Polyvinylpyrrolidone-Capped CuInS2 Colloidal Quantum Dots: Synthesis, Optical and Structural Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. PVP-Capped CuInS2 QDs Synthesis
2.3. Methods
2.4. Computational Details
2.5. Cell Culture and Processing
2.6. Imaging and Signal Analysis
3. Results and Discussion
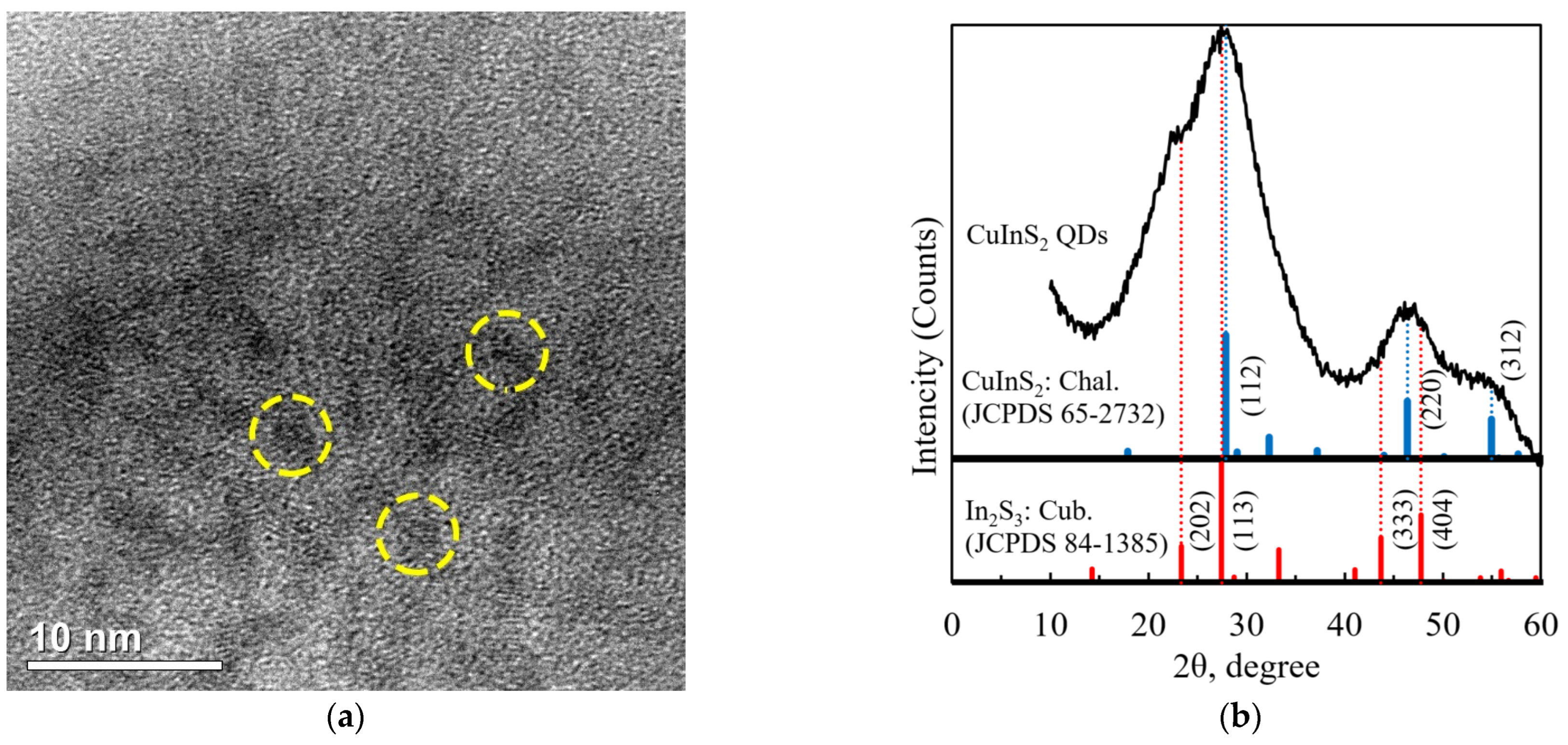
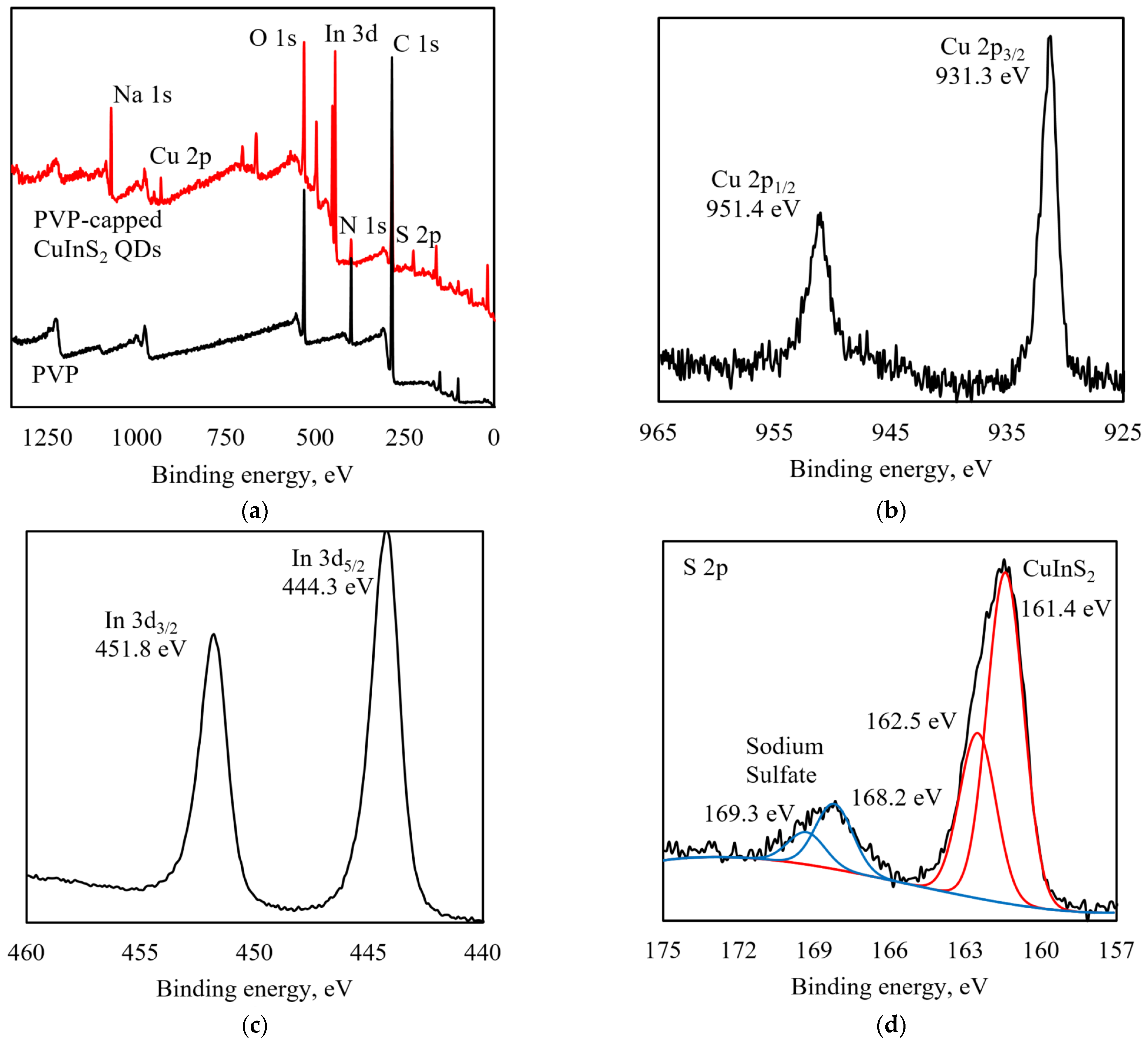
3.1. Structure Characterization
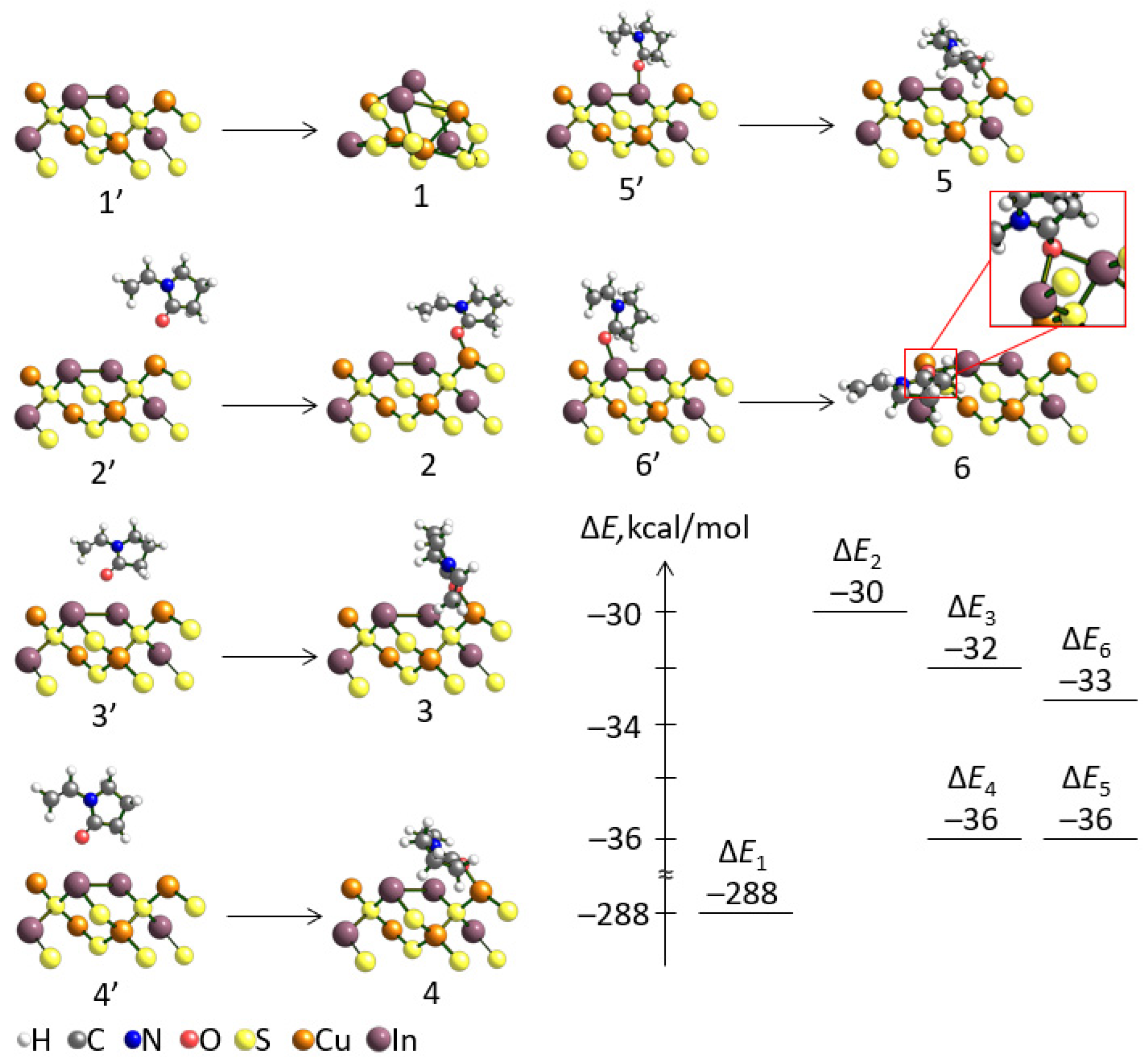
3.2. Theoretical Calculations
3.3. Optical Properties of PVP-Capped CuInS2 QDs
3.4. Live Cell Imaging Using PVP-Capped CuInS2 QDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFT | Density functional theory |
| FTIR | Fourier-transform infrared |
| PL | Photoluminescence |
| PVP | Polyvinylpyrrolidone |
| QDs | Quantum dots |
| TEM | Transmission electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Castro, S.L.; Bailey, S.G.; Raffaelle, R.P.; Banger, K.K.; Hepp, A.F. Synthesis and Characterization of Colloidal CuInS2 Nanoparticles from a Molecular Single-Source Precursor. J. Phys. Chem. B 2004, 108, 12429–12435. [Google Scholar] [CrossRef]
- Kino, T.; Kuzuya, T.; Itoh, K.; Sumiyama, K.; Wakamatsu, T.; Ichidate, M. Synthesis of Chalcopyrite Nanoparticles via Thermal Decomposition of Metal-Thiolate. Mater. Trans. 2008, 49, 435–438. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, Y.; Ye, M.; He, Y.; Ye, J.; He, C.; Yang, C.; Li, Y. Controlled Synthesis and Optical Properties of Colloidal Ternary Chalcogenide CuInS 2Nanocrystals. Chem. Mater. 2008, 20, 6434–6443. [Google Scholar] [CrossRef]
- Uehara, M.; Watanabe, K.; Tajiri, Y.; Nakamura, H.; Maeda, H. Synthesis of CuInS2 Fluorescent Nanocrystals and Enhancement of Fluorescence by Controlling Crystal Defect. J. Chem. Phys. 2008, 129, 134709. [Google Scholar] [CrossRef]
- Nose, K.; Omata, T.; Otsuka-Yao-Matsuo, S. Colloidal Synthesis of Ternary Copper Indium Diselenide Quantum Dots and Their Optical Properties. J. Phys. Chem. C 2009, 113, 3455–3460. [Google Scholar] [CrossRef]
- Korepanov, O.A.; Mazing, D.S.; Aleksandrova, O.A.; Moshnikov, V.A. Synthesis and Study of Colloidal Nanocrystals Based on Ternary Chalcogenides for Active Media of Heavy Metal Ions Sensors. In Proceedings of the 2019 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (EIConRus), Saint Petersburg and Moscow, Russia, 28–31 January 2019; pp. 771–773. [Google Scholar]
- Chen, B.; Zhong, H.; Zhang, W.; Tan, Z.; Li, Y.; Yu, C.; Zhai, T.; Bando, Y.; Yang, S.; Zou, B. Highly Emissive and Color-Tunable CuInS2-Based Colloidal Semiconductor Nanocrystals: Off-Stoichiometry Effects and Improved Electroluminescence Performance. Adv. Funct. Mater. 2012, 22, 2081–2088. [Google Scholar] [CrossRef]
- Aldakov, D.; Lefrançois, A.; Reiss, P. Ternary and Quaternary Metal Chalcogenide Nanocrystals: Synthesis, Properties and Applications. J. Mater. Chem. C 2013, 1, 3756. [Google Scholar] [CrossRef]
- Korepanov, O.A.; Mazing, D.S.; Aleksandrova, O.A.; Moshnikov, V.A.; Komolov, A.S.; Lazneva, E.F.; Kirilenko, D.A. Formation of AgInS2/ZnS Colloidal Nanocrystals and Their Photoluminescence Properties. Phys. Solid State 2019, 61, 2325–2328. [Google Scholar] [CrossRef]
- Jain, S.; Bharti, S.; Bhullar, G.K.; Tripathi, S.K. I-III-VI Core/Shell QDs: Synthesis, Characterizations and Applications. J. Lumin. 2020, 219, 116912. [Google Scholar] [CrossRef]
- Moodelly, D.; Kowalik, P.; Bujak, P.; Pron, A.; Reiss, P. Synthesis, Photophysical Properties and Surface Chemistry of Chalcopyrite-Type Semiconductor Nanocrystals. J. Mater. Chem. C 2019, 7, 11665–11709. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Armin, A.; Meredith, P.; Sargent, E.H. Solution-Processed Semiconductors for next-Generation Photodetectors. Nat. Rev. Mater. 2017, 2, 16100. [Google Scholar] [CrossRef]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building Devices from Colloidal Quantum Dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Yang, J.; Hyeon, T.; Kim, D.-H. Flexible Quantum Dot Light-Emitting Diodes for next-Generation Displays. npj Flex. Electron. 2018, 2, 10. [Google Scholar] [CrossRef]
- Xu, J.; Voznyy, O.; Liu, M.; Kirmani, A.R.; Walters, G.; Munir, R.; Abdelsamie, M.; Proppe, A.H.; Sarkar, A.; García de Arquer, F.P.; et al. 2D Matrix Engineering for Homogeneous Quantum Dot Coupling in Photovoltaic Solids. Nat. Nanotechnol. 2018, 13, 456–462. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.; Wang, W.; Qian, N.; Ji, X. Highly Efficient Zn–Cu–In–Se Quantum Dot-Sensitized Solar Cells through Surface Capping with Ascorbic Acid. ACS Appl. Mater. Interfaces 2019, 11, 6927–6936. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Matea, C.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.-Y. Synthetic Strategies and Biomedical Applications of I–III–VI Ternary Quantum Dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef]
- Zrazhevskiy, P.; Gao, X. Quantum Dot Imaging Platform for Single-Cell Molecular Profiling. Nat. Commun. 2013, 4, 1619. [Google Scholar] [CrossRef]
- Benninger, R.K.P.; Piston, D.W. Two-Photon Excitation Microscopy for the Study of Living Cells and Tissues. Curr. Protoc. Cell Biol. 2013, 59, 4–11. [Google Scholar] [CrossRef]
- Xu, H.; Xu, J.; Wang, X.; Wu, D.; Chen, Z.G.; Wang, A.Y. Quantum Dot-Based, Quantitative, and Multiplexed Assay for Tissue Staining. ACS Appl. Mater. Interfaces 2013, 5, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Liu, J.; Haley, K.N.; Treadway, J.A.; Larson, J.P.; Ge, N.; Peale, F.; Bruchez, M.P. Immunofluorescent Labeling of Cancer Marker Her2 and Other Cellular Targets with Semiconductor Quantum Dots. Nat. Biotechnol. 2003, 21, 41–46. [Google Scholar] [CrossRef]
- Korepanov, O.; Aleksandrova, O.; Firsov, D.; Kalazhokov, Z.; Kirilenko, D.; Kozodaev, D.; Matveev, V.; Mazing, D.; Moshnikov, V. Polyvinylpyrrolidone as a Stabilizer in Synthesis of AgInS2 Quantum Dots. Nanomaterials 2022, 12, 2357. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-Vinyl Pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M. Poly(Vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym.-Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Xian, J.; Hua, Q.; Jiang, Z.; Ma, Y.; Huang, W. Size-Dependent Interaction of the Poly(N-Vinyl-2-Pyrrolidone) Capping Ligand with Pd Nanocrystals. Langmuir 2012, 28, 6736–6741. [Google Scholar] [CrossRef]
- Mdluli, P.S.; Sosibo, N.M.; Revaprasadu, N.; Karamanis, P.; Leszczynski, J. Surface Enhanced Raman Spectroscopy (SERS) and Density Functional Theory (DFT) Study for Understanding the Regioselective Adsorption of Pyrrolidinone on the Surface of Silver and Gold Colloids. J. Mol. Struct. 2009, 935, 32–38. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree–Fock and Hybrid DFT Calculations. A ‘Chain-of-Spheres’ Algorithm for the Hartree–Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Lemeshko, P.; Korepanov, O.; Podkovyrina, E.; Spivak, Y.; Moshnikov, V.; Kozodaev, D. Porous Silicon Photoluminescence Enhancement by Silver Dendrites Registered with Multiphoton Microscopy. Opt. Laser Technol. 2025, 181, 111825. [Google Scholar] [CrossRef]
- Katerski, A.; Danilson, M.; Mere, A.; Krunks, M. Effect of the Growth Temperature on Chemical Composition of Spray-Deposited CuInS2 Thin Films. Energy Procedia 2010, 2, 103–107. [Google Scholar] [CrossRef]
- Yarema, O.; Yarema, M.; Bozyigit, D.; Lin, W.M.M.; Wood, V. Independent Composition and Size Control for Highly Luminescent Indium-Rich Silver Indium Selenide Nanocrystals. ACS Nano 2015, 9, 11134–11142. [Google Scholar] [CrossRef]
- Seoudi, R.; Fouda, A.A.; Elmenshawy, D.A. Synthesis, Characterization and Vibrational Spectroscopic Studies of Different Particle Size of Gold Nanoparticle Capped with Polyvinylpyrrolidone. Phys. B Condens. Matter 2010, 405, 906–911. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Wang, X.; Ding, S. Mechanisms of PVP in the Preparation of Silver Nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Snee, P.T. DFT Calculations of InP Quantum Dots: Model Chemistries, Surface Passivation, and Open-Shell Singlet Ground States. J. Phys. Chem. C 2021, 125, 11765–11772. [Google Scholar] [CrossRef]
- Butera, V. Density Functional Theory Methods Applied to Homogeneous and Heterogeneous Catalysis: A Short Review and a Practical User Guide. Phys. Chem. Chem. Phys. 2024, 26, 7950–7970. [Google Scholar] [CrossRef]
- Zaveri, J.; Dhanushkodi, S.R.; Fowler, M.W.; Peppley, B.A.; Taler, D.; Sobota, T.; Taler, J. Development of Deep Learning Simulation and Density Functional Theory Framework for Electrocatalyst Layers for PEM Electrolyzers. Energies 2025, 18, 1022. [Google Scholar] [CrossRef]
- van der Stam, W.; de Graaf, M.; Gudjonsdottir, S.; Geuchies, J.J.; Dijkema, J.J.; Kirkwood, N.; Evers, W.H.; Longo, A.; Houtepen, A.J. Tuning and Probing the Distribution of Cu+ and Cu2+ Trap States Responsible for Broad-Band Photoluminescence in CuInS2 Nanocrystals. ACS Nano 2018, 12, 11244–11253. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Zhang, B.; Fu, L.; Zou, G. Promising Mercaptobenzoic Acid-Bridged Charge Transfer for Electrochemiluminescence from CuInS2@ZnS Nanocrystals via Internal Cu+/Cu2+ Couple Cycling. J. Phys. Chem. Lett. 2019, 10, 5408–5413. [Google Scholar] [CrossRef]
- Kowalik, P.; Mucha, S.G.; Matczyszyn, K.; Bujak, P.; Mazur, L.M.; Ostrowski, A.; Kmita, A.; Gajewska, M.; Pron, A. Heterogeneity Induced Dual Luminescence Properties of AgInS2 and AgInS2–ZnS Alloyed Nanocrystals. Inorg. Chem. Front. 2021, 8, 3450–3462. [Google Scholar] [CrossRef]
- Arshad, A.; Akram, R.; Iqbal, S.; Batool, F.; Iqbal, B.; Khalid, B.; Khan, A.U. Aqueous Synthesis of Tunable Fluorescent, Semiconductor CuInS2 Quantum Dots for Bioimaging. Arab. J. Chem. 2019, 12, 4840–4847. [Google Scholar] [CrossRef]
- Fuhr, A.S.; Yun, H.J.; Makarov, N.S.; Li, H.; McDaniel, H.; Klimov, V.I. Light Emission Mechanisms in CuInS2 Quantum Dots Evaluated by Spectral Electrochemistry. ACS Photonics 2017, 4, 2425–2435. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.-H.; Zunger, A.; Katayama-Yoshida, H. Defect Physics of the CuInSe 2 Chalcopyrite Semiconductor. Phys. Rev. B 1998, 57, 9642–9656. [Google Scholar] [CrossRef]
- Zang, H.; Li, H.; Makarov, N.S.; Velizhanin, K.A.; Wu, K.; Park, Y.-S.; Klimov, V.I. Thick-Shell CuInS2/ZnS Quantum Dots with Suppressed ″Blinking″ and Narrow Single-Particle Emission Line Widths. Nano Lett. 2017, 17, 1787–1795. [Google Scholar] [CrossRef]
- Van Der Stam, W.; Berends, A.C.; Rabouw, F.T.; Willhammar, T.; Ke, X.; Meeldijk, J.D.; Bals, S.; De Mello Donega, C. Luminescent CuInS2 Quantum Dots by Partial Cation Exchange in Cu2–xS Nanocrystals. Chem. Mater. 2015, 27, 621–628. [Google Scholar] [CrossRef]
- Fuhr, A.; Yun, H.J.; Crooker, S.A.; Klimov, V.I. Spectroscopic and Magneto-Optical Signatures of Cu1+ and Cu2+ Defects in Copper Indium Sulfide Quantum Dots. ACS Nano 2020, 14, 2212–2223. [Google Scholar] [CrossRef]
- Berends, A.C.; Rabouw, F.T.; Spoor, F.C.M.; Bladt, E.; Grozema, F.C.; Houtepen, A.J.; Siebbeles, L.D.A.; De Mello Donegá, C. Radiative and Nonradiative Recombination in CuInS 2 Nanocrystals and CuInS2-Based Core/Shell Nanocrystals. J. Phys. Chem. Lett. 2016, 7, 3503–3509. [Google Scholar] [CrossRef]
- Piston, D. Imaging Living Cells and Tissues by Two-Photon Excitation Microscopy. Trends Cell Biol. 1999, 9, 66–69. [Google Scholar] [CrossRef]
- Jaiswal, J.K.; Goldman, E.R.; Mattoussi, H.; Simon, S.M. Use of Quantum Dots for Live Cell Imaging. Nat. Methods 2004, 1, 73–78. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Xu, H.; Wang, Z.; Dai, Z. Amino Acid-Capped Water-Soluble Near-Infrared Region CuInS2/ZnS Quantum Dots for Selective Cadmium Ion Determination and Multicolor Cell Imaging. Anal. Chem. 2019, 91, 8987–8993. [Google Scholar] [CrossRef]
- An, X.; Zhang, Y.; Wang, J.; Kong, D.; He, X.; Chen, L.; Zhang, Y. The Preparation of CuInS2-ZnS-Glutathione Quantum Dots and Their Application on the Sensitive Determination of Cytochrome c and Imaging of HeLa Cells. ACS Omega 2021, 6, 17501–17509. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Peng, S.; Zhao, Y.; Tang, T.; Guo, J.; Cui, R.; Sun, T.; Zhang, M. Improving Three-Photon Fluorescence of Near-Infrared Quantum Dots for Deep Brain Imaging by Suppressing Biexciton Decay. Nano Lett. 2024, 24, 6706–6713. [Google Scholar] [CrossRef] [PubMed]
- Maestro, L.M.; Rodríguez, E.M.; Rodríguez, F.S.; La Cruz, M.C.I.; Juarranz, A.; Naccache, R.; Vetrone, F.; Jaque, D.; Capobianco, J.A.; Solé, J.G. CdSe Quantum Dots for Two-Photon Fluorescence Thermal Imaging. Nano Lett. 2010, 10, 5109–5115. [Google Scholar] [CrossRef]
- Wang, S.; Fan, Y.; Li, D.; Sun, C.; Lei, Z.; Lu, L.; Wang, T.; Zhang, F. Anti-Quenching NIR-II Molecular Fluorophores for in Vivo High-Contrast Imaging and pH Sensing. Nat. Commun. 2019, 10, 1058. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korepanov, O.; Aleksandrova, O.; Botnar, A.; Firsov, D.; Kalazhokov, Z.; Kirilenko, D.; Lemeshko, P.; Matveev, V.; Mazing, D.; Moskalenko, I.; et al. Polyvinylpyrrolidone-Capped CuInS2 Colloidal Quantum Dots: Synthesis, Optical and Structural Assessment. Colloids Interfaces 2025, 9, 33. https://doi.org/10.3390/colloids9030033
Korepanov O, Aleksandrova O, Botnar A, Firsov D, Kalazhokov Z, Kirilenko D, Lemeshko P, Matveev V, Mazing D, Moskalenko I, et al. Polyvinylpyrrolidone-Capped CuInS2 Colloidal Quantum Dots: Synthesis, Optical and Structural Assessment. Colloids and Interfaces. 2025; 9(3):33. https://doi.org/10.3390/colloids9030033
Chicago/Turabian StyleKorepanov, Oleg, Olga Aleksandrova, Anna Botnar, Dmitrii Firsov, Zamir Kalazhokov, Demid Kirilenko, Polina Lemeshko, Vasilii Matveev, Dmitriy Mazing, Ivan Moskalenko, and et al. 2025. "Polyvinylpyrrolidone-Capped CuInS2 Colloidal Quantum Dots: Synthesis, Optical and Structural Assessment" Colloids and Interfaces 9, no. 3: 33. https://doi.org/10.3390/colloids9030033
APA StyleKorepanov, O., Aleksandrova, O., Botnar, A., Firsov, D., Kalazhokov, Z., Kirilenko, D., Lemeshko, P., Matveev, V., Mazing, D., Moskalenko, I., Novikov, A., Ulasevich, S., & Moshnikov, V. (2025). Polyvinylpyrrolidone-Capped CuInS2 Colloidal Quantum Dots: Synthesis, Optical and Structural Assessment. Colloids and Interfaces, 9(3), 33. https://doi.org/10.3390/colloids9030033





