Rapid Removal of Sizing Agent from Carbon Fiber Surface by Liquid-Phase Plasma Electrolysis
Abstract
1. Introduction
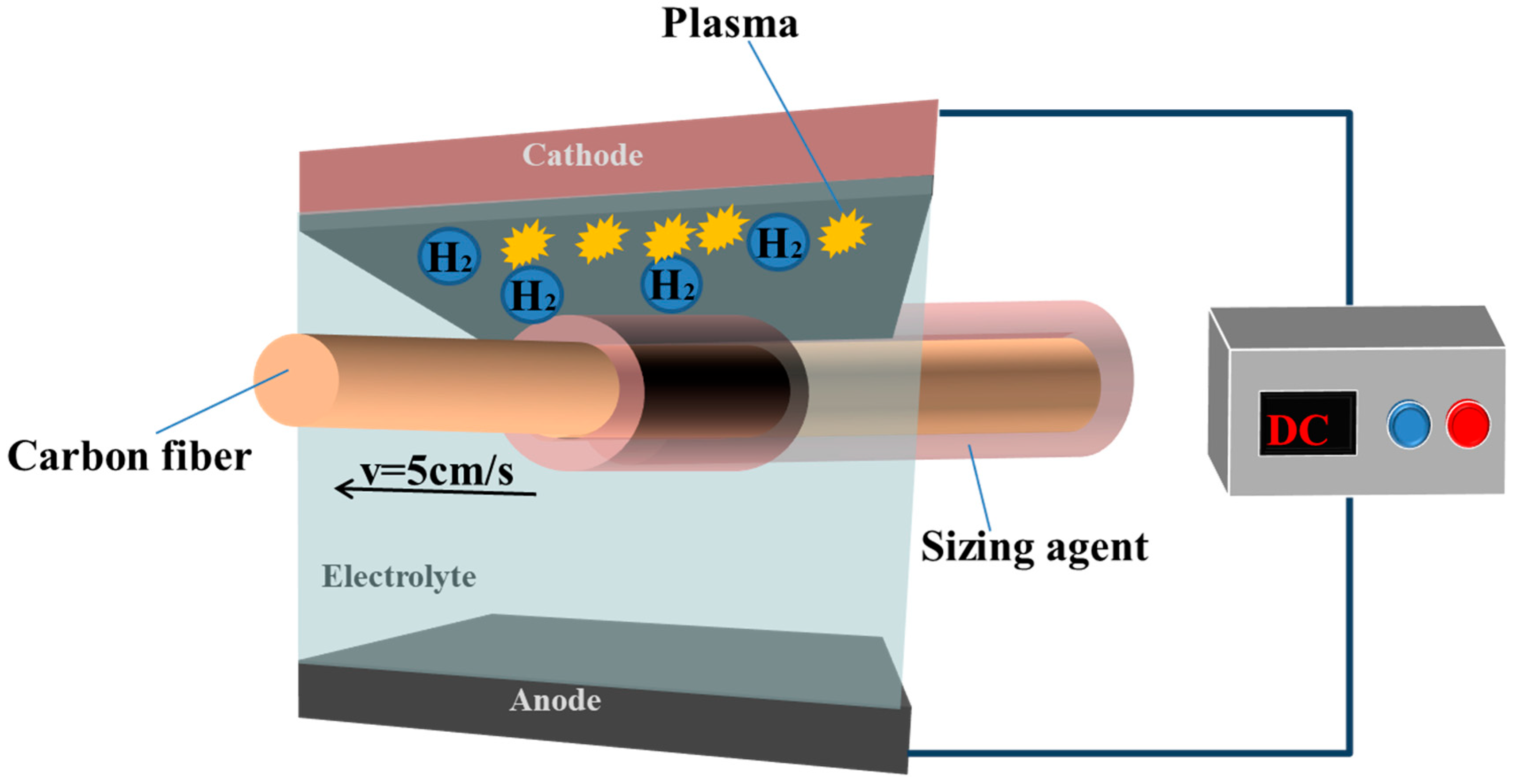
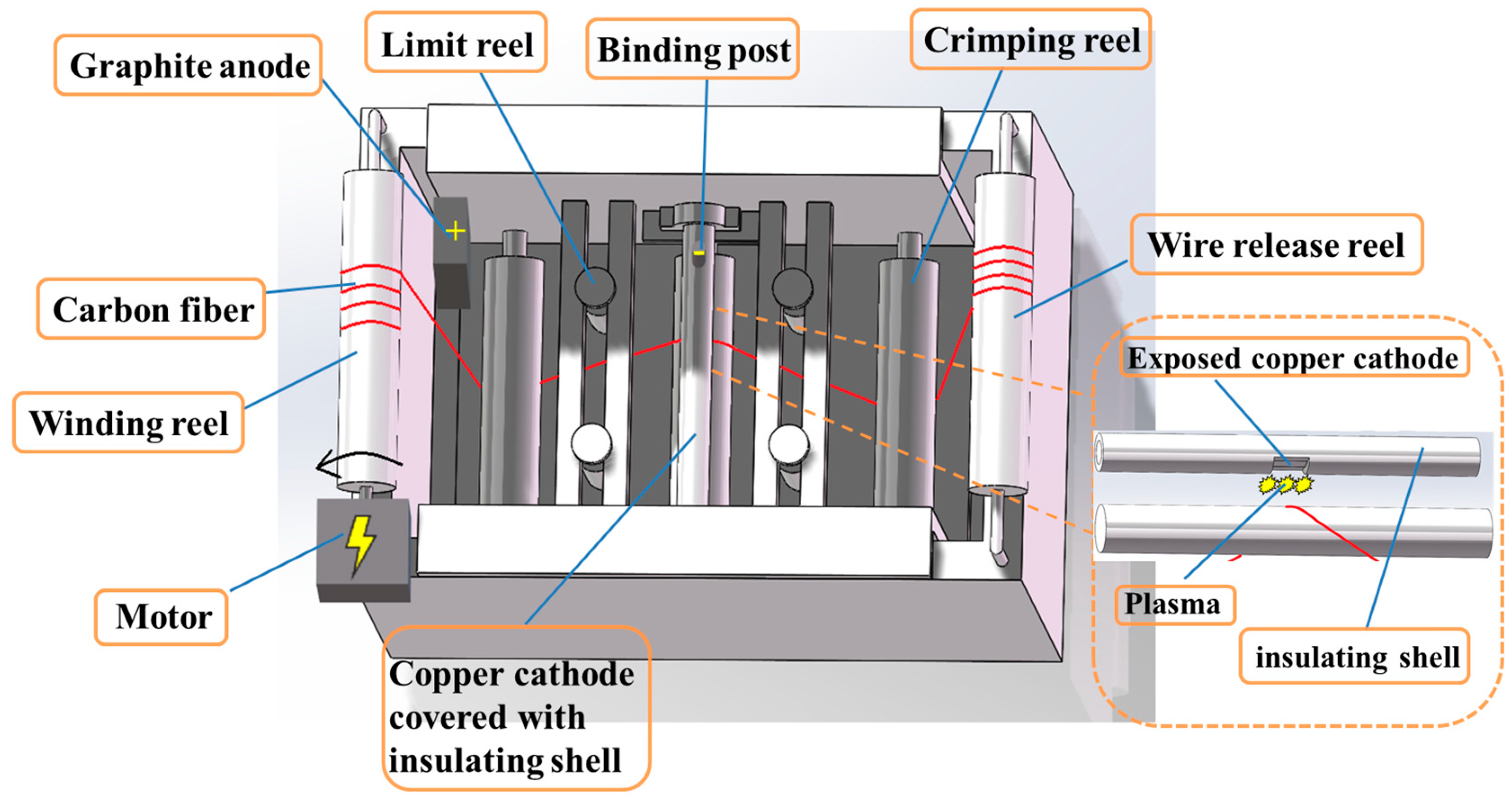
2. Experimental Section
2.1. LPE Process
2.2. Characterization
3. Results and Discussion
3.1. Morphology and Sizing Agent Content Estimation
3.2. Thermogravimetric Analysis
3.3. XPS
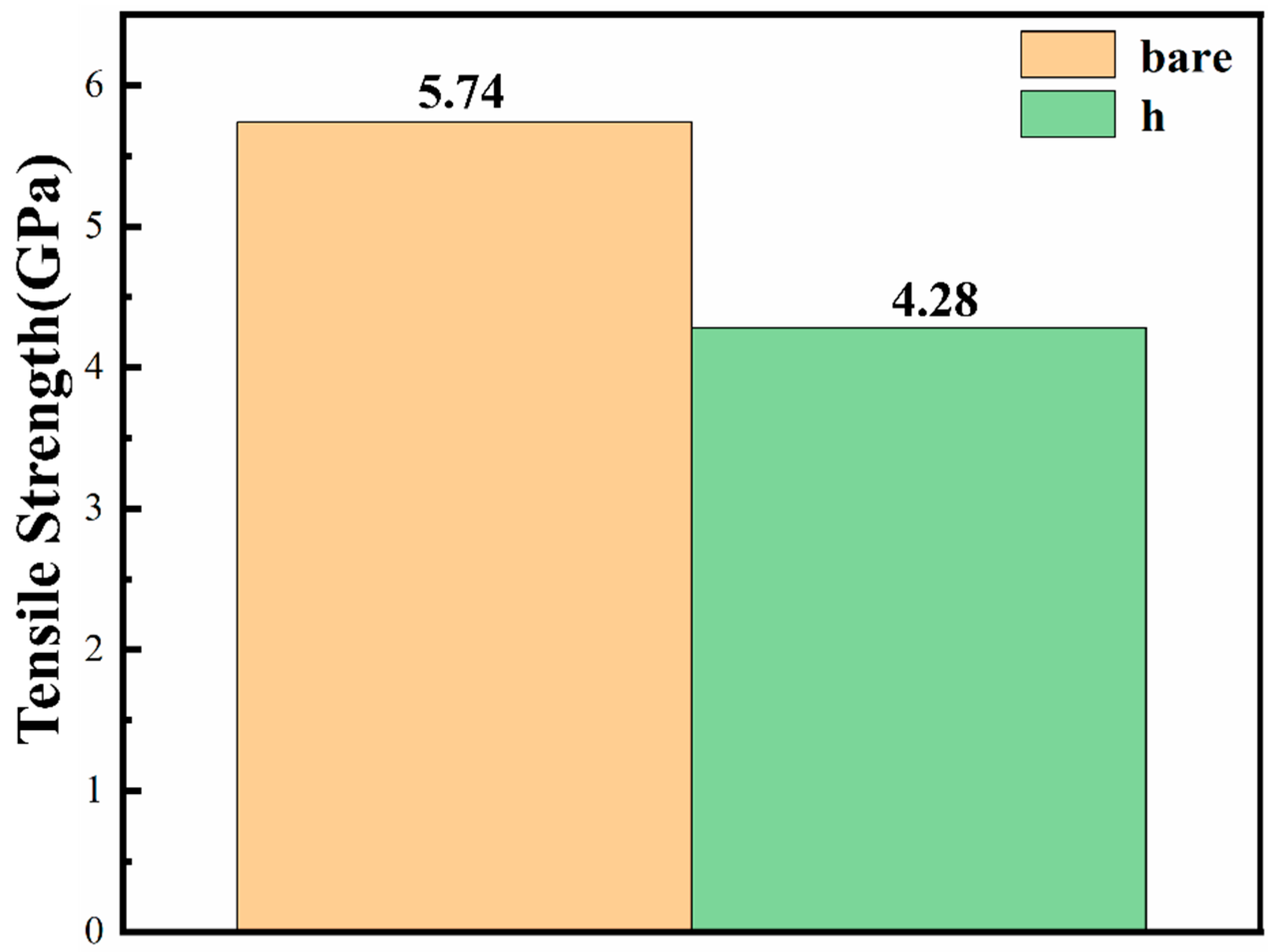
3.4. The Tensile Strength of Fibers
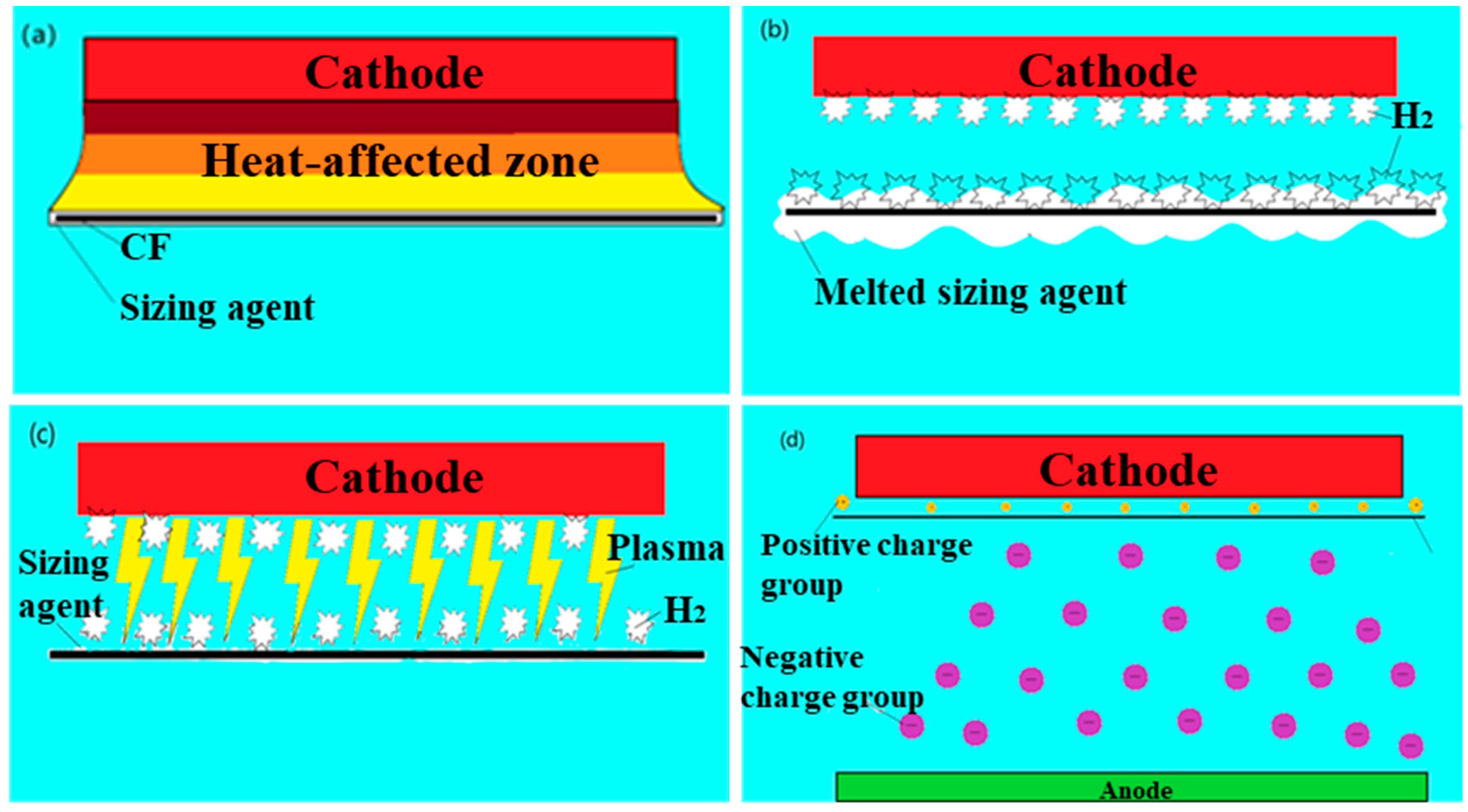
3.5. Mechanism of LPE in Removing Sizing Agents from the Surface of Carbon Fibers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savino, R.; Criscuolo, L.; Di Martino, G.D.; Mungiguerra, S. Aero-thermo-chemical characterization of ultra-high-temperature ceramics for aerospace applications. J. Eur. Ceram. Soc. 2018, 38, 2937–2953. [Google Scholar] [CrossRef]
- Tiwary, A.; Kumar, R.; Chohan, J.S. A review on characteristics of composite and advanced materials used for aerospace applications. Mater. Today Proc. 2022, 51, 865–870. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Zhang, Z.; Cheng, L.; Ma, H.; Yang, W. Advances in modifications and high-temperature applications of silicon carbide ceramic matrix composites in aerospace: A focused review. J. Eur. Ceram. Soc. 2021, 41, 4671–4688. [Google Scholar] [CrossRef]
- Voronina, S.Y.; Shalygina, T.A.; Voronchikhin, V.D.; Vlasov, A.Y.; Ovchinnikov, A.N.; Grotskaya, N.N. Data for determining the surface properties of carbon fiber in contact interaction with polymeric binders. Data Brief 2021, 35, 106847. [Google Scholar] [CrossRef]
- Albano, M.; Alifanov, O.M.; Budnik, S.A.; Morzhukhina, A.V.; Nenarokomov, A.V.; Titov, D.M.; Gabrielli, A.; Ianelli, S.; Marchetti, M. Carbon/carbon high thickness shell for advanced space vehicles. Int. J. Heat Mass Transf. 2019, 128, 613–622. [Google Scholar] [CrossRef]
- Du, C.; Zhang, X.; Zhou, R.; Sang, Z.; Jiang, Y. Multi-scale modeling and performance study of carbon fiber heterogeneous electrode batteries based on electrochemical-mechanical coupling. J. Power Sources 2025, 648, 237404. [Google Scholar] [CrossRef]
- Salimkhani, H.; Palmeh, P.; Khiabani, A.B.; Hashemi, E.; Matinpour, S.; Salimkhani, H.; Asl, M.S. Electrophoretic deposition of spherical carbonyl iron particles on carbon fibers as a microwave absorbent composite. Surf. Interfaces 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Movassagh-Alanagh, F.; Bordbar-Khiabani, A.; Ahangari-Asl, A. Fabrication of a ternary PANI@Fe3O4@CFs nanocomposite as a high performance electrode for solid-state supercapacitors. Int. J. Hydrogen Energy 2019, 44, 26794–26806. [Google Scholar] [CrossRef]
- Shin, S.; Jang, J.; Yoon, S.H.; Mochida, I. A study on the effect of heat treatment on functional groups of pitch based activated carbon fiber using FTIR. Carbon 1997, 35, 1739–1743. [Google Scholar] [CrossRef]
- Fanning, P.E.; Vannice, M.A. A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon 1993, 31, 721–730. [Google Scholar] [CrossRef]
- Lee, S.; Ham, S.; Youn, S.J.; Chung, Y.S.; Lee, S. Effect of Textile PAN-Based Carbon Fibers with Rough Surface on Interfacial Adhesion in PA6 Composites. Ind. Eng. Chem. Res. 2021, 60, 9088–9097. [Google Scholar] [CrossRef]
- Wang, H.; Jin, K.; Tao, J. Improving the interfacial shear strength of carbon fibre and epoxy via mechanical interlocking effect. Compos. Sci. Technol. 2020, 200, 108423. [Google Scholar] [CrossRef]
- Zhu, P.; Shi, J.; Bao, L. Effect of polyetherimide nanoparticle coating on the interfacial shear strength between carbon fiber and thermoplastic resins. Appl. Surf. Sci. 2020, 509, 145395. [Google Scholar] [CrossRef]
- Fukunaga, A.; Ueda, S. Anodic surface oxidation for pitch-based carbon fibers and the interfacial bond strengths in epoxy matrices. Compos. Sci. Technol. 2000, 60, 249–254. [Google Scholar] [CrossRef]
- Tran, M.Q.; Ho, K.K.C.; Kalinka, G.; Shaffer, M.S.P.; Bismarck, A. Carbon fibre reinforced poly(vinylidene fluoride): Impact of matrix modification on fibre/polymer adhesion. Compos. Sci. Technol. 2008, 68, 1766–1776. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, F.; Zhang, C.; Wang, J.; Jia, Z.; Hui, D.; Qiu, Y. Tensile and interfacial properties of polyacrylonitrile-based carbon fiber after different cryogenic treated condition. Compos. Part B Eng. 2016, 99, 358–365. [Google Scholar] [CrossRef]
- Sharma, M.; Gao, S.; Mäder, E.; Sharma, H.; Wei, L.Y.; Bijwe, J. Carbon fiber surfaces and composite interphases. Compos. Sci. Technol. 2014, 102, 35–50. [Google Scholar] [CrossRef]
- Lee, S.; Hoang, Q.N.; Lee, H.; Chung, Y.S.; Park, H.; Lee, S. Effect of plasma modification on the anisotropic surface structure of PAN-based graphitic carbon fiber. Carbon 2025, 242, 120429. [Google Scholar] [CrossRef]
- Lee, S.-C.; White, S.; Grzesik, J.A. Effective radiative properties of fibrous composites containing spherical particles. J. Thermophys. Heat Transf. 1994, 8, 400–405. [Google Scholar] [CrossRef]
- Fang, H.; Sheng, Z.; Wang, W.; Wei, C.; Li, S.; Geng, X.; Li, X.; Zhu, N.; Wen, G.; Dong, S.; et al. Formation and mechanism of carbon coating on carbon fibers through glucose-to-carbon conversion and its effect on the mechanical properties of Cf/ZrB2-SiC composites. J. Eur. Ceram. Soc. 2025, 45, 117569. [Google Scholar] [CrossRef]
- Patra, N.; Ramesh, P.; Ravi, G.; Țălu, Ș. Electroless deposition of nanoscale Ni-Co-Fe-P coatings on carbon fibers for multifunctional applications. Diam. Relat. Mater. 2025, 158, 112602. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Liu, W.; Zhou, M.; Li, W.; Hui, D.; Qiu, Y. Influence of cryogenic treatment on mechanical and interfacial properties of carbon nanotube fiber/bisphenol-F epoxy composite. Compos. Part B Eng. 2017, 125, 195–202. [Google Scholar] [CrossRef]
- Zafeiropoulos, N.E.; Baillie, C.A.; Hodgkinson, J.M. Engineering and characterisation of the interface in flax fibre/polypropylene composite materials. Part II. The effect of surface treatments on the interface. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1185–1190. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. Influence of tungsten on the activity of a Mn/Ce/W/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. A Gen. 2015, 497, 160–166. [Google Scholar] [CrossRef]
- Li, H. Efficient reduction of L-infinity geometry problems. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, CVPR 2009, Miami, FL, USA, 20–25 June 2009; pp. 2695–2702. [Google Scholar]
- Donnet, J.B.; Brendle, M.; Dhami, T.L.; Bahl, O.P. Plasma treatment effect on the surface energy of carbon and carbon fibers. Carbon 1986, 24, 757–770. [Google Scholar] [CrossRef]
- Shi, D.; Lian, J.; He, P.; Wang, L.M.; Xiao, F.; Yang, L.; Schulz, M.J.; Mast, D.B. Plasma coating of carbon nanofibers for enhanced dispersion and interfacial bonding in polymer composites. Appl. Phys. Lett. 2003, 83, 5301–5303. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, Z.; Yang, M.; Wu, J.; Chen, L.; Xue, W. Efficient synthesis and lithium storage performance of SiO2/TiO2 composite film anode by plasma electrolytic oxidation. Mater. Lett. 2024, 371, 136902. [Google Scholar] [CrossRef]
- Bokhari, S.W.; Wei, S.; Gao, W. Synthesis of bimetallic MoS2/VS2 nano-urchins-reduced graphene oxide hybrid nanocomposite for high performance supercapacitor application. Electrochim. Acta 2021, 398, 139300. [Google Scholar] [CrossRef]
- Lee, E.-s.; Lee, C.-h.; Chun, Y.-S.; Han, C.-j.; Lim, D.-S. Effect of hydrogen plasma-mediated surface modification of carbon fibers on the mechanical properties of carbon-fiber-reinforced polyetherimide composites. Compos. Part B Eng. 2017, 116, 451–458. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.; Zhao, Z.; Liu, J.; Chen, Q.; Wang, X. Rapid and Continuous Atmospheric Plasma Surface Modification of PAN-Based Carbon Fibers. ACS Omega 2022, 7, 10963–10969. [Google Scholar] [CrossRef] [PubMed]
- Moosburger-Will, J.; Bauer, M.; Schubert, F.; Kunzmann, C.; Lachner, E.; Zeininger, H.; Maleika, M.; Hönisch, B.; Küpfer, J.; Zschoerper, N.; et al. Methyltrimethoxysilane plasma polymerization coating of carbon fiber surfaces. Surf. Coat. Technol. 2017, 311, 223–230. [Google Scholar] [CrossRef]
- GB/T 29761-2022; Carbon Fibre—Determination of Sizing Content. 7.2 Method B: High-Temperature Decomposition Method. National Standardization Administration Committee, State Administration for Market Regulation: Beijing, China, 2022.
- Zhang, Y.; Chen, C.; Chen, W.; Cheng, H.; Wang, L. A novel aqueous plasma electrolysis for carbon fiber. Chem. Eng. J. 2016, 304, 426–430. [Google Scholar] [CrossRef]
- Gupta, P.; Tenhundfeld, G.; Daigle, E.O.; Ryabkov, D. Electrolytic plasma technology: Science and engineering—An overview. Surf. Coat. Technol. 2007, 201, 8746–8760. [Google Scholar] [CrossRef]

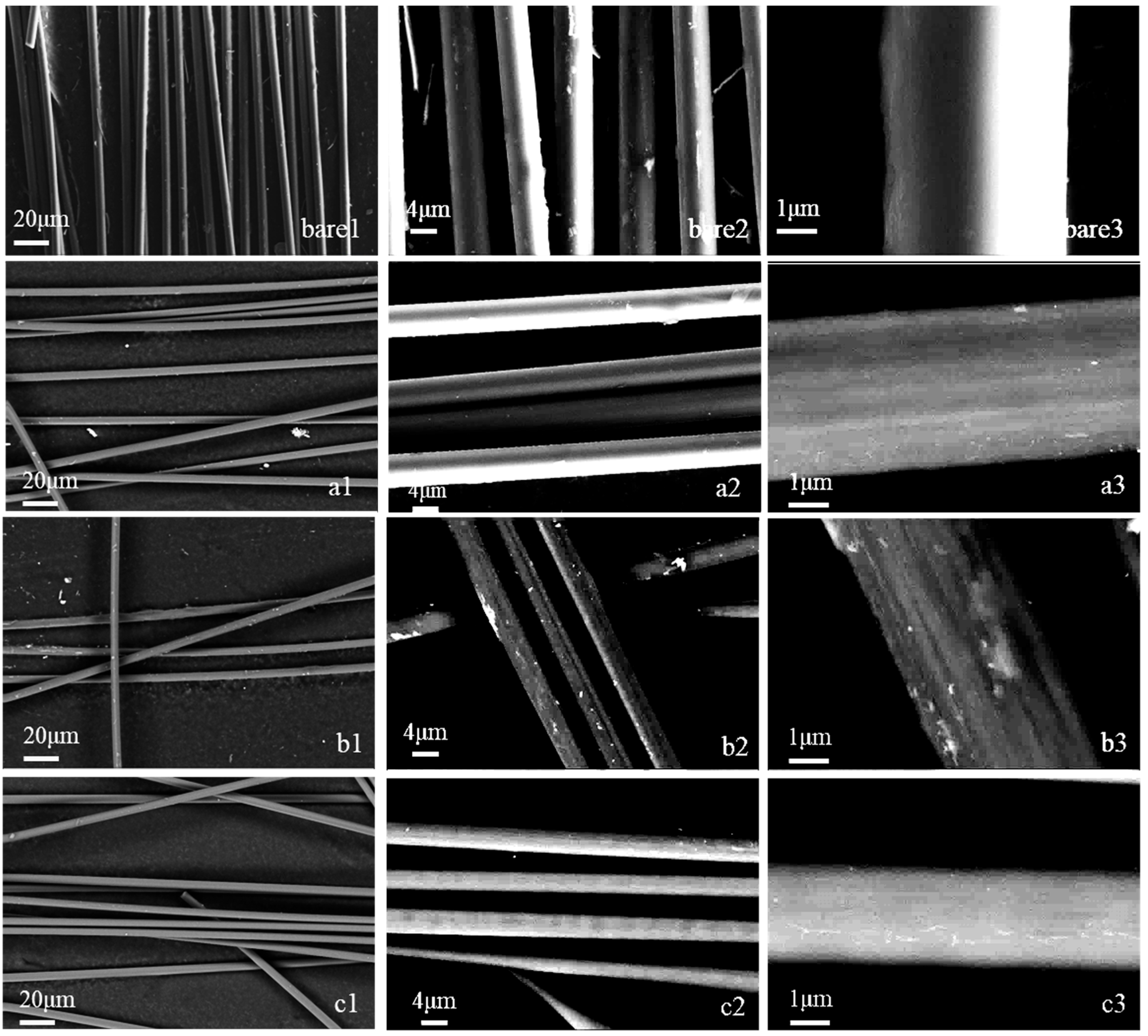
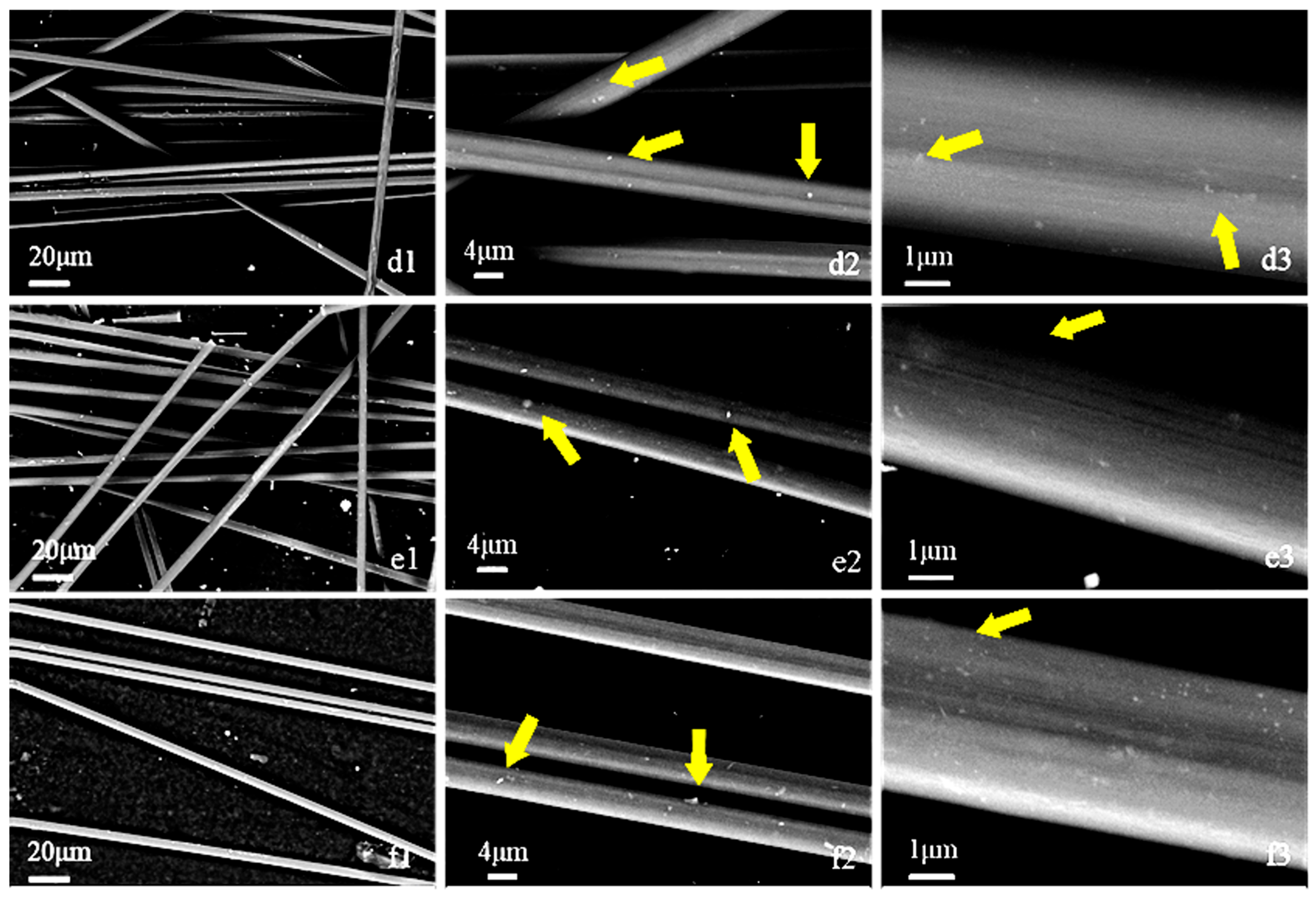





| Arcing Voltage | 185 V | 200 V | 215 V | |
|---|---|---|---|---|
| Electrode Spacing | ||||
| 20 mm | a | b | c | |
| 15 mm | d | e | f | |
| 10 mm | g | h | i | |
| Sample | Binding Energy/eV | Peak Height | Full Width at Half Maximum (FWHM) | Peak Area | Percentage of Peak Area |
|---|---|---|---|---|---|
| h | 284.0 (C-Csp2) 285.64 (C-O) | 6490 2630 | 1.71 1.76 | 13,057 4811 | 77.4 22.6 |
| CF after 700 °C /N2/1 h | 284.0 (C-Csp2) | 13,928 | 1.28 | 25,678 | 100 |
| bare | 284.0 (C-Csp2) 285.73 (C-O) | 6513 4691 | 1.33 1.40 | 10,441 7432 | 58.4 41.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Zhou, Q.; Li, M.; Wei, X.; Li, D.; He, X.; Chen, W. Rapid Removal of Sizing Agent from Carbon Fiber Surface by Liquid-Phase Plasma Electrolysis. Colloids Interfaces 2025, 9, 57. https://doi.org/10.3390/colloids9050057
Huang C, Zhou Q, Li M, Wei X, Li D, He X, Chen W. Rapid Removal of Sizing Agent from Carbon Fiber Surface by Liquid-Phase Plasma Electrolysis. Colloids and Interfaces. 2025; 9(5):57. https://doi.org/10.3390/colloids9050057
Chicago/Turabian StyleHuang, Chiyuhao, Qian Zhou, Maoyuan Li, Xiaolin Wei, Dongqin Li, Xin He, and Weiwei Chen. 2025. "Rapid Removal of Sizing Agent from Carbon Fiber Surface by Liquid-Phase Plasma Electrolysis" Colloids and Interfaces 9, no. 5: 57. https://doi.org/10.3390/colloids9050057
APA StyleHuang, C., Zhou, Q., Li, M., Wei, X., Li, D., He, X., & Chen, W. (2025). Rapid Removal of Sizing Agent from Carbon Fiber Surface by Liquid-Phase Plasma Electrolysis. Colloids and Interfaces, 9(5), 57. https://doi.org/10.3390/colloids9050057







