Abstract
The contamination of water resources with heavy metals creates problems for using it as a source of drinking water. Adsorption is one of the most promising methods for heavy metal ion removal from natural and wastewater. The process of removing copper (II) from aqueous solutions using SiO2 xerogel as an adsorbent has been studied. The xerogel was thoroughly characterized by transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and argon adsorption–desorption isotherms, revealing an amorphous structure with a high surface area (~347 m2/g) and uniform mesoporosity (2–14 nm pore size). The surface chemistry, dominated by silanol groups, was confirmed by XPS analysis. The adsorption process is influenced by electrostatic interactions between the positively charged Cu(II) ions and the negatively charged surface groups, with the optimal performance near neutral pH. Batch adsorption experiments demonstrated that the silica xerogel effectively removes Cu(II) ions from aqueous solutions, with removal efficiency exceeding 99% at pH values above 4.0. The maximum adsorption capacity of copper (II) ions on SiO2 xerogel is 67.5 mg/L.
1. Introduction
Currently, the contamination of water resources with various pollutants, including heavy metals, pesticides, pharmaceuticals, dyes, etc., and the resulting problem of the inability to use contaminated water as a source of drinking water, are some of the most pressing issues of modern times [1,2,3,4]. The main sources of various pollutants entering natural waters are industrial discharges, agricultural activities, and urban wastewater containing numerous toxic inorganic and organic pollutants. The presence of various pollutants in drinking water above permissible levels leads to increased toxicity and health problems for the population when such water is used for the drinking water supply [5,6,7].
Heavy metals, when present in drinking water at concentrations exceeding permissible levels, have a serious impact on the human body. Various methods (such as ion exchange, solvent extraction, adsorption, and precipitation) are currently used for removing heavy metals to meet the growing demand for drinking water from natural sources [8,9,10]. Among the methods of removing heavy metals, adsorption is recognized as an effective and economical method [11,12,13,14,15]. A large number of different materials have been proposed for use as sorbents for removing heavy metals, including activated carbon [16,17], zeolites [18,19], silica gel [20,21,22], aluminum oxide [23,24], and natural clay minerals (kaolinite, bentonite, illite, stevensite, rectorite, calcite, etc.) [25,26,27,28,29]. The adsorption process is flexible in operation and supplies high-quality purified drinking water. Additionally, adsorption is sometimes reversible, and adsorbents can be regenerated by a suitable desorption process [30,31,32].
Among the known methods for removing copper from natural water and wastewater, adsorption is the most attractive, effective, and economically feasible method [33,34]. Various inorganic and organic adsorbents, including some of natural origin [35], have been used to extract copper. Among the most studied materials as adsorbents for removing heavy metal ions are carbon-based adsorbents [36,37] including biochar [38], chitosan [39], natural zeolites [40], industrial waste [41], cellulose [42,43], graphene oxide [44], carbon nanotubes [45], alginate [46], metal oxides [47], etc.
Silica xerogel and aerogel-based sorbents have attracted considerable attention from researchers due to their non-toxic nature, high specific surface area, regular pore structure, and the possibility of surface modification [48,49,50,51,52,53,54]. The use of SiO2 xerogel synthesized from various precursors for removing heavy metal ions from aqueous solutions is considered promising due to its high porosity, low density, and high adsorption capacity [55,56,57]. SiO2 xerogel-based sorbents are also used to remove a variety of more persistent pollutants, including PFAS [58,59,60], pharmaceuticals [61,62], and personal care items [63,64]. The advantages and disadvantages of SiO2 xerogel use as a sorbent are presented in Table S1.
The most widely used precursors in the sol–gel synthesis of SiO2 xerogel are tetramethyl orthosilicate (TMOS) and tetraethyl orthosilicate (TEOS) [65]. Their use in the sol–gel synthesis of SiO2 has been well studied [66]. Researchers have paid particular attention to obtaining SiO2 aerogels using SiO2 xerogel as a precursor, as they possess a large specific surface area [67,68,69]. However, their production involves the use of complex equipment associated with drying SiO2 xerogel in a supercritical carbon dioxide environment [70,71].
At the same time, there is little attention paid in the literature to studying the removal of copper (II) ions by adsorption in SiO2 xerogels. The presence of a large number of hydroxyl groups on the surface of the synthesized SiO2 xerogel may lead to the precipitation of heavy metal ions as hydroxides [72]. The most promising materials for the removal of heavy metals, including copper (II) ions, are mesoporous silica-based sorbents (SiO2, such as silicate and silica gel) [48,49]. These materials have garnered attention from researchers as effective adsorbents for the removal of heavy metal ions [50]. The surface and pores of silica exhibit a substantial number of hydroxyl groups, which facilitate the efficient removal of Cu2+ ions [35]. The removal of Cu2+ ions to silica-based adsorbents is primarily associated with electrostatic interactions and ion exchange processes [73].
There is little information in the literature on the adsorption of SiO2 xerogel obtained from TMOS or TEOS without modification. Unmodified SiO2 xerogel has a low adsorption capacity to heavy metal ions. Table S2 shows the comparative characteristics of the adsorption of copper ions by SiO2 xerogel-based sorbents with and without modification.
This work investigates the process of copper (II) ion adsorption on freshly prepared SiO2 xerogel, which ensures a consistently high pH level. The structural characteristics and the influence of various parameters on the copper (II) ion adsorption process in SiO2 xerogel have been studied.
2. Materials and Methods
2.1. Synthesis of SiO2 Xerogel
A sample of SiO2 xerogel was prepared from TEOS in two stages of the sol–gel process, involving acid hydrolysis using hydrochloric acid in an alcohol medium, followed by gel formation with an ammonium hydroxide solution [74]. The molar ratios at the first stage were 1 mol TEOS: 3.9 mol ethanol: 1 mol H2O: 12 × 10−4 mol HCl (1.5 h at 60 °C, pH = 3). At the second stage, the ratio was 2.5 mol H2O: 7 × 10−3 mol NH4OH. After gel formation, the samples were soaked in ethanol twice for 24 h [75]. Subsequently, to obtain xerogels, the sample was dried in a drying oven at a temperature of 60 °C for 24 h, then at 150 °C (1 h) and 170 °C (1 h) [76].
2.2. Characterization of SiO2 Xerogel
Morphological studies, structural analysis of samples, and elemental mapping were carried out using a transmission electron microscope JEOL JEM-F200 (JEOL, Tokyo, Japan) with an electron accelerating voltage range from 20 to 200 kV. X-ray diffraction (XRD) studies were performed using an Empyrean PANalytical X-ray diffractometer (Almelo, The Netherlands) in the radiation of a copper anode with a nickel filter, with radiation wavelength λ(CuKα) = 0.154051 nm. Data processing was performed using the High Score Plus application program, included in the instrument software, and the diffraction database ICSD (PDF-2). The surface composition was carried out by an AXIS SupraTM X-ray photoelectron spectrometer (XPS) (Kratos Analytical Ltd., Manchester, UK). The data were processed by CasaXPS v.2.3.23 software (Casa Software Ltd., Wilmslow, UK). The changes that occurred in the surface properties of silica xerogels using different synthesis methods, as well as before and after adsorption, were identified using Fourier-transform infrared spectroscopy in the range from 400 cm−1 to 4000 cm−1 using an IR spectrometer Varian 640 (Varian, Inc., Palo Alto, CA, USA). The samples were prepared in the form of pellets with potassium bromide. The specific surface area was determined using the “Chemisorb-2750” instrument (Micromeritics, Norcross, GA, USA). Prior to measurement, the sample was heated at 200 °C to remove moisture and other adsorbed gases. Measurements were carried out using the Brunauer–Emmett–Teller method by adsorbing Ar at a temperature of −196 °C. High-resolution transmission electron microscopy (HRTEM), selected area electron diffraction (SAED), and energy dispersive X-ray spectroscopy (EDX) analyses were conducted using JEOL JEM-F200 (JEOL Ltd., Tokyo, Japan), which is operated at a 200 kV accelerating voltage and equipped with a cold field-emission gun (CFEG) and high-resolution CMOS AMT camera.
2.3. Adsorption Properties
The adsorption properties of the obtained samples were investigated in relation to copper (II) ions depending on pH, temperature, and contact time. Adsorption and kinetic curves were plotted. To study the effect of pH on the removal of Cu2+ ions, 7 samples of copper (II) sulfate solutions with different pH values ranging from 2 to 8, an initial concentration of Cu2+ C0 = 300 mg/L, and a solution volume of 100 mL were used. The pH value was determined using the Multitest IPL-101 pH meter (Moscow, Russia) with a built-in temperature sensor. The pH meter was calibrated using standard buffer solutions with pH values: 1.65; 3.56; 6.86; and 9.18. After adding 0.2 g of xerogel samples, the sample was kept for 30 min and filtered, followed by determination of the residual concentration of Cu2+ ions. The initial copper sulfate solution had pH = 5. The solution was acidified with a 0.1 M HCl solution and alkalized with 0.1 M NaOH. To prevent the absorption of carbon dioxide from the air, the flasks with solutions were tightly closed with stoppers. To determine the point of zero charge, the powder addition method was used, as described in [77,78].
The copper (II) content in the solution was determined via copper (II) complexes with ammonia using a photometric colorimeter SF-2000 (Biomer, Krasnoobsk, Russia) [73]. The adsorption capacity (qe, mg/g) was calculated using Equation (1) and the percentage removal (α, %) using Equation (2):
where C0 is the initial concentration of Cu2+ ions, mg/L, Ce is the equilibrium concentration of Cu2+ ions, and mg/L and m are the mass of the sorbent, g.
3. Results
3.1. Characterization of the Adsorbent
The morphology of the synthesized SiO2 xerogel samples was characterized using transmission electron microscopy (Figure 1). As seen from the obtained data, the SiO2 xerogel samples represent an agglomeration of non-spherical particles. In this case, SiO2 particles of arbitrary shapes are formed, different from Stoeber-like synthesis, where spherical silica particles are formed. A single SiO2 particle in Figure 1a has an irregularly formed spherical shape. The micrograph of a SiO2 xerogel segment, shown in Figure 1b, indicates the absence of any crystalline structures. The SiO2 xerogel obtained from TEOS is characterized by an amorphous structure. Elemental mapping of the isolated microparticle with a size of approximately 2 µm and energy-dispersive spectra shows the presence of only elements characteristic of SiO2 (Figure 1d–f). The data obtained indicate that the investigated microparticle of the SiO2 xerogel only exhibits the presence of oxygen and silicon (Figure 1d,e). The EDX spectra (Figure 1f) only showed the presence of silicon and oxygen in the synthesized xerogel sample. The presence of hydroxide ions on the surface of the SiO2 xerogel is an advantage in this case and will contribute to good binding of copper (II) ions.
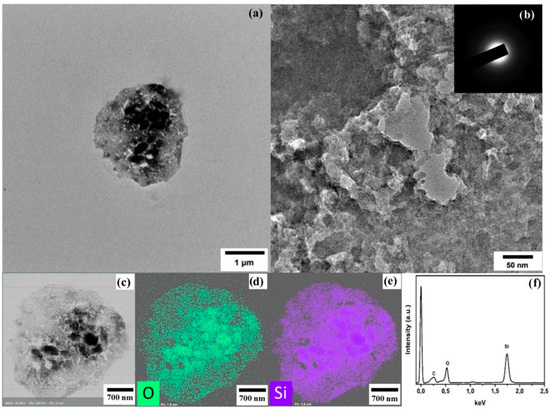
Figure 1.
(a,b) TEM, STEM images, EDX elemental mapping images (c,d,e), and EDX spectra of SiO2 xerogels (f). Insert—selected area electron diffraction.
The porosity of the SiO2 xerogel is fairly uniform in the size range of 2–14 nm, as illustrated in Figure S1, which shows the nitrogen sorption isotherm (Figure S1a) and particle size distribution (Figure S1b). The specific surface area of silica xerogel synthesized from TEOS was determined as 346.75 m2/g. The pore volumes of silica xerogel were determined as 0.796 cm3/g.
The absence of crystalline structures in the synthesized SiO2 xerogel is confirmed by the X-ray diffraction spectra presented in Figure 2. The X-ray diffraction pattern exhibits a broad peak between 17° and 30° with a maximum at 22°, characteristic of typical amorphous silica [79]. As confirmed by the data of other researchers, SiO2 synthesized using sol–gel technology from TEOS has an amorphous structure, and the maximum absorption peak can be in the range from 21.8° to 23° for different researchers [80,81]. At the same time, depending on the ratio of TEOS and water, quartz-like or cristobalite-like structures can also be formed during the synthesis of SiO2 [80].
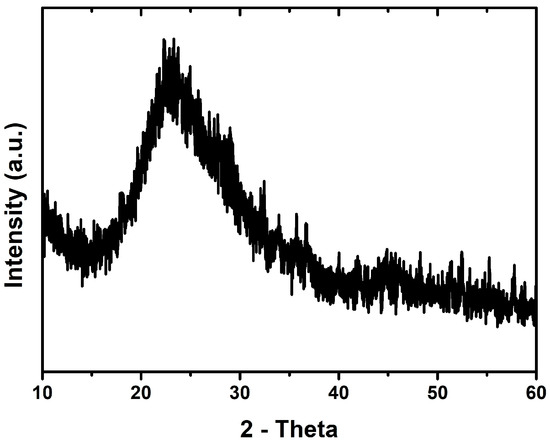
Figure 2.
XRD pattern of SiO2 xerogel.
The chemical composition and surface state of SiO2 play a crucial role in the adsorption process of heavy metal ions. Therefore, the surface of the sorbent was investigated using X-ray photoelectron spectroscopy (XPS) in the range of 0 to 1400 eV. An overview spectrum of the SiO2 surface is presented in Figure 3a, showing the presence of peaks C1s, O1s, Si2s, and Si2p. High-resolution XPS spectra of Si2p demonstrate only one peak at 103.6 eV (Figure 3b), corresponding to SiO2 [82]. Deconvolution of the experimental XPS spectra for Si2p ionization revealed two component peaks at 103.2 eV and 103.8 eV, attributed to the bonds between silicon and oxygen (Si–O–Si) and hydroxyl groups adsorbed on the SiO2 surface (–Si–OH) [83,84].
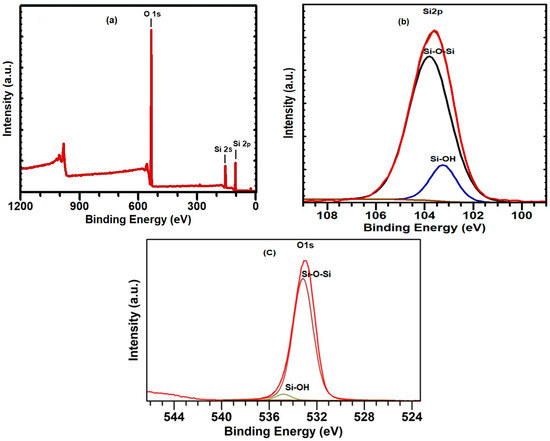
Figure 3.
XPS spectra of the SiO2 xerogel sample: (a) full spectra; (b) Si2p spectra; and (c) O1s spectra.
XPS spectra for oxygen O1s ionization are presented in Figure 3c. Oxygen is the most abundant element in the SiO2 xerogel sample. The center of the oxygen O1s spectrum was located at 533 eV. The O1s spectra were deconvoluted using two peaks, also showing contributions of oxygen atoms in the hydroxyl group (Si–OH) at 534.8 eV [85] and those associated with oxygen in silicon (–Si–O–Si–) or similar SiO2 at 533.1 eV [86]. The small area of the O1s peak at 534.8 eV, corresponding to hydroxyl groups, indicates that there are few hydroxyl groups on the xerogel surface.
3.2. Adsorption of Cu2+
The removal efficiency for Cu2+ ions, calculated based on the experimental data, for each pH value is shown in Figure 4. As seen in Figure 4a, the removal efficiency of copper ions sharply increases in the pH range transition of 2–3 and remains consistently high around pH 5. The residual concentration of Cu2+ ions averaged 0.2 mg/L. The obtained dependence of removal efficiency on pH shows that the optimal pH value for removing Cu2+ ions fall within a broad range, starting from 4 to 6. At low pH values, the effect of competition between H+ and Cu2+ ions for the active centers of the adsorbent leads to low values of adsorption efficiency. At pH increases, the concentration of H+ ions decreases, reducing competition between metal ions and hydrogen ions for active sites on the xerogel surface. Moreover, the surface charge also affects the adsorption process of Cu2+ ions.
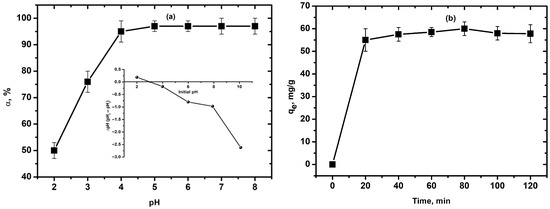
Figure 4.
(a) Effect of solution pH on the Cu2+ removal efficiency by adsorption onto SiO2 xerogel sample (C0 = 300 mg/L, T = 25 °C, time 30 min, and 0.2 g SiO2 xerogel); (b) adsorption capacity of Cu2+ ions as a function of contact time (C0 = 300 mg/L, T = 25 °C, 0.2 g SiO2 xerogel, and pH = 5).
As shown in Figure 4a (insert), the pHpzc for SiO2 xerogel was close to 2.9 and the adsorbent surface was positively charged, which promotes the adsorption of copper ions at pH > pHpzc. Hence, at low pH values, low adsorption levels are observed. Increasing the pH can significantly improve the adsorption property of xerogel for removing Cu2+ ions. At pH levels of 2–6, the metal is present in the solution as an ion. Further increases in the solution pH above 6 leads to the precipitation of Cu2+ ions as poorly soluble hydroxides. To avoid sediment formation, a pH value of 5.0 was chosen for the subsequent study of adsorption processes. The selected pH value of 5 is above the point of zero charge (pzc) for the SiO2 xerogel (pHpzc = 2.9). Due to the negatively charged surface of the xerogel, electrostatic interactions between Cu2+ ions and the surface in addition to ion exchange can also be the driving forces leading to adsorption. The mechanism of the physical and chemical adsorption of Cu2+ is shown in Figure S2.
To study the dependence of Cu2+ ion adsorption on temperature, experiments were conducted with three samples at temperatures of 25, 30, and 40 °C. After adding xerogel, allowing the samples to stand for 30 min, and further filtering the solutions, the residual concentration of Cu2+ ions was determined. The research results showed that with increasing temperature, the degree of Cu2+ ion adsorption on silicon dioxide xerogel increased by an average of 0.45% per degree [74].
The adsorption capacity of xerogels was determined by immersing them in a copper-containing solution for 120 min. Samples were taken every 20 min. After separating the xerogel by filtration, the solutions were analyzed for Cu2+ ion content. As shown in the results (Figure 4b), there is an increase in adsorption capacity with increased contact time between phases, especially up to 20 min of contact for the SiO2 xerogel sample. With further increases in contact time between phases, the increase in the adsorption capacity is insignificant. The optimal contact time for the studied sample was found to be 80 min.
The calculation showed that, over a contact time of 80 min, the maximum adsorption capacity for Cu2+ ions was 67.5 mg/g, with an initial concentration of Cu2+ ions in the solution being 500 mg/L (Figure 5a).
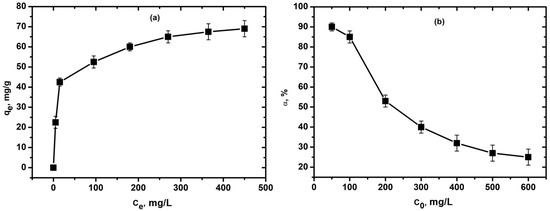
Figure 5.
(a) Isotherm plots of adsorption Cu2+ ions on SiO2 xerogel (C0 = 300 mg/L, T = 25 °C, time 80 min, 0.2 g SiO2 xerogel, and pH = 5); (b) the effect of the initial concentration on the efficiency of Cu2+ ions removal onto the SiO2 xerogel sample (T = 25 °C, time 80 min, 0.2 g SiO2 xerogel, and pH = 5).
By its appearance, the adsorption isotherm for Cu2+ ions can be classified as type I Langmuir isotherms. For isotherms of this class, the initial part is curved relative to the concentration axis, as with an increase in the fraction of occupied adsorption sites, it becomes more difficult for molecules of the adsorbate to find vacant space. At a certain concentration of the adsorbate, adsorption reaches a limit. In this case, physical adsorption takes place. The almost vertical portion of the isotherm at low concentrations indicates a strong affinity of the adsorbate for the adsorbent and extremely strong adsorption [87]. According to the Langmuir isotherm, only one layer of molecules can be adsorbed on the surface of the adsorbent. As the concentration of copper ions exceeds 300 mg/L, the surface of the xerogel becomes saturated with adsorbate ions.
The adsorption data of copper ions by silica xerogel in Figure 5a were investigated by Langmuir and Freundlich adsorption isotherm models. The Langmuir and Freundlich isotherm plots for the adsorption of Cu2+ onto SiO2 xerogel are presented in Figure 6a,b. Of the two linearized plots of the Langmuir and Freundlich isotherms, the Langmuir isotherm provides the best fit to the experimental data with a regression coefficient R2 of 0.9949, while for the Freundlich isotherm value is 0.9026.
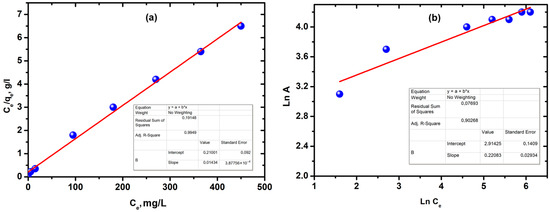
Figure 6.
(a) The Langmuir isotherm plots for the adsorption of Cu2+ onto SiO2 xerogel; (b) the Freundlich isotherm plots for the adsorption of Cu2+ ions onto SiO2 xerogel (C0 = 300 mg/L, T = 25 °C, time 80 min, 0.2 g SiO2 xerogel, and pH = 5).
This model assumes that the adsorption process was carried out by the monolayer adsorption of copper ions on the active sites of silica xerogel with equal affinity. The parameters of the adsorption isotherms are listed in Table 1.

Table 1.
Parameters of the adsorption isotherm of copper (II) ions.
The Langmuir model is based on the fact that a monomolecular layer of adsorbate is formed on the surface of the sorbent, and all active centers have equal energy and enthalpy.
The linear form of the Langmuir equation is as follows:
KL and Am—the constants of the Langmuir equation; A, Am—the amount of Cu2+ sorbed per 1 g of sorbent at equilibrium and the adsorption capacity of the sorbent at saturation, respectively, mg/g; and Ce—the equilibrium concentration of copper in the solution, mg/L.
The constants of the equation were calculated from the slope and intersection of the lines on the graph in the corresponding coordinates of the linear equation Ce/A from Ce.
Using the Langmuir adsorption model, we estimated the dimensionless equilibrium parameter (RL) shown in Equation (3).
The adsorption favorability of Cu2+ by silica xerogel was evaluated based on the RL value, which describes the adsorption as unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), and irreversible (RL = 0). In this study, the RL value was 0.75, indicating a favorable adsorption process [36,88]. Using the Langmuir adsorption isotherm model, the maximum adsorption capacity was calculated to be 71.4 mg/g, which is not much different from the practically obtained value. There is a dependence of the dimensionless parameter RL on the initial concentration of copper (II) ions for TEOS silica xerogel (Figure S3). According to Figure S3, it is evident that the values of the parameter RL are between 0.17 and 0.39, which means that the adsorption process of copper (II) ions is favorable and follows monolayer adsorption [50,51].
Based on the values of (RL) obtained from the approximation of the Langmuir–Koble (KL) equation using Equation 3, there is a dependence on the initial concentration of Cu2+ ions (Figure S3). With increasing concentrations of Cu2+ ions, the RL values decrease, indicating a reduction in the number of free active adsorption centers. At the same time, the RL values remain within the range of 0 to 1, suggesting that active centers are still in excess compared to the Cu2+ ions, and adsorption can occur either as monolayer adsorption or multilayer adsorption. However, at high concentrations, precipitation is more likely to occur, which we observed visually when the concentration of Cu2+ ions were significantly elevated.
The maximum removal efficiency for copper (II) ions was 90%. From Figure 5b, it can be seen that at low initial metal ion concentrations (from 0 to 50–100 mg/L), removal is better, then it sharply decreases (from 100 to 200 mg/L) and gradually decreases with further increases in concentration.
Elemental mapping of the SiO2 xerogel sample (Figure S4) and energy-dispersive spectra (Figure S4b) shows the adsorption of Cu2+ ions on the surface of the sorbent. The obtained data allow for a qualitative assessment of Cu2+ adsorption. For a more detailed understanding of the sorption mechanism and processes occurring on the surface of SiO2 xerogel, images of the surface of the adsorbent particles after adsorption were obtained. TEM images of the SiO2 xerogel sample after adsorption are presented in Figure 7. As can be seen from the obtained data, darker areas in the images correspond to copper ions and their compounds formed on the surface of SiO2 (Figure 7a). The highlighted area of the sorbent shows the formation of copper compounds with a crystalline structure on the surface of the xerogel (Figure 7b).
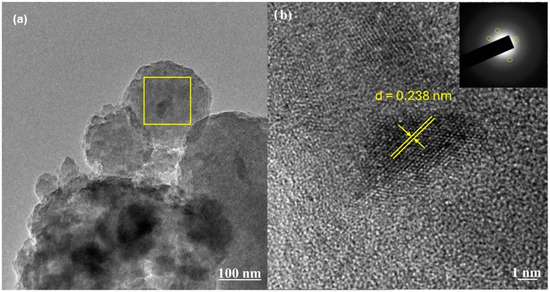
Figure 7.
(a,b) TEM images of SiO2 xerogels after Cu2+ adsorption (C0 = 300 mg/L, T = 25 °C, time 80 min, 0.2 g SiO2 xerogel, and pH = 5).
To determine the nature of the formed compound, XPS spectra of the xerogel sample after adsorption were obtained. Figure 8a presents O1s spectra for the SiO2 sample. Unlike the original O1s peak, a new peak at 532.7 eV appears during deconvolution, corresponding to metal hydroxides [89], and the peak corresponding to the hydroxyl group shifts by 0.6 eV. Weak adsorption occurs on the surface of the xerogel, and since the penetration depth of XPS analysis is small, it is not always possible to see the complete picture.
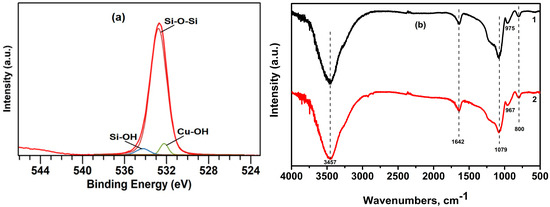
Figure 8.
(a) XPS O1s spectra of the SiO2 xerogel sample after Cu2+ sorption; (b) IR spectra of SiO2 xerogel samples before (1) and after (2) adsorption under optimal conditions (C0 = 300 mg/L, T = 25 °C, time 80 min, 0.2 g SiO2 xerogel, and pH = 5).
To study the mechanism of copper (II) ion sorption by xerogels, IR spectra of both the initial adsorbent samples and those after use were obtained. The experimental data obtained are shown in Figure 8b.
In Figure 8b, the broad peaks at 3423–3451 cm−1 are due to the stretching vibrations of O-H groups on the xerogel surface and adsorbed water molecules. The peak at 1631–642 cm−1 is due to the deformation vibrations of O-H groups and indicates the presence of physically sorbed water. The bands at 1079–1094 cm−1 and 800 cm−1 are due to the asymmetric and symmetric vibrations of Si-O-Si bonds. The bands at 975 cm−1 are attributed to the stretching vibrations of Si-O groups [90]. From the analysis of the spectra, it follows that the samples correspond to silicon dioxide in the amorphous state [91,92].
The sample before adsorption shows a high transmittance at 1079 cm−1, which indicates a high degree of a three-dimensional network of the Si–O–Si xerogel. After adsorption, a shift in the peak at 975 cm−1, corresponding to the silanol groups Si–OH, occurs, which is associated with the adsorption process. The sample after adsorption is characterized by a decrease in the peaks at 1079–1089 cm−1, which indicates the partial destruction of the silica matrix during the adsorption process [93].
3.3. Competitive Ions
It is well established that competitive cations such as Zn2+ and Cd2+ are commonly present in wastewater and can influence the adsorption process of Cu2+ ions. To investigate the impact of these co-existing ions on Cu2+ removal, Zn2+ and Cd2+ were individually added to the solution, as well as tested under combined presence with Cu2+. The effect of these competing cations was studied at four concentrations (50, 100, 200, and 300 mg/L) by adding them to Cu2+ solutions and determining the removal efficiency of copper ions.
The experimental data presented in Figure 9a indicate that Zn2+ ions do not significantly affect Cu2+ adsorption under the investigated concentrations, with only a slight decrease observed in the percentage of adsorbed Cu2+ ions. In contrast, Cd2+ ions exhibit a more pronounced effect on Cu2+ removal compared to Zn2+. The adsorption efficiency of copper ions decreases noticeably in the presence of Cd2+, and this effect is observed at lower initial concentrations of copper ions. Notably, at a Cd2+ concentration of 200 mg/L, the removal rate of Cu2+ was reduced by half.
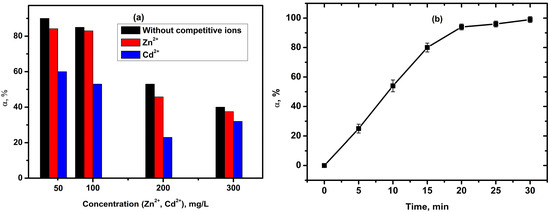
Figure 9.
(a) Effects of competing anions on the Cu2+ removal efficiency onto SiO2 xerogel (C0 = 300 mg/L, T = 25 °C, time 80 min, 0.2 g SiO2 xerogel, and pH = 5); (b) simulated plating wastewater treatment efficiency (C0 (Cu2+) = 38 mg/L, C0 (Zn2+) = 15 mg/L, T = 25 °C, 0.2 g SiO2 xerogel, and pH = 6.9).
The removal efficiency of copper ions reached only 84% and 60% in solutions with initial concentrations of 50 mg/L for Zn2+ and Cd2+, respectively. Furthermore, the removal rate decreased by 6% and 30% in solutions containing Zn2+–Cu2+ and Cd2+–Cu2+ systems.
The preferential adsorption of Cu2+ at high concentrations and low levels of competing ions can be attributed to several factors. Cu(II) has a smaller ionic radius and is more electronegative compared to Cd(II) and Zn(II), which may enhance its adsorption affinity under these conditions [94,95].
Based on the obtained results, the efficiency of removal for both Cu2+ and Zn2+ from model plating wastewater was investigated (Figure 9b). The use of the SiO2 xerogel at a copper ion concentration of 38 mg/L and zinc ion concentration of 15 mg/L allows them to be completely removed in 30 min. The removal efficiency is 98%.
3.4. Regeneration
The study of the desorption of adsorbed Cu2+ ions provides insights into the involvement of surface and intraporous hydroxyl groups, as well as the stability and regeneration potential of SiO2 xerogel. Practical applications of adsorbents necessitate the existence of crucial properties such as longevity and reusability after regeneration. The SiO2 xerogel was subjected to adsorption–regeneration cycles over the course of five cycles. Saturation of the SiO2 xerogel with Cu2+ ions was performed in a solution with an initial concentration of 300 mg/L under optimal adsorption conditions. Regeneration of the xerogel was conducted using a 0.1 M HCl solution. The obtained results are presented in Figure 10.
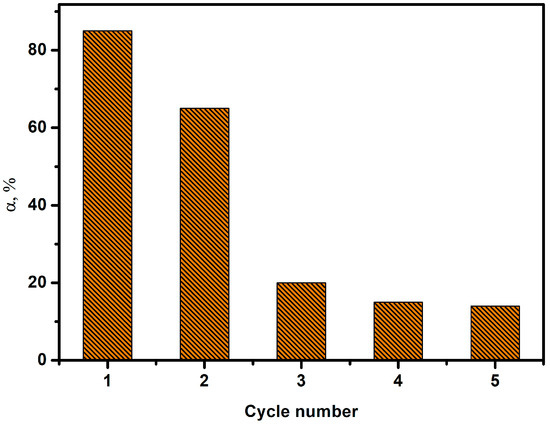
Figure 10.
Cycles of Cu2+ ions adsorption–desorption onto SiO2 xerogel (C0 = 300 mg/L, T = 25 °C, time 80 min, 0.2 g SiO2 xerogel, and pH = 5).
As depicted in Figure 10, the efficiency of Cu2+ removal by SiO2 xerogel decreased by 20% after the first regeneration cycle. During subsequent cycles, the adsorption efficiency declined to 14–15% following the fourth and fifth usage cycles. This reduction can likely be attributed to the inadequate recovery of hydroxyl groups within the pores during the first regeneration cycle. Furthermore, hydrochloric acid regeneration may also lead to the replacement of surface hydroxyl groups with chloride ions, which significantly reduces the effectiveness of the adsorption process for the removal of Cu2+ ions. This confirms that surface hydroxyl groups play a critical role in the removal process of Cu2+ ions.
4. Conclusions
Samples of SiO2 xerogels were synthesized using TEOS as a precursor. The SiO2 xerogel sample has a large specific surface area of 346.75 m2/g, which is an advantage when using the sorbent to remove heavy metal ions. The study of the adsorption properties showed that the obtained samples can be used in a wide range of pH values of the treated water. With increasing temperature, the adsorption capacity of the xerogels increases by 0.45% per degree. The optimal contact time of the xerogel samples was 80–120 min. The obtained experimental data on the adsorption removal of copper (II) ions using the synthesized samples indicate a fairly high efficiency of the sorption removal of Cu2+ from aqueous solutions. The maximum value of the adsorption capacity of the SiO2 xerogel is 67.5 mg/L. In their appearance, the adsorption isotherms for Cu2+ ions for the xerogel samples can be compared to Langmuir I isotherms. In the region of low equilibrium concentrations, a vertical initial section of the isotherms is observed, which indicates chemisorption on the surface of the sorbents. In the region of low initial concentrations of copper (II) ions, the maximum degree of removal is observed at around 90%. Analysis of SiO2 xerogel samples after copper (II) adsorption from aqueous solutions using XPS, EDX, and IR spectroscopy showed that adsorption mainly occurs through the substitution of hydroxyl ions associated with silicon atoms. The adsorption process occurs through the process of physical sorption. However, the decrease in adsorption capacity upon repeated use suggests that further optimization—such as improving regeneration techniques or enhancing structural stability—is necessary to ensure durability and cost-effectiveness in practical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/colloids9050058/s1, Table S1: Advantages and Disadvantages of SiO2 xerogel use as a sorbent; Table S2: The comparative characteristics on the adsorption of copper ions by SiO2 xerogels based sorbents; Figure S1: The Langmuir isotherm plots for the adsorption of Cu2+ onto SiO2 xerogels; Figure S2: The Freundlich isotherm plots for the adsorption of Cu2+ ions SiO2 xerogels; Figure S3: RL values versus of Cu2+ ions initial concentration; and Figure S4: STEM images, EDX mapping and EDX spectra of SiO2 xerogel particle after Cu2+ sorption.
Author Contributions
Conceptualization, A.S. and I.A.; methodology, A.S.; software, I.A., S.R. and P.I.; validation, A.S. and I.A.; formal analysis, A.S. and I.A.; investigation, A.S., S.R., P.I. and T.V.; resources, I.A., P.I., S.R. and T.V.; data curation, I.A., A.S. and P.I.; writing—original draft preparation, A.S. and I.A.; writing—review and editing, I.A. and T.V.; visualization, A.S., I.A. and P.I.; supervision, I.A. and T.V.; project administration, I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors are grateful to the Shared Use Center “High-Resolution Transmission Electron Microscopy” (SFedU) for conducting the TEM and EDX studies. XPS spectra were recorded on the equipment of the Shared Research Center “Surface and novel materials” of UdmFRC UB RAS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shah, A.; Arjunan, A.; Baroutaji, A.; Zakharova, J. A Review of Physicochemical and Biological Contaminants in Drinking Water and Their Impacts on Human Health. Water Sci. Eng. 2023, 16, 333–344. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; Shebl, A.; Abo El-Dahab, S.A. Drinking Water Treatment Sludge as an Efficient Adsorbent for Heavy Metals Removal. Appl. Clay Sci. 2017, 146, 343–349. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy Metals in Drinking Water: Occurrences, Implications, and Future Needs in Developing Countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Isaev, A.B.; Nabi, S.; Omarov, G.; Gulov, R.; Isaeva, M.A.; Nidheesh, P.V.; Oturan, M.A. Recent Progress in the Removal of Arsenic Using Iron Oxide and Oxyhydroxide Based Sorbents. Sep. Purif. Technol. 2025, 360, 131220. [Google Scholar] [CrossRef]
- Bora, A.J.; Dutta, R.K. Removal of Metals (Pb, Cd, Cu, Cr, Ni, and Co) from Drinking Water by Oxidation-Coagulation-Absorption at Optimized PH. J. Water Process Eng. 2019, 31, 100839. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of Three Dimensional Porous Aerogels as Adsorbent for Removal of Heavy Metal Ions from Water/Wastewater: A Review Study. Adv. Colloid. Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of Heavy Metals from Water Sources in the Developing World Using Low-Cost Materials: A Review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global Profile of Heavy Metals and Semimetals Adsorption Using Drinking Water Treatment Residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Senanu, L.D.; Kranjac-Berisavljevic, G.; Cobbina, S.J. The Use of Local Materials to Remove Heavy Metals for Household-Scale Drinking Water Treatment: A Review. Environ. Technol. Innov. 2023, 29, 103005. [Google Scholar] [CrossRef]
- Ismail, U.M.; Vohra, M.S.; Onaizi, S.A. Adsorptive Removal of Heavy Metals from Aqueous Solutions: Progress of Adsorbents Development and Their Effectiveness. Environ. Res. 2024, 251, 118562. [Google Scholar] [CrossRef]
- Li, B.; Li, K.; Li, X. Fabrication of Abundantly Functionalized Dendritic Biochar Composites as Adsorbents for the High-Efficiency Removal of Heavy Metal Ions and Dyes. Sep. Purif. Technol. 2024, 337, 126368. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy Metal Removal from Aqueous Solution by Advanced Carbon Nanotubes: Critical Review of Adsorption Applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Du, X.; Cui, S.; Fang, X.; Wang, Q.; Liu, G. Adsorption of Cd(II), Cu(II), and Zn(II) by Granules Prepared Using Sludge from a Drinking Water Purification Plant. J. Environ. Chem. Eng. 2020, 8, 104530. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul, A.K.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent Advances in Activated Carbon Modification Techniques for Enhanced Heavy Metal Adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Simate, G.S.; Maledi, N.; Ochieng, A.; Ndlovu, S.; Zhang, J.; Walubita, L.F. Coal-Based Adsorbents for Water and Wastewater Treatment. J. Environ. Chem. Eng. 2016, 4, 2291–2312. [Google Scholar] [CrossRef]
- Ugwu, E.I.; Othmani, A.; Nnaji, C.C. A Review on Zeolites as Cost-Effective Adsorbents for Removal of Heavy Metals from Aqueous Environment. Int. J. Environ. Sci. Technol. 2021, 19, 8061–8084. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkova, P.; Nickolov, R. Modified and unmodified silica gel used for heavy metal ions removal from aqueous solutions. J. Univ. Chem. Technol. Metall. 2012, 47, 498–504. [Google Scholar]
- Najafi, M.; Yousefi, Y.; Rafati, A.A. Synthesis, Characterization and Adsorption Studies of Several Heavy Metal Ions on Amino-Functionalized Silica Nano Hollow Sphere and Silica Gel. Sep. Purif. Technol. 2012, 85, 193–205. [Google Scholar] [CrossRef]
- Xia, K.; Ferguson, R.Z.; Losier, M.; Tchoukanova, N.; Brüning, R.; Djaoued, Y. Synthesis of Hybrid Silica Materials with Tunable Pore Structures and Morphology and Their Application for Heavy Metal Removal from Drinking Water. J. Hazard. Mater. 2010, 183, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Ezati, F.; Sepehr, E.; Ahmadi, F. The Efficiency of Nano-TiO2 and γ-Al2O3 in Copper Removal from Aqueous Solution by Characterization and Adsorption Study. Sci. Rep. 2021, 11, 18831. [Google Scholar] [CrossRef]
- Bhutto, A.A.; Baig, J.A.; uddin, S.; Kazi, T.G.; Sierra-Alvarez, R.; Akhtar, K.; Perveen, S.; Afridi, H.I.; Ali, H.E.; Hol, A.; et al. Biosynthesis of Aluminium Oxide Nanobiocomposite and Its Application for the Removal of Toxic Metals from Drinking Water. Ceram. Int. 2023, 49, 14615–14623. [Google Scholar] [CrossRef]
- Ewis, D.; Ba-Abbad, M.M.; Benamor, A.; El-Naas, M.H. Adsorption of Organic Water Pollutants by Clays and Clay Minerals Composites: A Comprehensive Review. Appl. Clay Sci. 2022, 229, 106686. [Google Scholar] [CrossRef]
- Novikau, R.; Lujaniene, G. Adsorption Behaviour of Pollutants: Heavy Metals, Radionuclides, Organic Pollutants, on Clays and Their Minerals (Raw, Modified and Treated): A Review. J. Environ. Manag. 2022, 309, 114685. [Google Scholar] [CrossRef] [PubMed]
- Otunola, B.O.; Ololade, O.O. A Review on the Application of Clay Minerals as Heavy Metal Adsorbents for Remediation Purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay Mineral Adsorbents for Heavy Metal Removal from Wastewater: A Review. Environ. Chem. Lett. 2018, 17, 629–654. [Google Scholar] [CrossRef]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent Advances in Applications of Low-Cost Adsorbents for the Removal of Heavy Metals from Water: A Critical Review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Jain, A.; Kumari, S.; Agarwal, S.; Khan, S. Water Purification via Novel Nano-Adsorbents and Their Regeneration Strategies. Process Saf. Environ. Prot. 2021, 152, 441–454. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of Heavy Metals from Aqueous Solution Using Carbon-Based Adsorbents: A Review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Pandey, L.M. Surface Engineering of Nano-Sorbents for the Removal of Heavy Metals: Interfacial Aspects. J. Environ. Chem. Eng. 2021, 9, 104586. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for Pollutants Removal: A Review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of Heavy Metals on Functionalized-Mesoporous Silica: A Review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Knight, A.W.; Tigges, A.B.; Ilgen, A.G. Adsorption of Copper (II) on Mesoporous Silica: The Effect of Nano-Scale Confinement. Geochem. Trans. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Fan, H.T.; Su, Z.J.; Fan, X.L.; Guo, M.M.; Wang, J.; Gao, S.; Sun, T. Sol-Gel Derived Organic-Inorganic Hybrid Sorbent for Removal of Pb2+, Cd2+ and Cu2+ from Aqueous Solution. J Solgel Sci Technol. 2012, 64, 418–426. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest Trends in Heavy Metal Removal from Wastewater by Biochar Based Sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Ziora, Z.M. Insights into Recent Advances of Chitosan-Based Adsorbents for Sustainable Removal of Heavy Metals and Anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Irannajad, M.; Kamran Haghighi, H. Removal of Heavy Metals from Polluted Solutions by Zeolitic Adsorbents: A Review. Environ. Process. 2020, 8, 7–35. [Google Scholar] [CrossRef]
- Kumar, R.; Laskar, M.A.; Hewaidy, I.F.; Barakat, M.A. Modified Adsorbents for Removal of Heavy Metals from Aqueous Environment: A Review. Earth Syst. Environ. 2019, 3, 83–93. [Google Scholar] [CrossRef]
- Mazibuko, M.T.; Onwubu, S.C.; Mokhothu, T.H.; Paul, V.; Mdluli, P.S. Unlocking Heavy Metal Remediation Potential: A Review of Cellulose–Silica Composites. Sustainability 2024, 16, 3265. [Google Scholar] [CrossRef]
- El Mahdaoui, A.; Radi, S.; Elidrissi, A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Moura, N.M.M. Progress in the Modification of Cellulose-Based Adsorbents for the Removal of Toxic Heavy Metal Ions. J. Environ. Chem. Eng. 2024, 12, 113870. [Google Scholar] [CrossRef]
- Duru, İ.; Ege, D.; Kamali, A.R. Graphene Oxides for Removal of Heavy and Precious Metals from Wastewater. J. Mater. Sci. 2016, 51, 6097–6116. [Google Scholar] [CrossRef]
- Liang, X.; Liu, S.; Wang, S.; Guo, Y.; Jiang, S. Carbon-Based Sorbents: Carbon Nanotubes. J. Chromatogr. A 2014, 1357, 53–67. [Google Scholar] [CrossRef]
- Sun, R.; Gao, S.; Zhang, K.; Cheng, W.T.; Hu, G. Recent Advances in Alginate-Based Composite Gel Spheres for Removal of Heavy Metals. Int. J. Biol. Macromol. 2024, 268, 131853. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy Metal Removal from Water/Wastewater by Nanosized Metal Oxides: A Review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Cardea, S.; Tabernero, A.; De Marco, I.; Valente, A.J.M.; Anastopoulos, I.; Brun, N. Porous Aerogels and Adsorption of Pollutants from Water and Air: A Review. Molecules 2021, 26, 4440. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Aznar, E.; Deveci, H.; Martínez-Máñez, R. Low-Cost Silica Xerogels as Potential Adsorbents for Ciprofloxacin Removal. Sustain. Chem. Pharm. 2021, 22, 100483. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Silica Aerogels/Xerogels Modified with Nitrogen-Containing Groups for Heavy Metal Adsorption. Molecules 2020, 25, 2788. [Google Scholar] [CrossRef]
- Jin, Z.; Lin, Y.; Zhang, Y.; Ying, J.; Gitis, V.; Yu, J. Development of Robust Fluorinated SiO2/PVDF Composite Hollow Fiber Membrane for Bromine Resources Recovery from Brine via Membrane Distillation. ACS ES T Water 2023, 3, 1874–1883. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Zhang, B.; Ye, Y.; Yang, T.; Zeng, H.; Luo, X. Enhanced Long-Term Antifouling Ability and Enrichment Effect of a Vertically Ordered Mesoporous Silica Film via Covalent Linkage of Chondroitin Sulfate for In Situ Detection of Cu2+ in Real Environmental Samples. ACS ES T Water 2023, 3, 2108–2119. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.K.; Park, H.H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Vareda, J.P.; Durães, L. Functionalized Silica Xerogels for Adsorption of Heavy Metals from Groundwater and Soils. J. Solgel Sci. Technol. 2017, 84, 400–408. [Google Scholar] [CrossRef]
- Dudás, Z.; Len, A.; Ianăși, C.; Paladini, G. Structural Modifications Caused by the Increasing MTES Amount in Hybrid MTES/TEOS-Based Silica Xerogels. Mater. Charact. 2020, 167, 110519. [Google Scholar] [CrossRef]
- Gizli, N.; Çok, S.S.; Koç, F. Aerogel, Xerogel, and Cryogel: Synthesis, Surface Chemistry, and Properties—Practical Environmental Applications and the Future Developments. In Advanced Materials for Sustainable Environmental Remediation: Terrestrial and Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–229. [Google Scholar] [CrossRef]
- Dudarko, O.; Budnyak, T.; Tkachenko, O.; Agback, T.; Agback, P.; Bonnet, B.; Ahrens, L.; Daniel, G.; Seisenbaeva, G. Removal of Poly- and Perfluoroalkyl Substances from Natural and Wastewater by Tailored Silica-Based Adsorbents. ACS ES T Water 2024, 4, 1303–1314. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, X.; Yun, X.; Yu, D.; Wang, B. Efficient Adsorption of Sodium P-Perfluorous Nonenoxybenzenesulfonate by Environmentally Friendly Self-Floating Amino Ordered Mesoporous Silica: Energy-Efficient Solid–Liquid Separation and Enhanced Adsorption Performance. Sep. Purif. Technol. 2025, 371, 133406. [Google Scholar] [CrossRef]
- Ateia, M.; Alsbaiee, A.; Karanfil, T.; Dichtel, W. Efficient PFAS Removal by Amine-Functionalized Sorbents: Critical Review of the Current Literature. Environ. Sci. Technol. Lett. 2019, 6, 688–695. [Google Scholar] [CrossRef]
- Lotfi, R.; Hayati, B.; Rahimi, S.; Shekarchi, A.A.; Mahmoodi, N.M.; Bagheri, A. Synthesis and Characterization of PAMAM/SiO2 Nanohybrid as a New Promising Adsorbent for Pharmaceuticals. Microchem. J. 2019, 146, 1150–1159. [Google Scholar] [CrossRef]
- Yu, Y.L.; Jin, H.F.; Shi, Y.; Cao, J. Synchronous Microextraction of Active and Toxic Compounds from Medicinal Plant Using Nano-SiO2 Assisted Miniaturized Matrix Solid-Phase Dispersion. Microchem. J. 2022, 183, 108099. [Google Scholar] [CrossRef]
- Tejedor, J.; Guerrero, V.H.; Vizuete, K.; Debut, A. Environmentally Friendly Synthesis of Silicon Dioxide Nanoparticles and Their Application for the Removal of Emerging Contaminants in Aqueous Media. J. Phys. Conf. Ser. 2022, 2238, 012005. [Google Scholar] [CrossRef]
- Tatar, D.K.; Jha, J.M. A Review on the Synthesis of Silica Oxide Nanoparticles (SiO2-NPs) and Their Use to Remove Pharmaceuticals and Personal Care Products (PPCPs) from Aqueous Solutions. In Advanced Oxidation Process-Based Integrated and Hybrid Technologies for Degradation of Pharmaceuticals and Personal Care Products; Elsevier: Amsterdam, The Netherlands, 2025; pp. 45–52. [Google Scholar] [CrossRef]
- Akhter, F.; Soomro, S.A.; Inglezakis, V.J. Silica Aerogels; a Review of Synthesis, Applications and Fabrication of Hybrid Composites. J. Porous Mater. 2021, 28, 1387–1400. [Google Scholar] [CrossRef]
- Lin, J.; Li, G.; Liu, W.; Qiu, R.; Wei, H.; Zong, K.; Cai, X. A Review of Recent Progress on the Silica Aerogel Monoliths: Synthesis, Reinforcement, and Applications. J. Mater. Sci. 2021, 56, 10812–10833. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Ye, W.; Shen, X.; Yuan, X.; Ma, C.; Cao, Y. Performance Regulation of Silica Aerogel Powder Synthesized by a Two-Step Sol-Gel Process with a Fast Ambient Pressure Drying Route. J. Non Cryst. Solids 2021, 567, 120923. [Google Scholar] [CrossRef]
- Faghihian, H.; Nourmoradi, H.; Shokouhi, M. Removal of Copper (II) and Nickel (II) from Aqueous Media Using Silica Aerogel Modified with Amino Propyl Triethoxysilane as an Adsorbent: Equilibrium, Kinetic, and Isotherms Study. Desalination Water Treat 2014, 52, 305–313. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Kazemi, M. Characterization of Modified Silica Aerogel Using Sodium Silicate Precursor and Its Application as Adsorbent of Cu2+, Cd2+, and Pb2+ Ions. Int. J. Ind. Chem. 2012, 3, 1–8. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Huang, S.; Kang, S.; Zhang, C.; Li, X. Low-Cost Route for Synthesis of Mesoporous Silica Materials with High Silanol Groups and Their Application for Cu(II) Removal. Mater. Chem. Phys. 2012, 132, 1053–1059. [Google Scholar] [CrossRef]
- NIST X-Ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/ (accessed on 4 June 2024).
- Diagboya, P.N.E.; Dikio, E.D. Silica-Based Mesoporous Materials; Emerging Designer Adsorbents for Aqueous Pollutants Removal and Water Treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Ammaeva, S.G.; Isaev, A.B.; Kharlamova, T.A. Preparation of Xerogel of Silicon Dioxide by Acid Hydrolysis of Tetraethoxysilane and Study into Its Sorption Properties. Chem. Probl. 2021, 1, 56–63. [Google Scholar] [CrossRef]
- Hegde, N.D.; Hirashima, H.; Venkateswara Rao, A. Two Step Sol-Gel Processing of TEOS Based Hydrophobic Silica Aerogels Using Trimethylethoxysilane as a Co-Precursor. J. Porous Mater. 2007, 14, 165–171. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Kalekar, A.S.; Harale, N.S.; Patil, S.S.; Dhere, S.L. Rapid Synthesis of Ambient Pressure Dried Tetraethoxysilane Based Silica Aerogels. J. Solgel Sci. Technol. 2021, 97, 5–10. [Google Scholar] [CrossRef]
- Akti, F.; Balci, S. Silica Xerogel and Iron Doped Silica Xerogel Synthesis in Presence of Drying Control Chemical Additives. Mater. Chem. Phys. 2023, 297, 127347. [Google Scholar] [CrossRef]
- Cristiano, E.; Hu, Y.J.; Siegfried, M.; Kaplan, D.; Nitsche, H. A Comparison of Point of Zero Charge Measurement Methodology. Clays Clay Min. 2011, 59, 107–115. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of Sorbent Point Zero Charge: Usefulness in Sorption Studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Bajat, J.B.; Milošev, I.; Jovanović, Ž.; Mišković-Stanković, V.B. Studies on Adhesion Characteristics and Corrosion Behaviour of Vinyltriethoxysilane/Epoxy Coating Protective System on Aluminium. Appl. Surf. Sci. 2010, 256, 3508–3517. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Xu, D.; Wang, D.; Xue, Y.; Su, W. Pressure-Induced Crystallization of Amorphous SiO2 with Silicon–Hydroxy Group and the Quick Synthesis of Coesite under Lower Temperature. High Press. Res. 2008, 28, 641–650. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of Amorphous SiO2 Particles and Their Properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Milošev, I.; Jovanović, Ž.; Bajat, J.B.; Jančić-Heinemann, R.; Mišković-Stanković, V.B. Surface Analysis and Electrochemical Behavior of Aluminum Pretreated by Vinyltriethoxysilane Films in Mild NaCl Solution. J. Electrochem. Soc. 2012, 159, C303–C311. [Google Scholar] [CrossRef]
- Todea, M.; Simon, V.; Muresan-Pop, M.; Vulpoi, A.; Rusu, M.M.; Simion, A.; Vasilescu, M.; Damian, G.; Petrisor, D.M.; Simon, S. Silica-Based Microspheres with Aluminum-Iron Oxide Shell for Diagnosis and Cancer Treatment. J. Mol. Struct. 2021, 1246, 131149. [Google Scholar] [CrossRef]
- Duval, Y.; Mielczarski, J.A.; Pokrovsky, O.S.; Mielczarski, E.; Ehrhardt, J.J. Evidence of the existence of three types of species at the quartz-aqueous solution interface at ph 0-10: Xps surface group quantification and surface complexation modeling. J. Phys. Chem. B 2002, 106, 2937–2945. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shimogaki, Y. X-Ray Photoelectron Spectroscopic Characterization of the Adhesion Behavior of Chemical Vapor Deposited Copper Films. J. Vac. Sci. Technol. A 2001, 19, 2642–2651. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R′′Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Elkady, M.F.; El-Aassar, M.R.; Hassan, H.S. Adsorption Profile of Basic Dye onto Novel Fabricated Carboxylated Functionalized Co-Polymer Nanofibers. Polymers 2016, 8, 177. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. Correlation between Physical Properties and Structure of Silica Xerogels. J. Non Cryst. Solids 2004, 347, 128–137. [Google Scholar] [CrossRef]
- Parvathy Rao, A.; Venkateswara Rao, A. Modifying the Surface Energy and Hydrophobicity of the Low-Density Silica Aerogels through the Use of Combinations of Surface-Modification Agents. J. Mater. Sci. 2010, 45, 51–63. [Google Scholar] [CrossRef]
- Halim, Z.A.A.; Yajid, M.A.M.; Hamdan, H. Effects of Solvent Exchange Period and Heat Treatment on Physical and Chemical Properties of Rice Husk Derived Silica Aerogels. Silicon 2021, 13, 251–257. [Google Scholar] [CrossRef]
- Wang, R.; Ng, D.H.L.; Liu, S. Recovery of Nickel Ions from Wastewater by Precipitation Approach Using Silica Xerogel. J. Hazard. Mater. 2019, 380, 120826. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Dong, X.; Gao, G.; Sha, F.; Xu, D. Microstructure and Adsorption Properties of MTMS / TEOS Co-Precursor Silica Aerogels Dried at Ambient Pressure. J. Non Cryst. Solids 2021, 562, 120778. [Google Scholar] [CrossRef]
- Fernández Pérez, B.; Ayala Espina, J.; Fernández González, M. de L. Á. Adsorption of Heavy Metals Ions from Mining Metallurgical Tailings Leachate Using a Shell-Based Adsorbent: Characterization, Kinetics and Isotherm Studies. Materials 2022, 15, 5315. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Matyjasik, W.; Matysik, J.; Szostak, K.; Śliwa, P.; Banach, M. Biocatalyst Based on Magnetic Nanoparticles with Cu(II), Mn(II), Zn(II) and Immobilised Catalase. J. Clust. Sci. 2024, 35, 143–158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).