APTES-Modified Interface Optimization in PbS Quantum Dot SWIR Photodetectors and Its Influence on Optoelectronic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PbS QDs
2.3. Ligand Exchange of PbS QDs
2.4. Preparation of APTES-Modified SWIR Photodetectors
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreels, I.; Justo, Y.; De Geyter, B.; Haustraete, K.; Martins, J.C.; Hens, Z. Size-Tunable, Bright, and Stable PbS Quantum Dots: A Surface Chemistry Study. ACS Nano 2011, 5, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Weidman, M.C.; Beck, M.E.; Hoffman, R.S.; Prins, F.; Tisdale, W.A. Monodisperse, Air-Stable PbS Nanocrystals via Precursor Stoichiometry Control. ACS Nano 2014, 8, 6363–6371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gu, Y.; Li, X.; Liu, Y.; Liu, F.; Wu, W. Recent Progress of Quantum Dot Infrared Photodetectors. Adv. Opt. Mater. 2023, 11, 2300970. [Google Scholar] [CrossRef]
- Zaini, M.S.; Liew, J.Y.C.; Alang Ahmad, S.A.; Mohmad, A.R.; Ahmad Kamarudin, M. Photoluminescence investigation of carrier localization in colloidal PbS and PbS/MnS quantum dots. ACS Omega 2020, 5, 30956–30962. [Google Scholar] [CrossRef] [PubMed]
- Venettacci, C.; Martín-García, B.; Prato, M.; Moreels, I.; De Iacovo, A. Increasing responsivity and air stability of PbS colloidal quantum dot photoconductors with iodine surface ligands. Nanotechnology 2019, 30, 405204. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Batmunkh, M.; Tricoli, A.; Qiao, S.Z.; Dai, S. Near-Infrared Active Lead Chalcogenide Quantum Dots: Preparation, Post-Synthesis Ligand Exchange, and Applications in Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 5202–5224. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Di Stasio, F.; Bi, Y.; Gupta, S.; Christodoulou, S.; Stavrinadis, A.; Konstantatos, G. High-efficiency colloidal quantum dot infrared light-emitting diodes via engineering at the supra-nanocrystalline level. Nat. Nanotechnol. 2019, 14, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Khan, J.; Deng, H.; Yuan, S.; Zhang, J.; Xia, Y.; Deng, F.; Zhou, X.; Umar, F.; et al. Hydroiodic Acid Additive Enhanced the Performance and Stability of PbS-QDs Solar Cells via Suppressing Hydroxyl Ligand. Nano-Micro Lett. 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli Dastjerdi, H.; Tavakoli, R.; Yadav, P.; Prochowicz, D.; Saliba, M.; Tavakoli, M.M. Oxygen plasma-induced p-type doping improves performance and stability of PbS quantum dot solar cells. ACS Appl. Mater. Interfaces 2019, 11, 26047–26052. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, R.; Tang, H.; Wienhold, K.S.; Li, N.; Jiang, Z.; Tang, J.; Jiang, X.; Kreuzer, L.P.; Liu, H.; et al. Operando structure degradation study of PbS quantum dot solar cells. Energy Environ. Sci. 2021, 14, 3420–3429. [Google Scholar] [CrossRef]
- Imamura, Y.; Yamada, S.; Tsuboi, S.; Nakane, Y.; Tsukasaki, Y.; Komatsuzaki, A.; Jin, T. Near-infrared emitting PbS quantum dots for in vivo fluorescence imaging of the thrombotic state in septic mouse brain. Molecules 2016, 21, 1080. [Google Scholar] [CrossRef] [PubMed]
- Benayas, A.; Ren, F.; Carrasco, E.; Marzal, V.; del Rosal, B.; Gonfa, B.A.; Juarranz, Á.; Sanz-Rodríguez, F.; Jaque, D.; García-Solé, J.; et al. PbS/CdS/ZnS Quantum Dots: A Multifunctional Platform for In Vivo Near-Infrared Low-Dose Fluorescence Imaging. Adv. Funct. Mater. 2015, 25, 6650–6659. [Google Scholar] [CrossRef]
- Zebibula, A.; Alifu, N.; Xia, L.; Sun, C.; Yu, X.; Xue, D.; Liu, L.; Li, G.; Qian, J. Ultrastable and Biocompatible NIR-II Quantum Dots for Functional Bioimaging. Adv. Funct. Mater. 2018, 28, 1703451. [Google Scholar] [CrossRef]
- Pejović, V.; Georgitzikis, E.; Lee, J.; Lieberman, I.; Cheyns, D.; Heremans, P.; Malinowski, P.E. Infrared Colloidal Quantum Dot Image Sensors. IEEE Trans. Electron Devices 2022, 69, 2840–2850. [Google Scholar] [CrossRef]
- Georgitzikis, E.; Malinowski, P.E.; Li, Y.; Maes, J.; Hagelsieb, L.M.; Guerrieri, S.; Hens, Z.; Heremans, P.; Cheyns, D. Integration of PbS Quantum Dot Photodiodes on Silicon for NIR Imaging. IEEE Sens. J. 2020, 20, 6841–6848. [Google Scholar] [CrossRef]
- Baranov, D.; Lynch, M.J.; Curtis, A.C.; Carollo, A.R.; Douglass, C.R.; Mateo-Tejada, A.M.; Jonas, D.M. Purification of Oleylamine for Materials Synthesis and Spectroscopic Diagnostics for trans Isomers. Chem. Mater. 2019, 31, 1223–1230. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, H.; Wang, X.; Pan, G.; Tang, H.; Fang, F.; Wu, J.; Wang, W.; Xu, L.; Tang, J.; et al. Hybrid-Size Quantum Dots in Hole Transport Layer Depress Dark Current Density of Short-Wave Infrared Photodetectors. ACS Photonics 2025, 12, 879–888. [Google Scholar] [CrossRef]
- Tang, H.; Zhong, J.; Chen, W.; Shi, K.; Mei, G.; Zhang, Y.; Wen, Z.; Müller-Buschbaum, P.; Wu, D.; Wang, K.; et al. Lead Sulfide Quantum Dot Photodetector with Enhanced Responsivity through a Two-Step Ligand-Exchange Method. ACS Appl. Nano Mater. 2019, 2, 6135–6143. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, F.; Zhong, H.; Huang, L.; Tang, H.; Chen, X.; Hao, J.; Zhang, L.; Cao, L.; Tang, J.; et al. Converting Perovskite Nanocrystals to PbS Quantum Dots Toward Short-Wave Infrared Photodetectors. Adv. Opt. Mater. 2025, 13, 2402740. [Google Scholar] [CrossRef]
- Chen, C.; Ao, L.; Wu, Y.T.; Cifliku, V.; Cardoso Dos Santos, M.; Bourrier, E.; Delbianco, M.; Parker, D.; Zwier, J.M.; Huang, L.; et al. Single-Nanoparticle Cell Barcoding by Tunable FRET from Lanthanides to Quantum Dots. Angew. Chem. Int. Ed. 2018, 57, 13686–13690. [Google Scholar] [CrossRef] [PubMed]
- Pietra, F.; van Dijk-Moes, R.J.A.; Ke, X.; Bals, S.; Van Tendeloo, G.; de Mello Donega, C.; Vanmaekelbergh, D. Synthesis of Highly Luminescent Silica-Coated CdSe/CdS Nanorods. Chem. Mater. 2013, 25, 3427–3434. [Google Scholar] [CrossRef]
- Koole, R.; Van Schooneveld, M.M.; Hilhorst, J.; de Mello Donegá, C.; Hart, D.C.; Van Blaaderen, A.; Vanmaekelbergh, D.; Meijerink, A. On the incorporation mechanism of hydrophobic quantum dots in silica spheres by a reverse microemulsion method. Chem. Mater. 2008, 20, 2503–2512. [Google Scholar] [CrossRef]
- Huang, P.Y.; Zhang, Y.Y.; Tsai, P.C.; Chung, R.J.; Tsai, Y.T.; Leung, M.K.; Lin, S.Y.; Fang, M.H. Interfacial Engineering of Quantum Dots–Metal–Organic Framework Composite Toward Efficient Charge Transport for a Short-Wave Infrared Photodetector. Adv. Opt. Mater. 2024, 12, 2302062. [Google Scholar] [CrossRef]
- Wu, Z.; Ou, Y.; Cai, M.; Wang, Y.; Tang, R.; Xia, Y. Short-wave infrared photodetectors and imaging sensors based on lead chalcogenide colloidal quantum dots. Adv. Opt. Mater. 2023, 11, 2201577. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, W.; Ning, Z. Integrated structure and device engineering for high performance and scalable quantum dot infrared photodetectors. Small 2020, 16, 2003397. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, C.; Guo, Y.; Yang, Y.; Xing, Y.; Que, W. PbS QD-based photodetectors: Future-oriented near-infrared detection technology. J. Mater. Chem. C 2021, 9, 417–438. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, J.-L.; Zhang, J.-Y.; Gao, X.; Zhong, Y.-N.; Wang, S.-D. Interface engineering for high photoresponse in PbS quantum-dot short-wavelength infrared photodiodes. IEEE Electron Device Lett. 2022, 43, 1275–1278. [Google Scholar] [CrossRef]
- Hines, M.A.; Scholes, G.D. Colloidal PbS Nanocrystals with Size-Tunable Near-Infrared Emission: Observation of Post-Synthesis Self-Narrowing of the Particle Size Distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Cao; Banin, U. Growth and properties of semiconductor core/shell nanocrystals with InAs cores. J. Am. Chem. Soc. 2000, 122, 9692–9702. [Google Scholar] [CrossRef]
- Luther, J.M.; Zheng, H.; Sadtler, B.; Alivisatos, A.P. Synthesis of PbS nanorods and other ionic nanocrystals of complex morphology by sequential cation exchange reactions. J. Am. Chem. Soc. 2009, 131, 16851–16857. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Protiere, M.; Li, L. Core/shell semiconductor nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Curry, R.J. Lead sulphide nanocrystal photodetector technologies. Nat. Photonics 2016, 10, 81–92. [Google Scholar] [CrossRef]
- Saengdee, P.; Promptmas, C.; Thanapitak, S.; Srisuwan, A.; Pankiew, A.; Thornyanadacha, N.; Chaisriratanakul, W.; Chaowicharat, E.; Jeamsaksiri, W. Optimization of 3-aminopropyltriethoxysilane functionalization on silicon nitride surface for biomolecule immobilization. Talanta 2020, 207, 120305. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seidler, P.; Wan, L.S.; Fill, C. Formation, structure, and reactivity of amino-terminated organic films on silicon substrates. J. Colloid Interface Sci. 2009, 329, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Howarter, J.A.; Youngblood, J.P. Optimization of Silica Silanization by 3-Aminopropyltriethoxysilane. Langmuir 2006, 22, 11142–11147. [Google Scholar] [CrossRef] [PubMed]


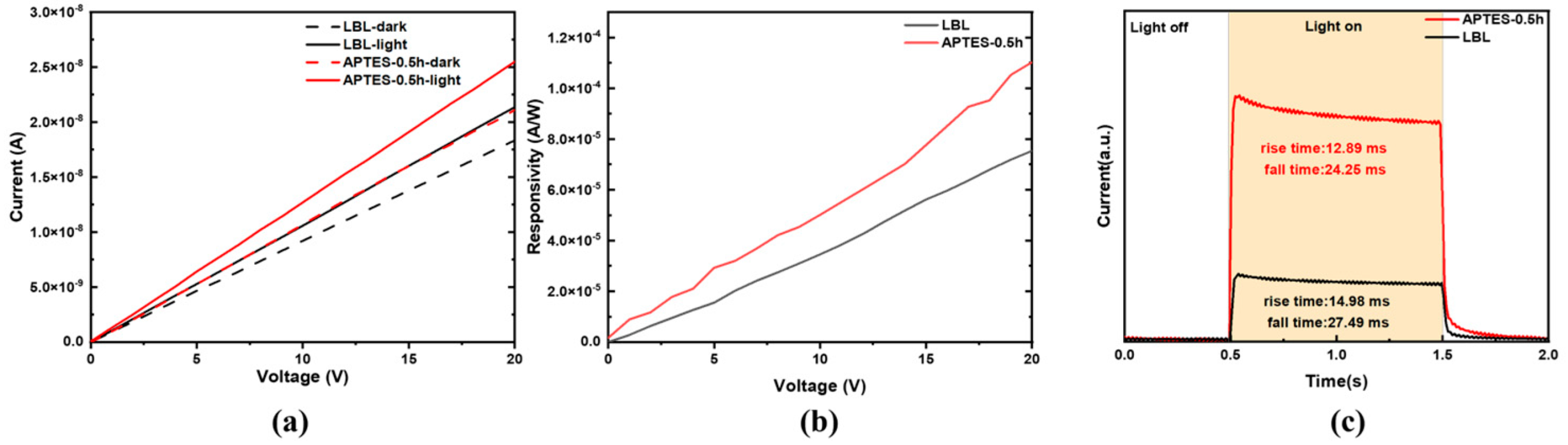
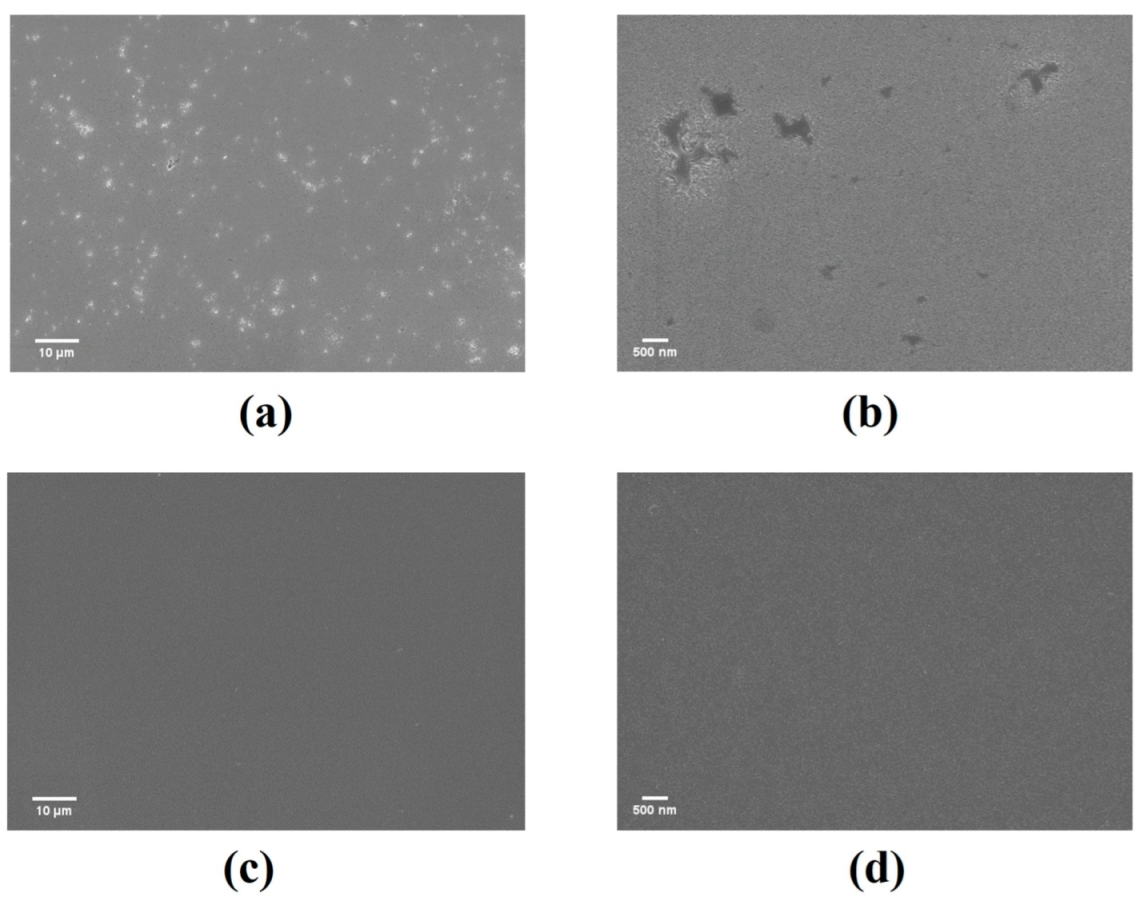


| Time (h) | Dark Current (A) | Light Current (A) | Responsivity(A/W) |
|---|---|---|---|
| 0 | 1.83 × 10−8 | 2.13 × 10−8 | 7.54 × 10−5 |
| 0.25 | 4.97 × 10−8 | 5.37 × 10−8 | 1.01 × 10−4 |
| 0.5 | 2.11 × 10−8 | 2.55 × 10−8 | 1.10 × 10−4 |
| 1.0 | 2.70 × 10−8 | 3.10 × 10−8 | 1.00 × 10−4 |
| 1.5 | 2.07 × 10−8 | 5.91 × 10−8 | 9.57 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Q.; Rao, L.; Deng, W.; Ao, X.; Fang, F.; Chen, W.; Cheng, J.; Tang, H.; Hao, J. APTES-Modified Interface Optimization in PbS Quantum Dot SWIR Photodetectors and Its Influence on Optoelectronic Properties. Colloids Interfaces 2025, 9, 49. https://doi.org/10.3390/colloids9040049
Lei Q, Rao L, Deng W, Ao X, Fang F, Chen W, Cheng J, Tang H, Hao J. APTES-Modified Interface Optimization in PbS Quantum Dot SWIR Photodetectors and Its Influence on Optoelectronic Properties. Colloids and Interfaces. 2025; 9(4):49. https://doi.org/10.3390/colloids9040049
Chicago/Turabian StyleLei, Qian, Lei Rao, Wencan Deng, Xiuqin Ao, Fan Fang, Wei Chen, Jiaji Cheng, Haodong Tang, and Junjie Hao. 2025. "APTES-Modified Interface Optimization in PbS Quantum Dot SWIR Photodetectors and Its Influence on Optoelectronic Properties" Colloids and Interfaces 9, no. 4: 49. https://doi.org/10.3390/colloids9040049
APA StyleLei, Q., Rao, L., Deng, W., Ao, X., Fang, F., Chen, W., Cheng, J., Tang, H., & Hao, J. (2025). APTES-Modified Interface Optimization in PbS Quantum Dot SWIR Photodetectors and Its Influence on Optoelectronic Properties. Colloids and Interfaces, 9(4), 49. https://doi.org/10.3390/colloids9040049








