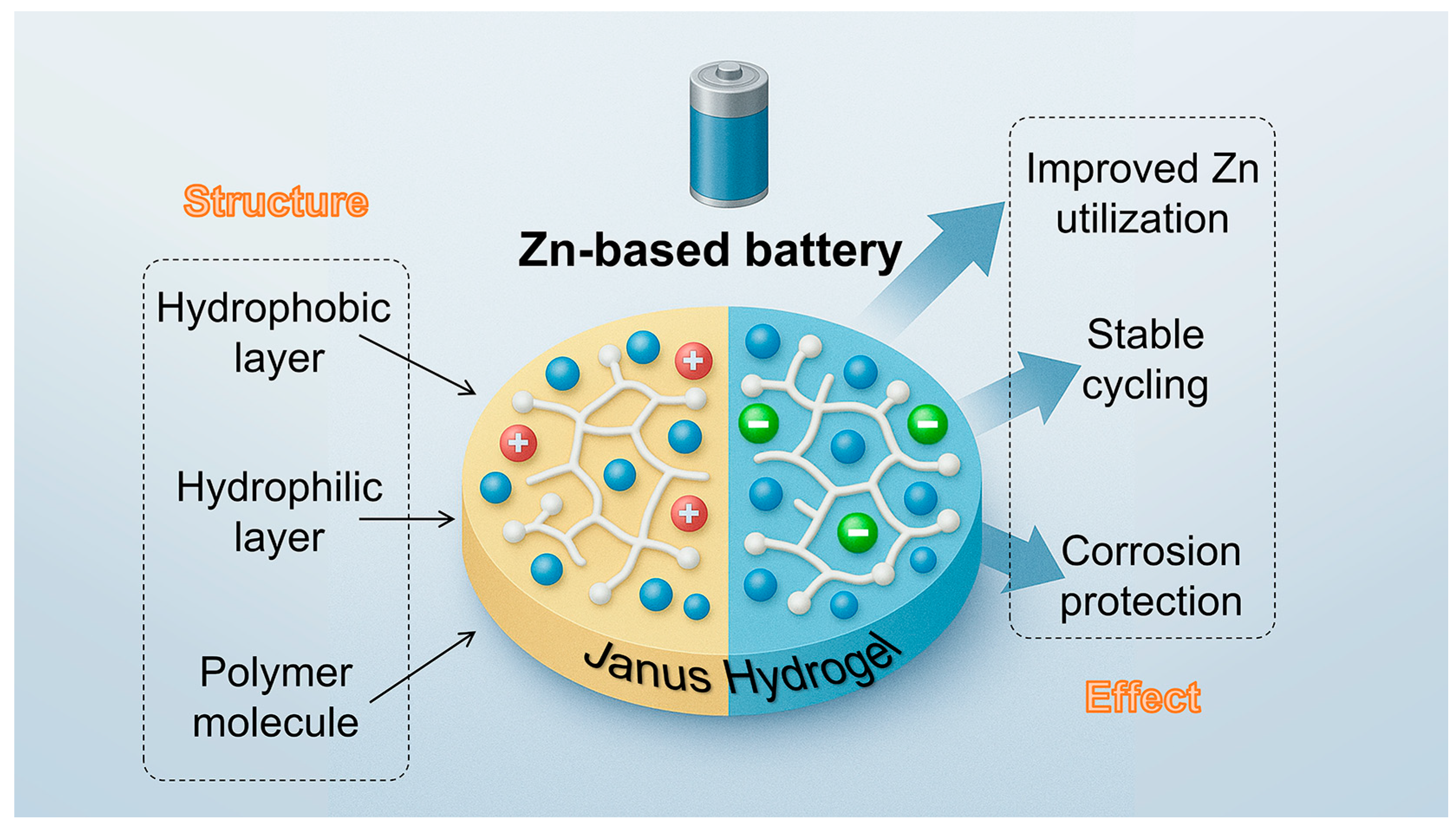
1. Introduction
The continued evolution of energy storage technologies demands materials that can offer not only high ionic conductivity and electrochemical stability but also mechanical flexibility, environmental adaptability, and precise interfacial control [
1,
2,
3]. Traditional hydrogel-based electrolytes—composed of hydrated polymer networks—have attracted widespread interest due to their high water content, tunable chemistry, and mechanical softness. However, most conventional hydrogels are structurally isotropic and functionally homogeneous, limiting their capacity to fulfill the complex, spatially heterogeneous requirements of modern battery architectures, particularly those involving metal anodes, flexible substrates, or multifunctional compartments [
4,
5].
Janus hydrogels, named after the Roman god Janus, who possesses two faces looking in opposite directions, provide a promising solution to these limitations. These hydrogels are characterized by asymmetric structures—either in chemical composition, physical morphology, or functional behavior—across distinct domains or surfaces. Inspired by biological systems such as cellular membranes or skin tissues, Janus hydrogels offer built-in spatial selectivity. This enables, for example, one side to provide ionic conduction while the other functions as a barrier layer, or one region to promote electrode contact while the other resists gas or moisture intrusion [
6,
7].
In energy storage systems, the dual-functional design offers multiple critical advantages by integrating complementary properties within a single structure. First, the presence of hydrophilicity gradients or asymmetrically distributed charged sites enables directional ion transport, effectively enhancing ion mobility and selectivity [
8,
9]. This is further complemented by selective interfacial compatibility, which facilitates improved contact with both electrodes [
10] and electrolytes [
11], thereby reducing interfacial resistance and promoting stable electrochemical performance. Additionally, such systems provide mechanical decoupling, allowing one layer to dissipate stress while preserving the electrochemical integrity of the other [
12,
13], a feature crucial for long-term cycling stability. Finally, their inherent thermal and chemical responsiveness imparts a self-regulating capability, enabling the system to adapt or shut down under abnormal conditions, thus enhancing safety and reliability [
14,
15].
Recent studies have demonstrated that Janus hydrogels can play multiple roles in zinc-based batteries, including functioning as quasi-solid-state electrolytes [
16], artificial solid electrolyte interphase (SEI) [
17], and self-healing interfaces [
18]. Unlike conventional electrolytes, the asymmetric architecture of Janus hydrogels enables the spatial separation of distinct functionalities across their two faces. In lithium–metal systems, one side can be engineered to form a stable and ion-conductive artificial SEI with lithiophilic components, while the opposite side may serve as a barrier to dendritic growth, thereby achieving simultaneous stabilization of both the electrode and electrolyte interface [
19]. Similarly, in aqueous zinc systems, the hydrophilic face can enhance ionic conductivity and wetting at the zinc surface, while the other face can incorporate proton-trapping or hydrogen-suppressing groups to minimize side reactions such as hydrogen evolution [
20]. This spatially directed functionality not only optimizes performance in metal batteries but also makes Janus hydrogels promising for emerging applications in wearable electronics [
21] and biointegrated energy systems [
22], where interface specificity and multifunctionality are crucial (
Figure 1).
This review presents a concise yet comprehensive examination of Janus hydrogels through the lens of advanced energy storage, with an emphasis on their evolving role as intelligent, interface-engineered materials in next-generation batteries. We investigate state-of-the-art fabrication strategies, reveal the intricate structure–property–performance interplays, and assess their impact across diverse battery architectures. Special attention is given to the emergent paradigm of interfacial asymmetry as a design principle for directional ion transport, multifunctional stability, and responsive behavior. This review concludes by outlining key scientific and engineering challenges, while charting a forward-looking roadmap towards scalable manufacturing, adaptive functionality, and integration into autonomous energy systems.
2. Fabrication and Structural Features of Janus Hydrogels
The functional capabilities of Janus hydrogels stem directly from their asymmetric structure. Over the past decade, several fabrication strategies have emerged to generate spatially heterogeneous hydrogels, each offering varying degrees of morphological control, process scalability, and compatibility with electroactive materials.
2.1. Interfacial Polymerization
Interfacial polymerization is a fundamental technique in which polymer formation occurs at the interface between two immiscible or partially miscible phases. This method enables the construction of distinct layered architectures with spatially localized functionalities. For example, in a previous study, a Janus asymmetric hydrogel electrolyte (AHE) was fabricated by sequentially integrating two polymer networks with tailored roles (
Figure 2a). Initially, a mixture of polyvinyl alcohol (PVA), poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) (PEDOT:PSS), and glutaraldehyde was cast and treated with acetic acid to form a dense, conductive PVA–PEDOT hydrogel with enhanced mechanical strength on the cathode-facing side. Subsequently, a sodium alginate–carrageenan solution was layered on top and crosslinked with Zn
2+ ions to generate a robust Carra-Zn-Alg hydrogel on the anode side. Strong hydrogen bonding at the interfacial region ensured tight adhesion and avoided delamination during swelling. This stepwise interfacial polymerization yielded a dual-layer hydrogel with asymmetric wettability and mechanical properties, significantly enhancing Zn
2+ transport and stabilizing the Zn/electrolyte interface in Zn–I
2 batteries [
23].
2.2. Gradient Curing
Gradient curing refers to a strategy that induces spatial variation in the degree of crosslinking or polymerization through differences in light exposure, initiator concentration, or component diffusion. Unlike sharply defined interfaces, this approach produces smooth, continuous gradients in regard to physical or chemical properties across the hydrogel. One notable example employed interfacial ignition driven by the density difference between a monomer precursor and a concentrated salt solution (e.g., LiCl or ZnCl
2). A redox-initiated polymerization front formed a barrier layer at the interface, resulting in a vertical gradient in crosslinking density—from high at the top to low at the bottom. The resulting Janus gradient hydrogel exhibited pressure-dependent conductivity, opening new possibilities for integrating sensing and energy storage functionalities [
24].
Figure 2.
Strategy for fabricating a Janus adhesive hydrogel: (
a) illustration of the preparation process for the AHE [
23]; (
b) stick–slip surface of the Janus adhesive hydrogel and its application [
25].
Figure 2.
Strategy for fabricating a Janus adhesive hydrogel: (
a) illustration of the preparation process for the AHE [
23]; (
b) stick–slip surface of the Janus adhesive hydrogel and its application [
25].
In another case, gradient crosslinking was achieved via masked or directional ultraviolet (UV) curing, where specific regions of a Pregel matrix were selectively exposed to UV light (
Figure 2b). Using this method, a Janus-structured conductive hydrogel was prepared by polymerizing sulfobetaine methacrylate (SBMA) into poly(sulfobetaine methacrylate) (PSBMA) under UV irradiation, with PVA forming a self-crosslinked backbone. Tannic acid (TA) was then introduced from one side through unidirectional dipping, forming a surface-specific modification (TA@PVA/PSBMA). The resulting hydrogel exhibited strong adhesion on the TA-modified side and excellent oil resistance on the PSBMA side, enabling antifouling and self-adhering capabilities in oil–water complex environments—ideal for wearable sensor applications [
25].
2.3. Layer-by-Layer Integration
Layer-by-layer (LbL) casting is a modular technique wherein distinct hydrogel precursors are sequentially cast to create multilayered structures with spatially defined mechanical and ionic functionalities. A notable example is the development of an accordion-structured hydrogel battery (ASHB), which employs a foldable paper–gel assembly inspired by the morphology of biological organs (
Figure 3a). The distinctive accordion geometry functions as an ionic isolation barrier, significantly improving the retention of ionic gradients compared to traditional laminated configurations. This structure minimizes spontaneous ion migration during storage and enables ionic gradient retention for over 30 h. The ASHB design represents a promising direction for next-generation flexible electronics by offering enhanced modularity, portability, and long-term electrochemical performance [
26].
Further advances have leveraged sacrificial templates and anisotropic swelling agents during gelation to construct uniaxial porosity gradients. These gradients form directional ion transport channels that enhance through-thickness conductivity while minimizing lateral ionic dispersion. Although not a morphologically Janus structure in the strictest sense, such anisotropic architectures embody a core Janus design principle: directionally resolved ion transport. For instance, incorporating layered double hydroxides (LDHs) into the porous matrix yielded a dual-network hydrogel with a uniaxial pore orientation that facilitated OH
− transport through a synergistic combination of the Grotthuss and vehicle mechanisms (
Figure 3b) [
27]. Computational studies based on COMSOL Multiphysics simulations and activation energy analysis revealed that the Grotthuss mechanism dominated OH
− migration within the LDH-containing hydrogel, evidenced by a significantly reduced ion transport activation energy (9.14 kJ·mol
−1) compared to control samples. Simulated electric field and concentration profiles further confirmed reduced internal resistance and steeper OH
− transport flux in the LDH/carboxymethyl cellulose (CMC)/polyacrylamide (PAM) system. Experimentally, this hydrogel demonstrated high ionic conductivity (145.93 mS·cm
−1 at room temperature), extended battery cycling (>160 h), and excellent mechanical resilience (530% strain, 0.19 MPa stress) when used as a Zn–air battery electrolyte. These characteristics stem from anisotropic ion pathways and justify its inclusion as a Janus-inspired design.
3. Key Functional Properties of Janus Hydrogels in Battery
Janus hydrogels, by virtue of their spatially resolved architectures, exhibit a suite of physicochemical properties that directly address critical limitations in both aqueous and solid-state battery systems. Their key functionalities include directional ion transport, interfacial stabilization, mechanical adaptability, and environmental responsiveness—each arising from the rational asymmetry encoded in the hydrogel design.
3.1. Directional Ion Transport
Conventional hydrogels typically allow isotropic ion movement, which can result in uncontrolled migration, ion crossover, or uneven electrodeposition. In contrast, Janus hydrogels exploit ionic selectivity and structural anisotropy to promote unidirectional ion flux. This is especially valuable in systems prone to dendritic growth or electrolyte decomposition [
28].
One common approach involves incorporating fixed charge groups—such as sulfonic acid (−SO
3−) [
29], carboxylate (−COO
−) [
30], or ammonium groups—into one region of the hydrogel [
31]. These fixed charges facilitate Donnan exclusion, in which counter ions (e.g., Zn
2+, Li
+) are preferentially absorbed and conducted while co-ions are repelled. When localized within a layered or gradient domain, this mechanism creates a chemical potential gradient that reinforces directional transport [
30,
32].
To overcome the limitations posed by high water reactivity in conventional aqueous and hydrogel electrolytes, particularly under elevated temperatures, a non-conventional, Janus-inspired hydrogel (HPG, a hydrogel composed of poly(ethylene glycol) methyl ether methacrylate (PMEM) and N-hydroxyethyl acrylamide (HEAA)) was rationally designed by Zhi et al. (
Figure 4a) [
33]. This system forms a dual-functional polymer network in which the flexible ether oxygen units of PMEM coordinate Zn
2+ transport, while the hydrophilic HEAA segments immobilize water molecules via hydrogen bonding, effectively reducing free water content and water-induced side reactions. While this hydrogel does not exhibit apparent morphological anisotropy, its molecular-level division of function between domains leads to a spatially resolved functional asymmetry. As a result, the HPG hydrogel achieves a high ionic conductivity of 3.9 × 10
−3 S·cm
−1 at 35 wt% water content and a significantly expanded electrochemical stability window (
Figure 4b).
This asymmetrically functionalized hydrogel demonstrates remarkable temperature adaptability: symmetric Zn||Zn and Zn||Ti cells achieved >7500 and 5500 h of stable cycling at room temperature, and around 99% coulombic efficiency even at 90 °C, highlighting its robustness under harsh thermal conditions. In full Zn||Zn0.25V2O5·nH2O (ZVO) batteries, the HPG electrolyte enabled 2300 cycles with 92% capacity retention at 0.7 A·g−1, and 90% retention after 750 cycles at 90 °C with about 100% Coulombic efficiency.
These findings confirm that molecularly engineered Janus hydrogels with distinct functional domains can simultaneously ensure efficient Zn2+ migration and thermal resilience, offering a new strategy for high-performance, environment-adaptable aqueous zinc batteries.
3.2. Interfacial Regulation and Electrochemical Stability
The success of batteries is often governed by the quality of the electrode–electrolyte interface. In Janus hydrogels, one face can be tailored to match the chemical and mechanical characteristics of the active electrode, forming a stable, adaptive contact layer. The other face can act as a moisture barrier, electronic insulator, or an anti-gas diffusion layer [
34].
In aqueous zinc–ion batteries (ZIBs), Janus hydrogels reduce hydrogen evolution reaction (HER) by spatially decoupling the Zn-plating surface from the bulk electrolyte. The hydrophobic or chemically inert outer side inhibits water penetration, while the inner layer supports ion transport and stabilizes Zn
2+ nucleation via chelation or adsorption. Hao et al. presented a design of a Janus hydrogel electrolyte based on a combination of deep hydrophilic eutectic solvent (DES, composed of choline chloride and ethylene glycol, [ChCl]EG) and a hydrophobic ionic liquid (IL, 1-allyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, [AMIm]TFSI), realized through a one-step UV polymerization strategy that transforms a biphasic liquid Janus system into a solid-state gel [
35]. The Janus architecture comprises a hydrophilic DES phase ([ChCl]EG) and a hydrophobic IL phase ([AMIm]TFSI), connected via a miscible interfacial transition layer formed by the copolymerization of acrylic acid (AA) and trifluoroethyl methacrylate (TFEMA). This transition layer ensures structural continuity and minimal interfacial impedance between the two chemically distinct regions. In function, the DES side exhibits excellent reduction resistance due to the hydrogen bonding between choline chloride and ethylene glycol, which restricts hydrogen atom mobility. Meanwhile, the IL side offers high oxidative stability, thanks to the inherent electrochemical robustness of the TFSI
− anion, providing superior compatibility with cathode materials. This dual functionality enables the Janus hydrogel to achieve a broad electrochemical stability window (ESW) of 3.6 V, along with outstanding thermal tolerance (operational from −20 to 90 °C), anti-freezing behavior (freezing point of −81.6 °C), and high solvent retention (97.47% at 25 °C over 21 days). Moreover, the hydrogel exhibits a high ionic conductivity of 3.57 mS·cm
−1 and excellent long-term cycling stability, with 80.6% capacity retention and 99.9% coulombic efficiency after 10,000 charge–discharge cycles (
Figure 4c,d). When used in a supercapacitor, it delivers an impressive energy density of 89.4 Wh·kg
−1 at a power density of 646.2 W·kg
−1.
3.3. Mechanical Modulation and Crack Suppression
Janus hydrogels frequently feature mechanical heterogeneity, where tough and stretchable components are asymmetrically distributed. This design provides built-in stress dissipation mechanisms, accommodating the swelling, shrinking, or volume changes in electrodes during cycling or thermal fluctuation.
A compelling example of utilizing structural engineering to enhance the mechanical robustness of gel-based electrolytes is demonstrated in a dual-network hydrogel system composed of poly(acrylic acid) (PAA) and PAM. Although not a classic Janus configuration in terms of compositional asymmetry, this system embodies the principle of functional compartmentalization that underpins the Janus design—achieving a balance between mechanical integrity and ionic transport through spatially differentiated structural features.
Specifically, Zhang et al. designed a PAA–PAM hydrogel that was fabricated via a stepwise crosslinking and secondary swelling strategy, forming a hierarchical pore architecture that spans from nanometer to micrometer scale [
36]. X-ray tomography revealed that the micropores generated during secondary swelling act as stress-dissipating nodes, enabling the gel to withstand pressures up to 984 kPa, a record-breaking mechanical strength for hydrogel electrolytes. Simultaneously, macro-porous channels formed within the polymer networks facilitate exceptional ionic conductivity of 205 mS·cm
−1, which is several times higher than conventional isotropic hydrogels. Although the structure is not explicitly layered, the resulting gradient in mechanical and transport properties across the gel creates a spatially anisotropic performance profile. When applied in zinc–air battery systems—including both button cells and flexible Zn–air batteries (FZABs)—this hydrogel electrolyte supported continuous operation for over 120 h, with stable voltage output and structural integrity (
Figure 4e,f).
Figure 4.
(
a) Schematic of the proposed hydrogel electrolyte. (
b) The comparison of ionic conductivity of different electrolytes: Introducing a 2,6-bis((propylimino)methyl)-4-chlorophenol (Hbimcp) ligand into the poly(propylene oxide) (PPO) polymer chain (PHP), heterolytic coordination polymer electrolytes (HCPE), Zn
2+-conducting solid-state electrolytes (ZCE), and precursor monomer to build in situ polymerized Zn
2+ SPEs (PPM) [
33]. (
c) Galvanostatic charge–discharge curves of the supercapacitor at 2 A/g for 10,000 cycles. The inserts are the zoomed-in figures on the initial and end localizations of the cycles. (
d) Cycling performance of the supercapacitors based on Janus gel and hetero-network gel as electrolytes [
35]. (
e) Electrochemical stability windows of PAA, PAM, and PAA-PAM. (
f) Stress–strain curves of polymer gels [
36].
Figure 4.
(
a) Schematic of the proposed hydrogel electrolyte. (
b) The comparison of ionic conductivity of different electrolytes: Introducing a 2,6-bis((propylimino)methyl)-4-chlorophenol (Hbimcp) ligand into the poly(propylene oxide) (PPO) polymer chain (PHP), heterolytic coordination polymer electrolytes (HCPE), Zn
2+-conducting solid-state electrolytes (ZCE), and precursor monomer to build in situ polymerized Zn
2+ SPEs (PPM) [
33]. (
c) Galvanostatic charge–discharge curves of the supercapacitor at 2 A/g for 10,000 cycles. The inserts are the zoomed-in figures on the initial and end localizations of the cycles. (
d) Cycling performance of the supercapacitors based on Janus gel and hetero-network gel as electrolytes [
35]. (
e) Electrochemical stability windows of PAA, PAM, and PAA-PAM. (
f) Stress–strain curves of polymer gels [
36].
The ability to fine-tune the pore architecture via swelling conditions offers a promising direction for the development of Janus-like hydrogels with gradient mechanical properties, where one layer may provide strain-adaptive compliance and the other reinforces load-bearing capability. This finding emphasizes the relevance of spatially organized dual-network or Janus-like hydrogels in suppressing material collapse during battery operation and provides a strategic framework for designing future electrolyte systems that simultaneously meet the demands of mechanical durability and high ionic transport.
4. Discussion
4.1. Zinc–Ion Batteries (ZIBs)
ZIBs have attracted considerable attention for grid-scale and flexible energy storage due to their intrinsic safety, low cost, and environmental friendliness. However, ZIBs are plagued by interfacial instability at the Zn anode, including uncontrolled dendrite formation, parasitic hydrogen evolution, and electrode passivation. These challenges stem from the aqueous nature of the electrolyte and the high charge density of Zn2+ ions. Specifically, the divalent nature and strong electrostatic interactions of Zn2+ lead to non-uniform ion flux and localized Zn2+ accumulation at the electrode interface during cycling, which promotes uneven nucleation and growth of Zn metal. This uneven deposition facilitates the formation of dendritic structures that can pierce the separator, posing serious safety and performance risks.
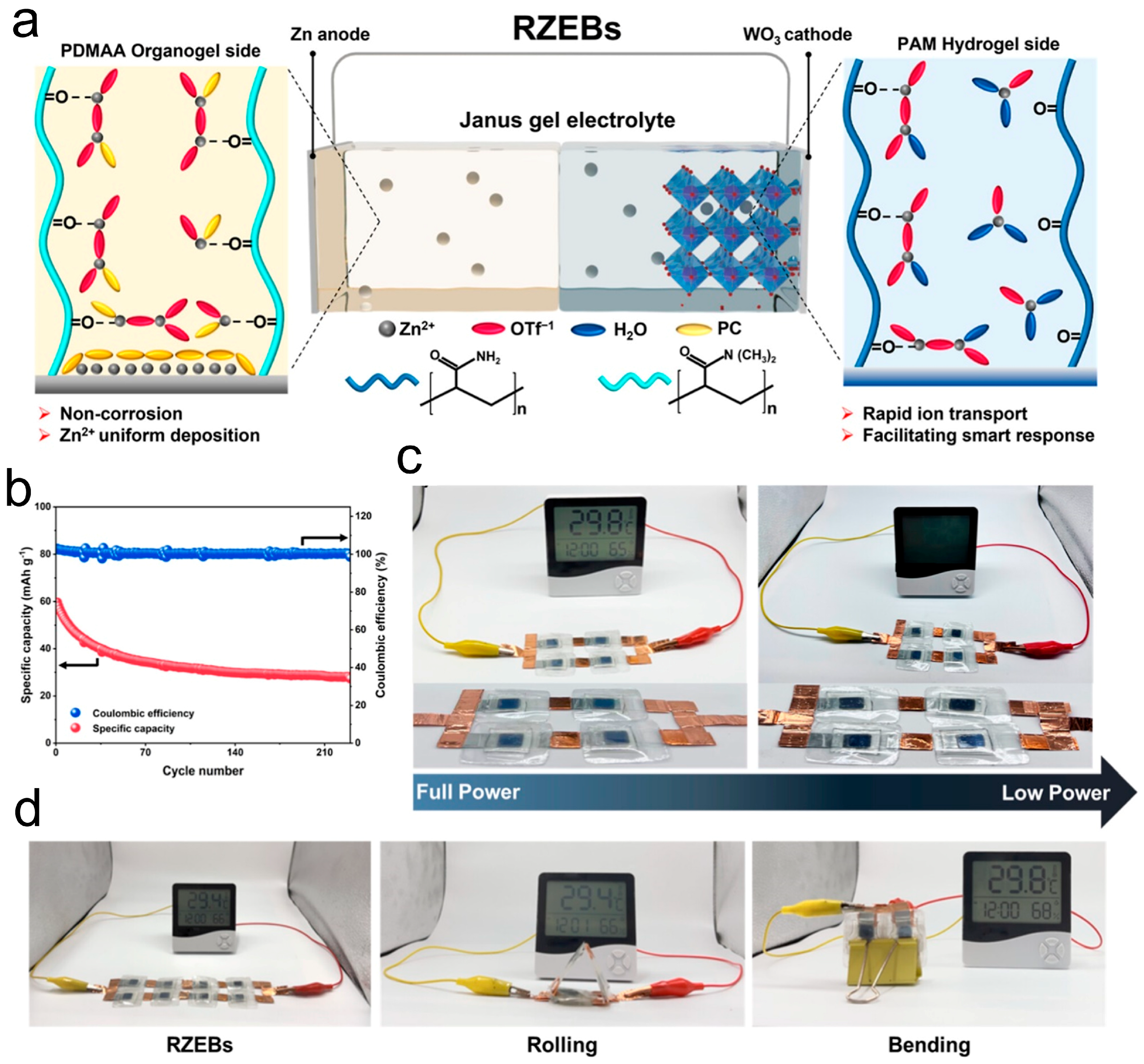
To address these persistent limitations, Janus hydrogel electrolytes offer a unique approach by enabling spatially resolved ion transport and interface protection within a single gel matrix. A compelling example is demonstrated in the development of rechargeable electrochromic Zn-ion batteries (RZEBs), where energy storage is coupled with dynamic color-switching functionality. Yan et al. designed a Janus gel electrolyte that was constructed by integrating a hydrophobic organogel layer composed of propylene carbonate-based poly(N,N-dimethylacrylamide) (PDMAA) and a hydrophilic hydrogel layer of PAM [
37]. This asymmetric structure effectively addresses the inherent limitations of aqueous Zn systems, such as dendrite formation, HER, and Zn corrosion.
The hydrophobic PDMAA organogel, positioned adjacent to the Zn anode, acts as a physical and chemical barrier that isolates water molecules, thereby mitigating parasitic reactions and suppressing dendrite growth. Moreover, the organogel contains zincophilic sites that enable uniform Zn deposition and contribute to a stabilized Zn interface. On the other side, the PAM hydrogel provides a high-ionic-conductivity environment for the rapid Zn
2+ insertion/extraction at the electrochromic cathode, ensuring efficient energy storage and color switching (
Figure 5a). This Janus configuration results in remarkable electrochemical performance, with Zn||Cu half-cells achieving a Coulombic efficiency of 97.91%, and Zn||WO
3 full batteries delivering a specific capacity of 51.2 mAh·g
−1 and maintaining 91.7% capacity retention after 100 cycles (
Figure 5b). Additionally, the battery demonstrated stable and reversible optical transitions between sky-blue and black states, confirming its applicability in transparent or smart display energy systems (
Figure 5c,d). This work reveals the multifunctional advantages of Janus hydrogels in aqueous Zn-ion batteries, namely simultaneously enhancing cycling stability, suppressing water-related degradation, and supporting fast ion transport, making them a powerful electrolyte design strategy for advanced multifunctional battery systems.
This example underscores how Janus hydrogels can effectively mitigate Zn anode degradation while enabling multifunctional energy storage, thus offering a strategic electrolyte design for next-generation aqueous ZIBs.
4.2. Zinc–Air Batteries (ZABs)
ZABs are promising for high-energy-density applications due to their lightweight design and abundant raw materials. However, traditional alkaline electrolytes suffer from several drawbacks, such as rapid water evaporation, dendrite growth, Zn anode passivation, and CO2 carbonation at the air cathode. These limitations become more severe under ambient air exposure and mechanical deformation, which are common in wearable applications.
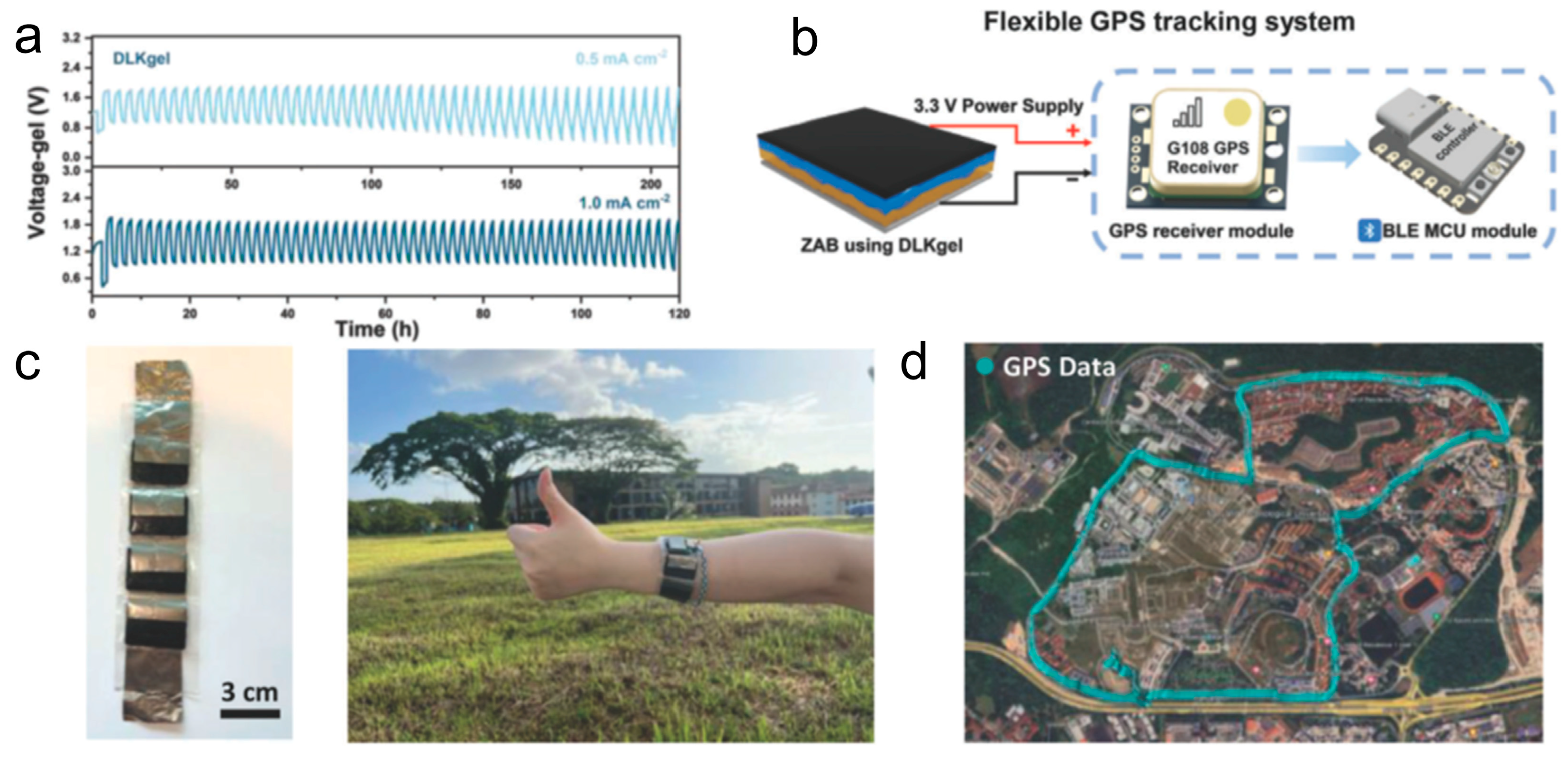
To overcome these limitations, Fan et al. developed a Janus-type, double-layer kosmotropic gel (DLKgel), leveraging the differing kosmotropic behaviors of ZnCl
2 and ZnSO
4 salts [
38]. The DLKgel comprises two functionally distinct layers within a single gel matrix: a ZnCl
2/CaCl
2 cellulose hydrogel positioned near the air cathode, and a ZnSO
4–Poly(acrylamide-acrylic acid-methylacrylamide) (PAAM) hydrogel adjacent to the Zn anode. This design enables spontaneous vertical phase separation without requiring a physical separator.
The ZnCl
2-rich layer promotes efficient Zn
2+ transport and mitigates the formation of irreversible by-products such as Zn hydroxysulfates. The ZnSO
4 layer stabilizes the Zn anode through strong sulfate coordination, suppressing passivation and corrosion. This spatially asymmetric structure decouples ion transport functions while enhancing overall electrochemical stability. Compared to conventional alkaline or neutral gel electrolytes, the DLKgel demonstrates superior cycling stability, maintaining smooth operation for over 200 h at 0.5 mA cm
−2 and 120 h at 1.0 mA cm
−2 (
Figure 6a).
As a practical demonstration, the DLKgel-powered flexible ZAB was implemented in a wearable global positioning system (GPS) tracker, the battery was engineered to deliver a consistent voltage of 3.3 V, which can sustain the operation of the GPS chip (
Figure 6b). These features highlight the potential of structural electrolyte engineering in extending the lifetime and practicality of ZABs for portable and wearable electronics (
Figure 6c,d).
This work demonstrates how Janus hydrogel electrolytes can address the dual challenges of electrolyte degradation and mechanical adaptability, thus enabling the development of reliable, application-specific ZABs.
4.3. Flexible and Wearable Batteries
The proliferation of wearable electronics such as health monitors, smart textiles, and epidermal sensors has created an urgent need for energy storage devices that are lightweight, skin-compatible, and resilient under mechanical stress. Conventional liquid or bulk polymer electrolytes struggle to meet these criteria due to issues such as leakage, rigidity, and poor ionic interface stability.
To resolve these challenges, Yang et al. developed a Janus poly(AM-co-Zn-AC-co-NIPAM-co-MBAA) (PAZPM) hydrogel with asymmetric wettability and gradient pore structures for Zn-ion pouch cells [
39]. The hydrophilic surface, with large pores, facilitates rapid proton insertion at the cathode, while the hydrophobic side, with dense pores, limits water access and stabilizes Zn deposition at the anode. This structural asymmetry balances ion accessibility with anode protection.
The Janus PAZPM hydrogel was employed in flexible Zn||(NH
4)
2V
10O
25·8H
2O (NVO) pouch cells (
Figure 7a). The resulting devices demonstrated outstanding mechanical flexibility and electrochemical performance. Pouch cells delivered a capacity of 48 mAh and retained 85% capacity after 150 cycles (
Figure 7b). To highlight real-world potential, two Zn||NVO pouch cells connected in series were shown to reliably power a 76-LED array (“DICP 504”) (
Figure 7c) as well as an electronic timer (
Figure 7d), even under bending, cutting, or weight-loading conditions. Beyond the NVO cathode, the Janus PAZPM hydrogel also enabled stable cycling in a flexible Zn||polyaniline (PANI) pouch cell, which delivered a high specific capacity of 154 mAh g
−1 and 80% retention after 2800 cycles at a current density of 2 A g
−1, demonstrating the broad compatibility of the Janus electrolyte with different cathode chemistries.
This example illustrates how Janus hydrogels enable multifunctionality in wearable battery systems, offering tunable ion transport, structural robustness, and environmental adaptability. Their self-healing and stress-responsive capabilities further enhance their potential in dynamic and biocompatible energy systems.
In all these systems, Janus hydrogels play a transformative role in decoupling conflicting electrolyte functions—such as ion conductivity versus electrode protection—through spatial asymmetry. By doing so, they unlock new design pathways for high-performance, safe, and application-specific energy storage technologies. Compared to conventional liquid electrolytes, Janus hydrogels offer substantial advantages in addressing interfacial instability, dendrite formation, and electrolyte leakage. Traditional aqueous electrolytes used in ZIBs typically operate at low current densities (<1 mA·cm−2) with moderate cycling lifespans (~200–500 h), whereas Janus gel-based systems demonstrate extended stability (>800 h) and high Zn utilization (>85%). In lithium–ion systems, standard carbonate-based electrolytes face challenges under mechanical strain and are prone to flammability. Solid polymer electrolytes (e.g., polyethylene oxide (PEO)-based) offer improved safety but suffer from poor room temperature conductivity (~10−5 S·cm−1). In contrast, Janus hydrogel electrolytes can achieve ionic conductivities exceeding 10−3 S·cm−1 under ambient conditions while maintaining structural flexibility and electrochemical resilience, particularly in wearable or deformable environments. These comparisons reveal that the Janus hydrogel architecture is not merely a novel structure but addresses system-level challenges across electrolyte performance, mechanical compliance, and operational durability.
5. Challenges and Outlook
Despite the significant progress in Janus hydrogel design and the demonstrated utility of such hydrogels across various battery systems, several critical challenges must be addressed to realize their practical deployment in commercial energy storage technologies.
One major hurdle lies in the scalability and manufacturing control of current fabrication methods. The above techniques are often labor-intensive, environmentally sensitive, and unsuitable for large-area or high-throughput production. Achieving consistent batch-to-batch reproducibility—particularly in terms of interfacial alignment and crosslinking uniformity—remains difficult. To enable industrial translation, future research must focus on scalable, roll-to-roll, or printing-compatible manufacturing approaches that retain spatial precision while improving throughput and cost-efficiency.
Another pressing issue is the mechanical integrity of Janus hydrogels, particularly interfacial delamination under operational stress. Swelling mismatch, thermal gradients, or directional ion flux during cycling can weaken interfacial adhesion between asymmetrical domains, leading to performance degradation or even device failure. Enhancing interfacial robustness through strategies such as semi-interpenetrating polymer networks, dynamic covalent bonding, or modulus-gradient design is essential for ensuring mechanical durability, especially in flexible or wearable applications.
Electrochemical and environmental stability also remain limiting factors. The water-rich nature of most Janus hydrogels restricts their compatibility with high-voltage or moisture-sensitive systems such as lithium-based chemistries. Challenges such as evaporation, hydrolysis, and parasitic reactions—particularly at reactive metal interfaces—can reduce cycling life and raise safety concerns. Potential solutions include the incorporation of ionic liquids, low-volatility additives, or protective encapsulation layers to expand the electrochemical window and improve operational stability. Furthermore, the long-term biostability and ionic leakage under ambient conditions in wearable devices are still underexplored and warrant deeper investigation.
From a fundamental standpoint, the lack of comprehensive mechanistic understanding limits the rational design of Janus hydrogel systems. The impact of structural asymmetry on ion transport, charge transfer, and failure pathways remains poorly quantified. Future research should leverage in situ or operando characterization tools—such as electrochemical impedance spectroscopy, neutron scattering, and cryo-TEM—alongside multiscale computational models to elucidate structure–property–performance relationships and inform predictive design strategies.
Regarding future prospects, the next generation of Janus hydrogels is expected to incorporate adaptive, self-healing, and stimuli-responsive features. Drawing inspiration from biological systems, integrating multifunctional layers (e.g., photothermal and ion-conductive) and intelligent responsiveness (e.g., humidity-activated conductivity) will unlock broader applications beyond energy storage, including soft electronics, biosensors, and robotics. Coupling Janus hydrogels with microfluidics, soft circuitry, or AI-assisted diagnostics may ultimately enable autonomous, self-regulating electrochemical platforms for real-time monitoring and therapeutic interventions. Janus hydrogels represent more than just separators or ion conductors; they are programmable, multifunctional materials with the potential to redefine the landscape of next-generation smart electrochemical systems.
6. Conclusions
Janus hydrogels, with their asymmetric structure and dual-function interfaces, have emerged as a promising frontier in the development of next-generation solid-state electrolytes. By enabling directional ion transport, enhanced interfacial stability, and effective dendrite suppression, they provide innovative solutions to longstanding challenges in lithium-based and zinc-based batteries. This review has summarized recent progress in their structural design and fabrication, revealing how spatial control over ion dynamics and mechanical gradients can be strategically harnessed for electrochemical performance enhancement.
In terms of future prospects, Janus hydrogels hold vast potential beyond current applications. Their inherent design flexibility makes them ideal candidates for integration into a wide spectrum of emerging battery systems—including flexible and wearable electronics, implantable biomedical devices, and high-energy-density solid-state batteries. Moreover, the ability to couple stimuli-responsive, self-healing, and adaptive functionalities further positions Janus hydrogels as transformative materials in intelligent energy storage platforms. Realizing their full potential will require advances in scalable fabrication, long-term electrochemical stability, and deeper understanding of the structure–function relationship at the interface. Nonetheless, the unique convergence of multifunctionality and asymmetry in Janus hydrogels offers a powerful blueprint for engineering the next generation of smart, safe, and high-performance battery systems.