper Removal from Aqueous Solution by Characterization and Adsorption Study. Sci. Rep. 2021, 11, 18831. [Google Scholar] [CrossRef]
- Bhutto, A.A.; Baig, J.A.; uddin, S.; Kazi, T.G.; Sierra-Alvarez, R.; Akhtar, K.; Perveen, S.; Afridi, H.I.; Ali, H.E.; Hol, A.; et al. Biosynthesis of Aluminium Oxide Nanobiocomposite and Its Application for the Removal of Toxic Metals from Drinking Water. Ceram. Int. 2023, 49, 14615–14623. [Google Scholar] [CrossRef]
- Ewis, D.; Ba-Abbad, M.M.; Benamor, A.; El-Naas, M.H. Adsorption of Organic Water Pollutants by Clays and Clay Minerals Composites: A Comprehensive Review. Appl. Clay Sci. 2022, 229, 106686. [Google Scholar] [CrossRef]
- Novikau, R.; Lujaniene, G. Adsorption Behaviour of Pollutants: Heavy Metals, Radionuclides, Organic Pollutants, on Clays and Their Minerals (Raw, Modified and Treated): A Review. J. Environ. Manag. 2022, 309, 114685. [Google Scholar] [CrossRef] [PubMed]
- Otunola, B.O.; Ololade, O.O. A Review on the Application of Clay Minerals as Heavy Metal Adsorbents for Remediation Purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay Mineral Adsorbents for Heavy Metal Removal from Wastewater: A Review. Environ. Chem. Lett. 2018, 17, 629–654. [Google Scholar] [CrossRef]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent Advances in Applications of Low-Cost Adsorbents for the Removal of Heavy Metals from Water: A Critical Review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Jain, A.; Kumari, S.; Agarwal, S.; Khan, S. Water Purification via Novel Nano-Adsorbents and Their Regeneration Strategies. Process Saf. Environ. Prot. 2021, 152, 441–454. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of Heavy Metals from Aqueous Solution Using Carbon-Based Adsorbents: A Review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Pandey, L.M. Surface Engineering of Nano-Sorbents for the Removal of Heavy Metals: Interfacial Aspects. J. Environ. Chem. Eng. 2021, 9, 104586. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for Pollutants Removal: A Review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of Heavy Metals on Functionalized-Mesoporous Silica: A Review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Knight, A.W.; Tigges, A.B.; Ilgen, A.G. Adsorption of Copper (II) on Mesoporous Silica: The Effect of Nano-Scale Confinement. Geochem. Trans. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Fan, H.T.; Su, Z.J.; Fan, X.L.; Guo, M.M.; Wang, J.; Gao, S.; Sun, T. Sol-Gel Derived Organic-Inorganic Hybrid Sorbent for Removal of Pb2+, Cd2+ and Cu2+ from Aqueous Solution. J Solgel Sci Technol. 2012, 64, 418–426. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest Trends in Heavy Metal Removal from Wastewater by Biochar Based Sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Ziora, Z.M. Insights into Recent Advances of Chitosan-Based Adsorbents for Sustainable Removal of Heavy Metals and Anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Irannajad, M.; Kamran Haghighi, H. Removal of Heavy Metals from Polluted Solutions by Zeolitic Adsorbents: A Review. Environ. Process. 2020, 8, 7–35. [Google Scholar] [CrossRef]
- Kumar, R.; Laskar, M.A.; Hewaidy, I.F.; Barakat, M.A. Modified Adsorbents for Removal of Heavy Metals from Aqueous Environment: A Review. Earth Syst. Environ. 2019, 3, 83–93. [Google Scholar] [CrossRef]
- Mazibuko, M.T.; Onwubu, S.C.; Mokhothu, T.H.; Paul, V.; Mdluli, P.S. Unlocking Heavy Metal Remediation Potential: A Review of Cellulose–Silica Composites. Sustainability 2024, 16, 3265. [Google Scholar] [CrossRef]
- El Mahdaoui, A.; Radi, S.; Elidrissi, A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Moura, N.M.M. Progress in the Modification of Cellulose-Based Adsorbents for the Removal of Toxic Heavy Metal Ions. J. Environ. Chem. Eng. 2024, 12, 113870. [Google Scholar] [CrossRef]
- Duru, İ.; Ege, D.; Kamali, A.R. Graphene Oxides for Removal of Heavy and Precious Metals from Wastewater. J. Mater. Sci. 2016, 51, 6097–6116. [Google Scholar] [CrossRef]
- Liang, X.; Liu, S.; Wang, S.; Guo, Y.; Jiang, S. Carbon-Based Sorbents: Carbon Nanotubes. J. Chromatogr. A 2014, 1357, 53–67. [Google Scholar] [CrossRef]
- Sun, R.; Gao, S.; Zhang, K.; Cheng, W.T.; Hu, G. Recent Advances in Alginate-Based Composite Gel Spheres for Removal of Heavy Metals. Int. J. Biol. Macromol. 2024, 268, 131853. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy Metal Removal from Water/Wastewater by Nanosized Metal Oxides: A Review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Cardea, S.; Tabernero, A.; De Marco, I.; Valente, A.J.M.; Anastopoulos, I.; Brun, N. Porous Aerogels and Adsorption of Pollutants from Water and Air: A Review. Molecules 2021, 26, 4440. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Aznar, E.; Deveci, H.; Martínez-Máñez, R. Low-Cost Silica Xerogels as Potential Adsorbents for Ciprofloxacin Removal. Sustain. Chem. Pharm. 2021, 22, 100483. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Silica Aerogels/Xerogels Modified with Nitrogen-Containing Groups for Heavy Metal Adsorption. Molecules 2020, 25, 2788. [Google Scholar] [CrossRef]
- Jin, Z.; Lin, Y.; Zhang, Y.; Ying, J.; Gitis, V.; Yu, J. Development of Robust Fluorinated SiO2/PVDF Composite Hollow Fiber Membrane for Bromine Resources Recovery from Brine via Membrane Distillation. ACS ES T Water 2023, 3, 1874–1883. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Zhang, B.; Ye, Y.; Yang, T.; Zeng, H.; Luo, X. Enhanced Long-Term Antifouling Ability and Enrichment Effect of a Vertically Ordered Mesoporous Silica Film via Covalent Linkage of Chondroitin Sulfate for In Situ Detection of Cu2+ in Real Environmental Samples. ACS ES T Water 2023, 3, 2108–2119. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.K.; Park, H.H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Vareda, J.P.; Durães, L. Functionalized Silica Xerogels for Adsorption of Heavy Metals from Groundwater and Soils. J. Solgel Sci. Technol. 2017, 84, 400–408. [Google Scholar] [CrossRef]
- Dudás, Z.; Len, A.; Ianăși, C.; Paladini, G. Structural Modifications Caused by the Increasing MTES Amount in Hybrid MTES/TEOS-Based Silica Xerogels. Mater. Charact. 2020, 167, 110519. [Google Scholar] [CrossRef]
- Gizli, N.; Çok, S.S.; Koç, F. Aerogel, Xerogel, and Cryogel: Synthesis, Surface Chemistry, and Properties—Practical Environmental Applications and the Future Developments. In Advanced Materials for Sustainable Environmental Remediation: Terrestrial and Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–229. [Google Scholar] [CrossRef]
- Dudarko, O.; Budnyak, T.; Tkachenko, O.; Agback, T.; Agback, P.; Bonnet, B.; Ahrens, L.; Daniel, G.; Seisenbaeva, G. Removal of Poly- and Perfluoroalkyl Substances from Natural and Wastewater by Tailored Silica-Based Adsorbents. ACS ES T Water 2024, 4, 1303–1314. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, X.; Yun, X.; Yu, D.; Wang, B. Efficient Adsorption of Sodium P-Perfluorous Nonenoxybenzenesulfonate by Environmentally Friendly Self-Floating Amino Ordered Mesoporous Silica: Energy-Efficient Solid–Liquid Separation and Enhanced Adsorption Performance. Sep. Purif. Technol. 2025, 371, 133406. [Google Scholar] [CrossRef]
- Ateia, M.; Alsbaiee, A.; Karanfil, T.; Dichtel, W. Efficient PFAS Removal by Amine-Functionalized Sorbents: Critical Review of the Current Literature. Environ. Sci. Technol. Lett. 2019, 6, 688–695. [Google Scholar] [CrossRef]
- Lotfi, R.; Hayati, B.; Rahimi, S.; Shekarchi, A.A.; Mahmoodi, N.M.; Bagheri, A. Synthesis and Characterization of PAMAM/SiO2 Nanohybrid as a New Promising Adsorbent for Pharmaceuticals. Microchem. J. 2019, 146, 1150–1159. [Google Scholar] [CrossRef]
- Yu, Y.L.; Jin, H.F.; Shi, Y.; Cao, J. Synchronous Microextraction of Active and Toxic Compounds from Medicinal Plant Using Nano-SiO2 Assisted Miniaturized Matrix Solid-Phase Dispersion. Microchem. J. 2022, 183, 108099. [Google Scholar] [CrossRef]
- Tejedor, J.; Guerrero, V.H.; Vizuete, K.; Debut, A. Environmentally Friendly Synthesis of Silicon Dioxide Nanoparticles and Their Application for the Removal of Emerging Contaminants in Aqueous Media. J. Phys. Conf. Ser. 2022, 2238, 012005. [Google Scholar] [CrossRef]
- Tatar, D.K.; Jha, J.M. A Review on the Synthesis of Silica Oxide Nanoparticles (SiO2-NPs) and Their Use to Remove Pharmaceuticals and Personal Care Products (PPCPs) from Aqueous Solutions. In Advanced Oxidation Process-Based Integrated and Hybrid Technologies for Degradation of Pharmaceuticals and Personal Care Products; Elsevier: Amsterdam, The Netherlands, 2025; pp. 45–52. [Google Scholar] [CrossRef]
- Akhter, F.; Soomro, S.A.; Inglezakis, V.J. Silica Aerogels; a Review of Synthesis, Applications and Fabrication of Hybrid Composites. J. Porous Mater. 2021, 28, 1387–1400. [Google Scholar] [CrossRef]
- Lin, J.; Li, G.; Liu, W.; Qiu, R.; Wei, H.; Zong, K.; Cai, X. A Review of Recent Progress on the Silica Aerogel Monoliths: Synthesis, Reinforcement, and Applications. J. Mater. Sci. 2021, 56, 10812–10833. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Ye, W.; Shen, X.; Yuan, X.; Ma, C.; Cao, Y. Performance Regulation of Silica Aerogel Powder Synthesized by a Two-Step Sol-Gel Process with a Fast Ambient Pressure Drying Route. J. Non Cryst. Solids 2021, 567, 120923. [Google Scholar] [CrossRef]
- Faghihian, H.; Nourmoradi, H.; Shokouhi, M. Removal of Copper (II) and Nickel (II) from Aqueous Media Using Silica Aerogel Modified with Amino Propyl Triethoxysilane as an Adsorbent: Equilibrium, Kinetic, and Isotherms Study. Desalination Water Treat 2014, 52, 305–313. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Kazemi, M. Characterization of Modified Silica Aerogel Using Sodium Silicate Precursor and Its Application as Adsorbent of Cu2+, Cd2+, and Pb2+ Ions. Int. J. Ind. Chem. 2012, 3, 1–8. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Huang, S.; Kang, S.; Zhang, C.; Li, X. Low-Cost Route for Synthesis of Mesoporous Silica Materials with High Silanol Groups and Their Application for Cu(II) Removal. Mater. Chem. Phys. 2012, 132, 1053–1059. [Google Scholar] [CrossRef]
- NIST X-Ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/ (accessed on 4 June 2024).
- Diagboya, P.N.E.; Dikio, E.D. Silica-Based Mesoporous Materials; Emerging Designer Adsorbents for Aqueous Pollutants Removal and Water Treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Ammaeva, S.G.; Isaev, A.B.; Kharlamova, T.A. Preparation of Xerogel of Silicon Dioxide by Acid Hydrolysis of Tetraethoxysilane and Study into Its Sorption Properties. Chem. Probl. 2021, 1, 56–63. [Google Scholar] [CrossRef]
- Hegde, N.D.; Hirashima, H.; Venkateswara Rao, A. Two Step Sol-Gel Processing of TEOS Based Hydrophobic Silica Aerogels Using Trimethylethoxysilane as a Co-Precursor. J. Porous Mater. 2007, 14, 165–171. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Kalekar, A.S.; Harale, N.S.; Patil, S.S.; Dhere, S.L. Rapid Synthesis of Ambient Pressure Dried Tetraethoxysilane Based Silica Aerogels. J. Solgel Sci. Technol. 2021, 97, 5–10. [Google Scholar] [CrossRef]
- Akti, F.; Balci, S. Silica Xerogel and Iron Doped Silica Xerogel Synthesis in Presence of Drying Control Chemical Additives. Mater. Chem. Phys. 2023, 297, 127347. [Google Scholar] [CrossRef]
- Cristiano, E.; Hu, Y.J.; Siegfried, M.; Kaplan, D.; Nitsche, H. A Comparison of Point of Zero Charge Measurement Methodology. Clays Clay Min. 2011, 59, 107–115. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of Sorbent Point Zero Charge: Usefulness in Sorption Studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Bajat, J.B.; Milošev, I.; Jovanović, Ž.; Mišković-Stanković, V.B. Studies on Adhesion Characteristics and Corrosion Behaviour of Vinyltriethoxysilane/Epoxy Coating Protective System on Aluminium. Appl. Surf. Sci. 2010, 256, 3508–3517. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Xu, D.; Wang, D.; Xue, Y.; Su, W. Pressure-Induced Crystallization of Amorphous SiO2 with Silicon–Hydroxy Group and the Quick Synthesis of Coesite under Lower Temperature. High Press. Res. 2008, 28, 641–650. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of Amorphous SiO2 Particles and Their Properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Milošev, I.; Jovanović, Ž.; Bajat, J.B.; Jančić-Heinemann, R.; Mišković-Stanković, V.B. Surface Analysis and Electrochemical Behavior of Aluminum Pretreated by Vinyltriethoxysilane Films in Mild NaCl Solution. J. Electrochem. Soc. 2012, 159, C303–C311. [Google Scholar] [CrossRef]
- Todea, M.; Simon, V.; Muresan-Pop, M.; Vulpoi, A.; Rusu, M.M.; Simion, A.; Vasilescu, M.; Damian, G.; Petrisor, D.M.; Simon, S. Silica-Based Microspheres with Aluminum-Iron Oxide Shell for Diagnosis and Cancer Treatment. J. Mol. Struct. 2021, 1246, 131149. [Google Scholar] [CrossRef]
- Duval, Y.; Mielczarski, J.A.; Pokrovsky, O.S.; Mielczarski, E.; Ehrhardt, J.J. Evidence of the existence of three types of species at the quartz-aqueous solution interface at ph 0-10: Xps surface group quantification and surface complexation modeling. J. Phys. Chem. B 2002, 106, 2937–2945. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shimogaki, Y. X-Ray Photoelectron Spectroscopic Characterization of the Adhesion Behavior of Chemical Vapor Deposited Copper Films. J. Vac. Sci. Technol. A 2001, 19, 2642–2651. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R′′Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Elkady, M.F.; El-Aassar, M.R.; Hassan, H.S. Adsorption Profile of Basic Dye onto Novel Fabricated Carboxylated Functionalized Co-Polymer Nanofibers. Polymers 2016, 8, 177. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. Correlation between Physical Properties and Structure of Silica Xerogels. J. Non Cryst. Solids 2004, 347, 128–137. [Google Scholar] [CrossRef]
- Parvathy Rao, A.; Venkateswara Rao, A. Modifying the Surface Energy and Hydrophobicity of the Low-Density Silica Aerogels through the Use of Combinations of Surface-Modification Agents. J. Mater. Sci. 2010, 45, 51–63. [Google Scholar] [CrossRef]
- Halim, Z.A.A.; Yajid, M.A.M.; Hamdan, H. Effects of Solvent Exchange Period and Heat Treatment on Physical and Chemical Properties of Rice Husk Derived Silica Aerogels. Silicon 2021, 13, 251–257. [Google Scholar] [CrossRef]
- Wang, R.; Ng, D.H.L.; Liu, S. Recovery of Nickel Ions from Wastewater by Precipitation Approach Using Silica Xerogel. J. Hazard. Mater. 2019, 380, 120826. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Dong, X.; Gao, G.; Sha, F.; Xu, D. Microstructure and Adsorption Properties of MTMS / TEOS Co-Precursor Silica Aerogels Dried at Ambient Pressure. J. Non Cryst. Solids 2021, 562, 120778. [Google Scholar] [CrossRef]
- Fernández Pérez, B.; Ayala Espina, J.; Fernández González, M. de L. Á. Adsorption of Heavy Metals Ions from Mining Metallurgical Tailings Leachate Using a Shell-Based Adsorbent: Characterization, Kinetics and Isotherm Studies. Materials 2022, 15, 5315. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Matyjasik, W.; Matysik, J.; Szostak, K.; Śliwa, P.; Banach, M. Biocatalyst Based on Magnetic Nanoparticles with Cu(II), Mn(II), Zn(II) and Immobilised Catalase. J. Clust. Sci. 2024, 35, 143–158. [Google Scholar] [CrossRef]




| Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|
| KL, l/mg | Am, mg/g | R2 | KF, (mg/g)·(l/mg)1/n | 1/n | R2 |
| 0.07 | 71.4 | 0.9949 | 17.8 | 0.21 | 0.9026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanaz, A.; Abdulgalim, I.; Richard, S.; Ilya, P.; Valery, T. Removal of Copper (II) from Aqueous Solutions Using Silica Xerogel as Sorbent: Adsorption Properties and Mechanism. Colloids Interfaces 2025, 9, 58. https://doi.org/10.3390/colloids9050058
Shanaz A, Abdulgalim I, Richard S, Ilya P, Valery T. Removal of Copper (II) from Aqueous Solutions Using Silica Xerogel as Sorbent: Adsorption Properties and Mechanism. Colloids and Interfaces. 2025; 9(5):58. https://doi.org/10.3390/colloids9050058
Chicago/Turabian StyleShanaz, Ammaeva, Isaev Abdulgalim, Schubert Richard, Pankov Ilya, and Talanov Valery. 2025. "Removal of Copper (II) from Aqueous Solutions Using Silica Xerogel as Sorbent: Adsorption Properties and Mechanism" Colloids and Interfaces 9, no. 5: 58. https://doi.org/10.3390/colloids9050058
APA StyleShanaz, A., Abdulgalim, I., Richard, S., Ilya, P., & Valery, T. (2025). Removal of Copper (II) from Aqueous Solutions Using Silica Xerogel as Sorbent: Adsorption Properties and Mechanism. Colloids and Interfaces, 9(5), 58. https://doi.org/10.3390/colloids9050058







