Determination of Particle Mixture Composition by Visible Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Ferrihydrite
2.2. Extraction of Natural Allophane
2.3. Morphological and Elemental Composition of Particles
2.4. Particle Size Distribution
2.5. Suspended Particle Quantification by UV–Visible Spectroscopy
2.6. Stability of Calibration Curves over Time
2.7. Analytical Quality Parameters
3. Results
3.1. Particle Characterization
3.2. Determination of Particle Mixtures Using UV–Visible Spectroscopy
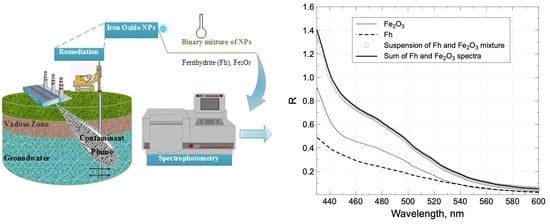
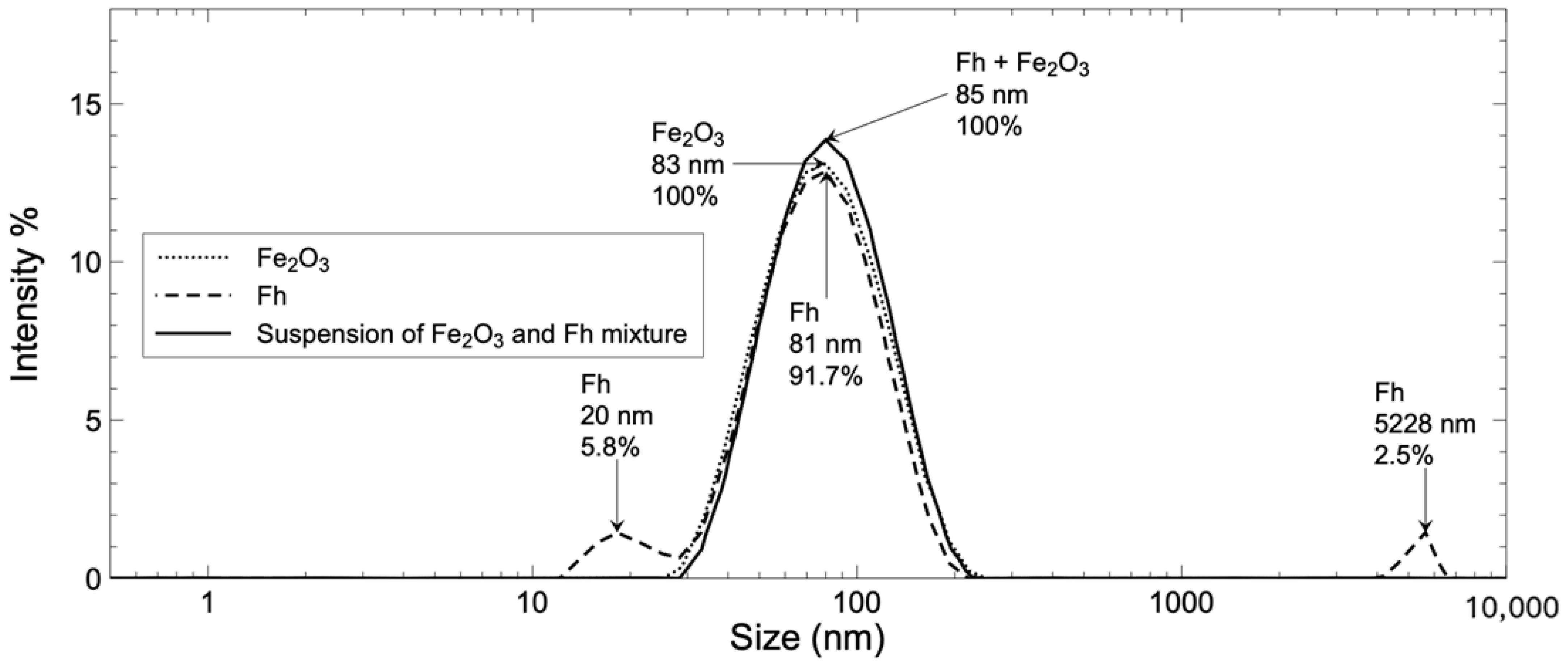
 ) of the spectra made with Fe2O3 and Fh agrees well with the spectrum obtained from a suspension made with both particles (◯) at same concentration. This behavior is consistent at each wavelength and is independent of the particle or medium composition if there is no reaction between either of the components. Another way to demonstrate the absence of interaction between the particles is to compare the DLS size distribution of the single particles to the distribution obtained for the mix (Figure S2). In the case of an interaction between the Fh and Fe2O3 particles, one should expect a strong size distribution shift from the single-particle to the mixed-particle system. This is not the case, since after combination the intense distribution located at 81 and 83 nm for the single-particle Fh and Fe2O3 DLS size distributions, respectively, (Figure 1) grew into a more intense distribution present for the mix at 85 nm (Figure S2). The secondary, less important distribution for Fh was not detected for the mix with Fe2O3, probably due to a dilution effect.
) of the spectra made with Fe2O3 and Fh agrees well with the spectrum obtained from a suspension made with both particles (◯) at same concentration. This behavior is consistent at each wavelength and is independent of the particle or medium composition if there is no reaction between either of the components. Another way to demonstrate the absence of interaction between the particles is to compare the DLS size distribution of the single particles to the distribution obtained for the mix (Figure S2). In the case of an interaction between the Fh and Fe2O3 particles, one should expect a strong size distribution shift from the single-particle to the mixed-particle system. This is not the case, since after combination the intense distribution located at 81 and 83 nm for the single-particle Fh and Fe2O3 DLS size distributions, respectively, (Figure 1) grew into a more intense distribution present for the mix at 85 nm (Figure S2). The secondary, less important distribution for Fh was not detected for the mix with Fe2O3, probably due to a dilution effect.3.3. Aging of Reference Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IONPs | Iron oxide-based nanoparticles |
| Fh | Ferrihydrite |
| NA | Natural allophane |
| FAAS | Flame Atomic Adsorption Spectroscopy |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectroscopy |
| DCB | Dithionite–citrate–bicarbonate |
| SEM | Scanning Electron Microscope |
| EDS | Energy dispersive spectroscopy |
| DLS | Dynamic Light Scattering |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| SEC-ICP-MS | Size Exclusion Chromatography Inductively Coupled Plasma Mass Spectrometry |
References
- Zaman, B.T.; Akbiyik, H.; Girgin, A.; Bozyigit, G.D.; Bakirdere, E.G.; Bakirdere, S. Removal of cadmium ions from synthetic wastewater samples by copper ferrite magnetic nanoparticle-assisted batch-type adsorption-based removal strategy. Environ. Monit. Assess. 2024, 196, 1210. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Liang, Y.; Bradford, S.A.; Simunek, J.; Heggen, M.; Vereecken, H.; Klumpp, E. Retention and Remobilization of Stabilized Silver Nanoparticles in an Undisturbed Loamy Sand Soil. Environ. Sci. Technol. 2013, 47, 12229–12237. [Google Scholar] [CrossRef] [PubMed]
- Arruda, S.C.C.; Silva, A.L.D.; Galazzi, R.M.; Azevedo, R.A.; Arruda, M.A.Z. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef]
- Otsuki, A.; Dodbiba, G.; Fujita, T. Two-Liquid Flotation for Separating Mixtures of Ultra-Fine Rare Earth Fluorescent Powders for Material Recycling—A Review. Colloids Interfaces 2018, 2, 7. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Feng, W.W.; Wang, H.Q.; Lang, X.H.; Yang, J.L.; Wu, X.; Wang, Q. Identification method of microplastics based on Raman—Infrared spectroscopy fusion. In Proceedings of the Conference on Optical Design and Testing XII, Electr Network, Online, 5–11 December 2022. [Google Scholar]
- Liu, J.B.; Du, Y.C.; Zhao, Y.H. Soil microplastics spectrum based on visible near-infrared spectroscopy. Bangladesh J. Bot. 2022, 51, 971–977. [Google Scholar] [CrossRef]
- Shang, S.M.; Guo, Y.W.; Song, J.; Liu, L.P. Study on marine microplastics monitoring based on infrared spectroscopy technology. Mater. Express 2023, 13, 1582–1589. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Souza, A.G.; Dresselhaus, G.; Saito, R. Raman spectroscopy on one isolated carbon nanotube. Phys. B-Condens. Matter 2002, 323, 15–20. [Google Scholar] [CrossRef]
- Graupner, R. Raman spectroscopy of covalently functionalized single-wall carbon nanotubes. J. Raman Spectrosc. 2007, 38, 673–683. [Google Scholar] [CrossRef]
- Babenco, M.G.; Tao, L.; Akinwande, D. Graphene Raman imaging and spectroscopy processing: Characterization of graphene growth. In Proceedings of the Conference on Instrumentation, Metrology, and Standards for Nanomanufacturing, Optics, and Semiconductors VI, San Diego, CA, USA, 13–14 August 2012. [Google Scholar]
- Yang, S.N.; Chen, Q.; Shi, M.Y.; Zhang, Q.Q.; Lan, S.K.; Maimaiti, T.; Li, Q.; Ouyang, P.; Tang, K.X.; Yang, S.T. Fast Identification and Quantification of Graphene Oxide in Aqueous Environment by Raman Spectroscopy. Nanomaterials 2020, 10, 770. [Google Scholar] [CrossRef]
- Patel, D.; Patel, B.; Patani, A.; Yadav, V.K.; Alharbi, S.A.; Alarfaj, A.A.; Choudhary, N.; Patel, A. Biogenic silver nanoparticles derived from the marine brown algae Iyengaria stellata for plant growth promotion under saline conditions. Physiol. Plant. 2024, 176, 14638. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Naseem, K.; Ali, F.; Batool, M.; Xiao, J.; Irfan, A. Applications of UV/Vis Spectroscopy in Characterization and Catalytic Activity of Noble Metal Nanoparticles Fabricated in Responsive Polymer Microgels: A Review. Crit. Rev. Anal. Chem. 2018, 48, 503–516. [Google Scholar] [CrossRef]
- Attar, A.; Yapaoz, M.A. Biosynthesis of palladium nanoparticles using Diospyros kaki leaf extract and determination of antibacterial efficacy. Prep. Biochem. Biotechnol. 2018, 48, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Kim, H.-J.; Phenrat, T.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Ionic Strength and Composition Affect the Mobility of Surface-Modified Fe0 Nanoparticles in Water-Saturated Sand Columns. Environ. Sci. Technol. 2008, 42, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Sirk, K.; Liu, Y.; Phenrat, T.; Dufour, B.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Surface Modifications Enhance Nanoiron Transport and NAPL Targeting in Saturated Porous Media. Environ. Eng. Sci. 2007, 24, 45–57. [Google Scholar] [CrossRef]
- Spek, S.; Haeuser, M.; Schaefer, M.M.; Langer, K. Characterisation of PEGylated PLGA nanoparticles comparing the nanoparticle bulk to the particle surface using UV/vis spectroscopy, SEC, H-1 NMR spectroscopy, and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2015, 347, 378–385. [Google Scholar] [CrossRef]
- Sanchez-Valdes, S.; Munoz-Jimenez, L.; Ramos-deValle, L.F.; Sanchez-Martinez, Z.V.; Flores-Gallardo, S.; Ramirez-Vargas, R.R.; Ramirez-Vargas, E.; Castaneda-Flores, M.; Betancourt-Galindo, R.; Martinez-Colunga, J.G.; et al. Antibacterial silver nanoparticle coating on oxo-biodegradable polyethylene film surface using modified polyethylene and corona discharge. Polym. Bull. 2018, 75, 3987–4002. [Google Scholar] [CrossRef]
- Yu, C.; Guo, X.; Gao, X.; Yu, Z.; Jiang, J. Transport of graphene quantum dots (GQDs) in saturated porous media. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124418. [Google Scholar] [CrossRef]
- Zhou, D.; Cai, Y.; Yang, Z. Transport of polystyrene microplastics in bare and iron oxide-coated quartz sand: Effects of ionic strength, humic acid, and co-existing graphene oxide. Sci. Total Environ. 2024, 946, 174270. [Google Scholar] [CrossRef]
- Sebby, K.B.; Mansfield, E. Determination of the surface density of polyethylene glycol on gold nanoparticles by use of microscale thermogravimetric analysis. Anal. Bioanal. Chem. 2015, 407, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Pumera, M. Simultaneous Direct Voltammetric Determination of Metal-Oxide Nanoparticles from Their Mixture (CuO/NiO). ChemElectroChem 2014, 1, 249–253. [Google Scholar] [CrossRef]
- Christian, P.; Von der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, properties, preparation and behaviour in environmental media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Applications and implications of manufactured nanoparticles in soils: A review. Eur. J. Soil Sci. 2012, 63, 437–456. [Google Scholar] [CrossRef]
- Nashaat, N.N. The application of nanoparticles for wastewater remediation. In Applications of Nanomaterials for Water Quality; Future Science Ltd.: London, UK, 2013; pp. 52–65. [Google Scholar] [CrossRef]
- Dickson, D. The Effect of Iron Oxide Nanoparticles on the Fate and Transformation of Arsenic in Aquatic Environments. Ph.D. Thesis, Florida International University, Miami, FL, USA, 2013. [Google Scholar]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Surampalli, R.Y. Engineered nanoparticles in wastewater and wastewater sludge—Evidence and impacts. Waste Manag. 2010, 30, 504–520. [Google Scholar] [CrossRef]
- Zhou, X.-X.; Liu, J.-F.; Geng, F.-L. Determination of metal oxide nanoparticles and their ionic counterparts in environmental waters by size exclusion chromatography coupled to ICP-MS. NanoImpact 2016, 1, 13–20. [Google Scholar] [CrossRef]
- Ali, A.M.; Hill, H.J.; Elkhouly, G.E.; Raya, N.R.; Tawfik, N.F.; Bakkar, M.R.; El-Basaty, A.B.; Stamataki, Z.; Abo-zeid, Y. Green and chemical synthesis of iron oxide nanoparticles: Comparative study for antimicrobial activity and toxicity concerns. J. Drug Deliv. Sci. Technol. 2025, 103, 106434. [Google Scholar] [CrossRef]
- Gaffet, E. Nanomaterials: A review of the definitions, applications, health effects. How to implement secure development. arXiv 2011, arXiv:1106.2206. [Google Scholar]
- Auffan, M.; Bottero, J.-Y.; Chaneac, C.; Rose, J. Inorganic manufactured nanoparticles: How their physicochemical properties influence their biological effects in aqueous environments. Nanomedicine 2010, 5, 999–1007. [Google Scholar] [CrossRef]
- Faria, J.M.D.; Morozesk, M.; Souza, I.D.; da Silva, V.C.; Bataus, L.A.M.; de Sabóia-Morais, S.M.T.; Fernandes, M.N. Glyphosate and glyphosate-based herbicides induce Poecilia reticulata to maintain redox equilibrium during and after coexposure to iron oxide nanoparticles (y-Fe2O3). Aquat. Toxicol. 2025, 279, 107175. [Google Scholar] [CrossRef]
- Ding, Y.F.; Sheng, A.X.; Li, X.X.; Liu, Y.Y.; Yan, M.Q.; Takahashi, Y.; Liu, J. Triplet-Excited Riboflavin Promotes Labile Fe(III) Accumulation and Changes Mineralization Pathways in Fe(II)-Catalyzed Ferrihydrite Transformation. Environ. Sci. Technol. 2024, 58, 22148–22158. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wu, D.; Nie, Y.L.; Yang, C.; Li, Y. Efficiently catalytic ozonation of 2,4-dichlorophenoxyacetic acid by natural ferrihydrite: A pH dependent and surface-OH group involved reaction mechanism. Environ. Res. 2025, 264, 120410. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Nanamura, K. Allophanes in the weathered volcanic ash deposits distributed in southern Kyushu, Japan and their ion adsorption characteristics. J. Mineral. Petrol. Sci. 2024, 119, 240729. [Google Scholar] [CrossRef]
- Cea, M.; Seaman, J.C.; Jara, A.A.; Fuentes, B.; Mora, M.L.; Diez, M.C. Adsorption behavior of 2,4-dichlorophenol and pentachlorophenol in an allophanic soil. Chemosphere 2007, 67, 1354–1360. [Google Scholar] [CrossRef]
- Jara, A.A.; Goldberg, S.; Mora, M.L. Studies of the surface charge of amorphous aluminosilicates using surface complexation models. J. Colloid Interface Sci. 2005, 292, 160–170. [Google Scholar] [CrossRef]
- Ben Moshe, S.; Gelman, F.; Furman, A. Noninvasive Investigation of Gold Nanoparticle Transport and Fate in Soil. ACS EST Water 2024, 4, 5787–5794. [Google Scholar] [CrossRef]
- Bouguer, M. Essai D’optique, sur la Gradation de la Lumière; Claude Jombert: Paris, France, 1729. [Google Scholar]
- Lambert, J.H. Photometria sive de Mensura et Gradibus Luminis, Colorum et Umbrae; Eberhardt Klett: Augsburg, Germany, 1760. [Google Scholar]
- Beer, A. Bestimmung der Absorption des rothen Lichts in farbigen Flüssigkeiten. Ann. Phys. 1852, 162, 78–88. [Google Scholar] [CrossRef]
- Morris, R. Spectrophotometry. Curr. Protoc. Essent. Lab. Tech. 2015, 11, 2.1.1–2.1.30. [Google Scholar] [CrossRef]
- Morais, I.P.; Tóth, I.V.; Rangel, A.O. Turbidimetric and nephelometric flow analysis: Concepts and applications. Spectrosc. Lett. 2006, 39, 547–579. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. General Preparative Techniques. In Iron Oxides in the Laboratory: Preparation and Characterization; Wiley-VCH: Weinheim, Germany, 2000; pp. 19–25. [Google Scholar] [CrossRef]
- Kunze, G.W. Pretreatment for mineralogical analysis. In Methods of Soil Analysis; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 568–577. [Google Scholar]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate A2—Ingerson, earl. In Clays and Clay Minerals, Proceedings of the Seventh National Conference on Clays and Clay Minerals, Washington, DC, USA, 20–23 October 1958; Pergamon: Washington, DC, USA, 1960; pp. 317–327. [Google Scholar] [CrossRef]
- Escudey, M.; Galindo, G.; Ervin, J. Effect of Iron Oxide Dissolution Treatment on the Isoelectric Point of Allophanic Soils. Clays Clay Miner. 1986, 34, 108–110. [Google Scholar] [CrossRef]
- de Kanter, M.; Meyer-Kirschner, J.; Viell, J.; Mitsos, A.; Kather, M.; Pich, A.; Janzen, C. Enabling the measurement of particle sizes in stirred colloidal suspensions by embedding dynamic light scattering into an automated probe head. Measurement 2016, 80, 92–98. [Google Scholar] [CrossRef]
- Freud, P.J. Nanoparticle Sizing—Dynamic Light Scattering Analysis in the Frequency Spectrum Mode. Available online: https://analysis.rs/wp-content/uploads/2022/01/0193_M_Nanoparticles-with-DLS_Nanotrac_EN_RevF-min.pdf (accessed on 9 March 2025).
- Christian, G.D. Química Analítica; Mcgraw-Hill/Interamericana: Ciudad de México, Mexico, 2009; pp. 111–113. [Google Scholar]
- Kaufhold, S.; Kaufhold, A.; Dohrmann, R. Comparison of the Critical Coagulation Concentrations of Allophane and Smectites. Colloids Interfaces 2018, 2, 12. [Google Scholar] [CrossRef]
- Belenkiy, A.; Vila Echagüe, E. History of one defeat: Reform of the Julian calendar as envisaged by Isaac Newton. Notes Rec. R. Soc. 2005, 59, 223–254. [Google Scholar] [CrossRef]

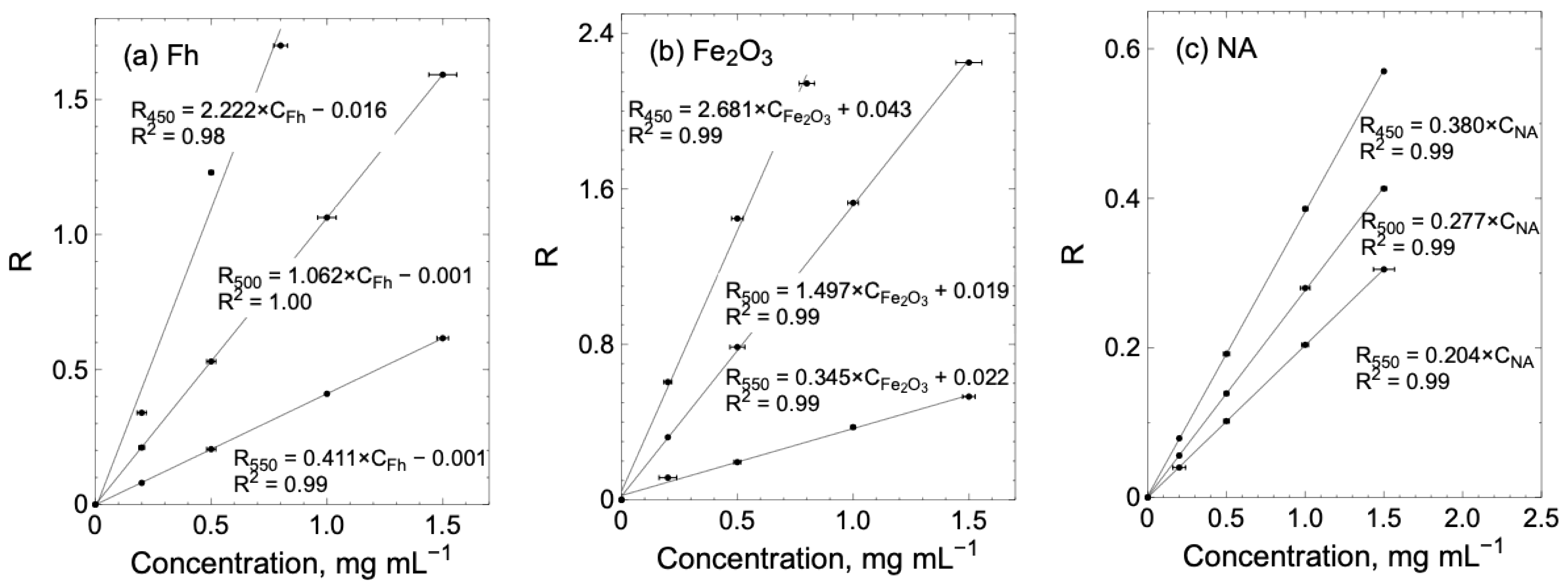

| K450 | K500 | K550 | |
| (mL mg−1) | |||
| Fh | - | 1.062 ± 0.001 | 0.411 ± 0.001 |
| Fe2O3 | 2.681 ± 0.109 | 1.497 ± 0.016 | 0.345 ± 0.016 |
| NA | 0.380 ± 0.002 | 0.277 ± 0.002 | 0.204 ± 0.000 |
| Mix | Theoretical Concentration (mg mL−1) | Rλ | Experimental Concentration ± Standard Deviation (%error) (mg mL−1) | |||
|---|---|---|---|---|---|---|
| Fe2O3 | Fh | 500 nm | 550 nm | Fe2O3 | Fh | |
| M1 | 0.2 | 1.0 | 1.388 | 0.491 | 0.20 ± 0.00 (0.0) | 1.03 ± 0.06 (+3.0) |
| M2 | 0.6 | 0.6 | 1.575 | 0.465 | 0.61 ± 0.03 (+1.7) | 0.61 ± 0.02 (+1.7) |
| M3 | 1.0 | 0.2 | 1.748 | 0.434 | 1.03 ± 0.08 (+3.0) | 0.19 ± 0.02 (−5.0) |
| Fe2O3 | NA | 500 nm | 550 nm | Fe2O3 | NA | |
| M4 | 0.2 | 1.0 | 0.606 | 0.281 | 0.21 ± 0.00 (+5.0) | 0.99 ± 0.07 (−1.0) |
| M5 | 0.6 | 0.6 | 1.126 | 0.353 | 0.60 ± 0.04 (0.0) | 0.61 ± 0.02 (+1.7) |
| M6 | 1.0 | 0.2 | 1.676 | 0.433 | 1.03 ± 0.06 (+3.0) | 0.23 ± 0.05 (+15.0) |
| Fe2O3 | NA | 450 nm | 550 nm | Fe2O3 | NA | |
| M7 | 0.2 | 1.0 | 0.974 | 0.281 | 0.23 ± 0.00 (+15.0) | 0.96 ± 0.05 (−4.0) |
| M8 | 0.6 | 0.6 | 1.905 | 0.353 | 0.63 ± 0.01 (+5.0) | 0.58 ± 0.01 (−3.3) |
| M9 | 1.0 | 0.2 | 2.501 | 0.433 | 0.83 ± 0.03 (−17.0) | 0.72 ± 0.02 (+260.0) |
| LOD (mg L−1) | LOQ (mg L−1) | |||||
|---|---|---|---|---|---|---|
| Wavelengths (nm) | 450 | 500 | 550 | 450 | 500 | 550 |
| Fh | - | 1.5 | 3.0 | - | 5.1 | 9.8 |
| Fe2O3 | 0.2 | 1.1 | 3.5 | 0.8 | 3.6 | 11.7 |
| NA | 1.6 | 5.9 | 6.0 | 5.3 | 19.5 | 19.8 |
| Fh | Fe2O3 | ||||
|---|---|---|---|---|---|
| Fresh | One-Week Old | Fresh | One-Week Old | ||
| K500 | (mL mg−1) | 1.062 | 1.069 | 1.497 | 1.525 |
| K550 | 0.411 | 0.416 | 0.345 | 0.346 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudey, M.; Cáceres-Jensen, L.; Gacitúa, M. Determination of Particle Mixture Composition by Visible Spectroscopy. Colloids Interfaces 2025, 9, 16. https://doi.org/10.3390/colloids9020016
Escudey M, Cáceres-Jensen L, Gacitúa M. Determination of Particle Mixture Composition by Visible Spectroscopy. Colloids and Interfaces. 2025; 9(2):16. https://doi.org/10.3390/colloids9020016
Chicago/Turabian StyleEscudey, Mauricio, Lizethly Cáceres-Jensen, and Manuel Gacitúa. 2025. "Determination of Particle Mixture Composition by Visible Spectroscopy" Colloids and Interfaces 9, no. 2: 16. https://doi.org/10.3390/colloids9020016
APA StyleEscudey, M., Cáceres-Jensen, L., & Gacitúa, M. (2025). Determination of Particle Mixture Composition by Visible Spectroscopy. Colloids and Interfaces, 9(2), 16. https://doi.org/10.3390/colloids9020016