Bio-Based Interpolyelectrolyte Complexes for the Stabilization of Pickering-like Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Preparation of the Dispersions Containing IPECs
2.2.2. Preparation of the Pickering-like Emulsions
2.2.3. Characterization of the Dispersions Containing IPECs
2.2.4. Characterization of the Pickering-like Emulsions Stabilized by IPECs
3. Results
3.1. Assembly of IPECs in Solution
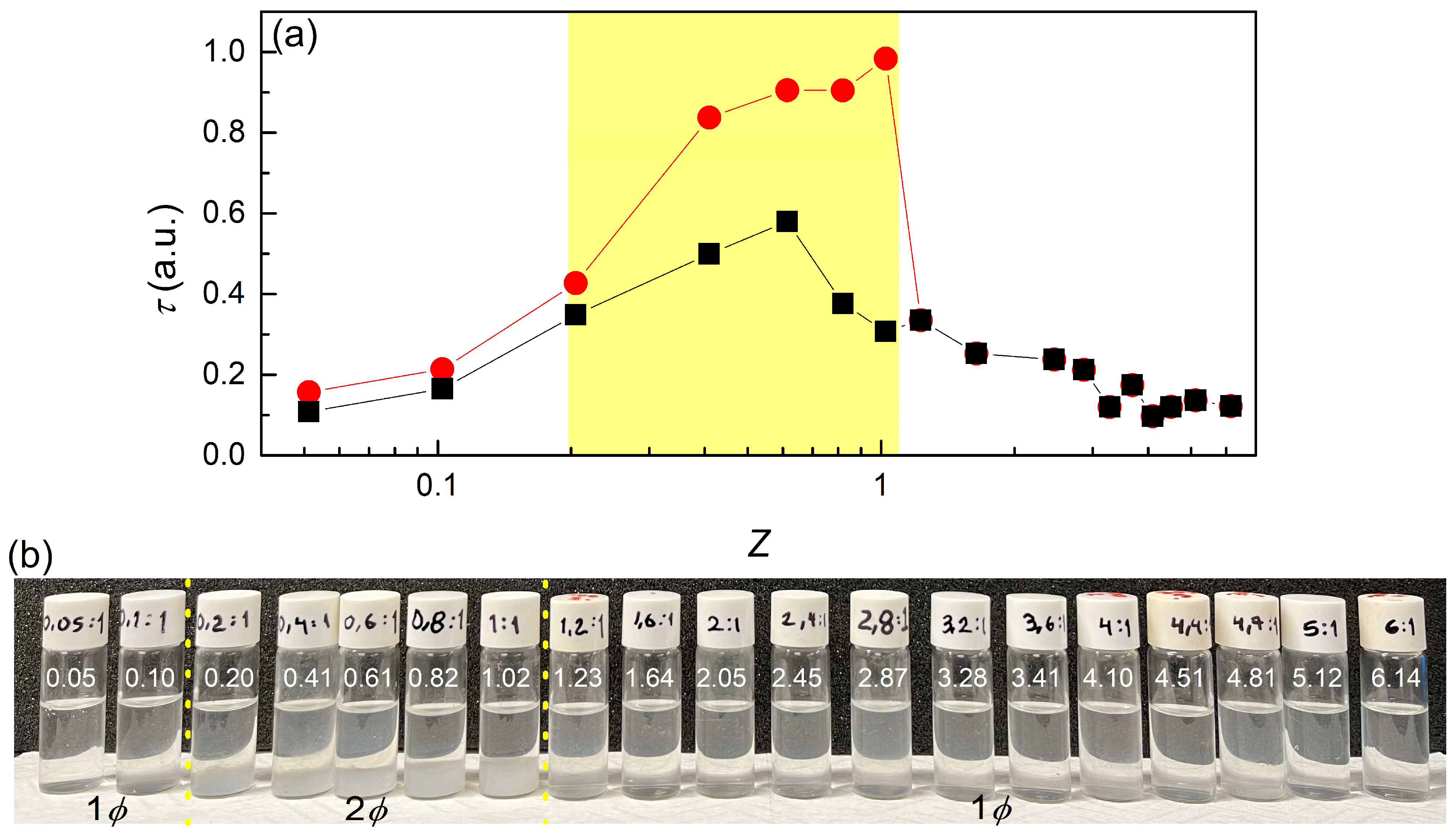
3.1.1. Turbidity Measurements
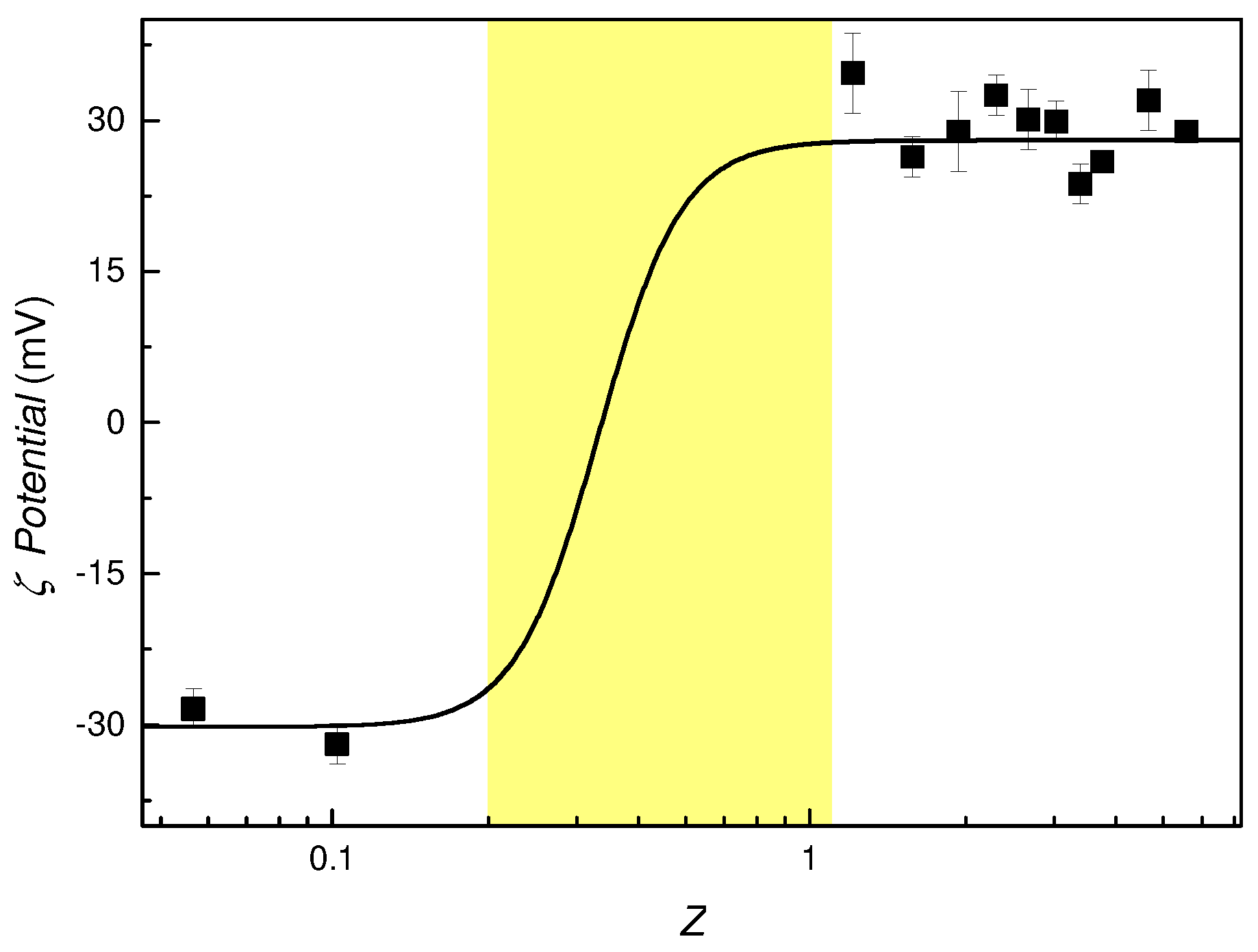
3.1.2. ζ-Potential Measurements
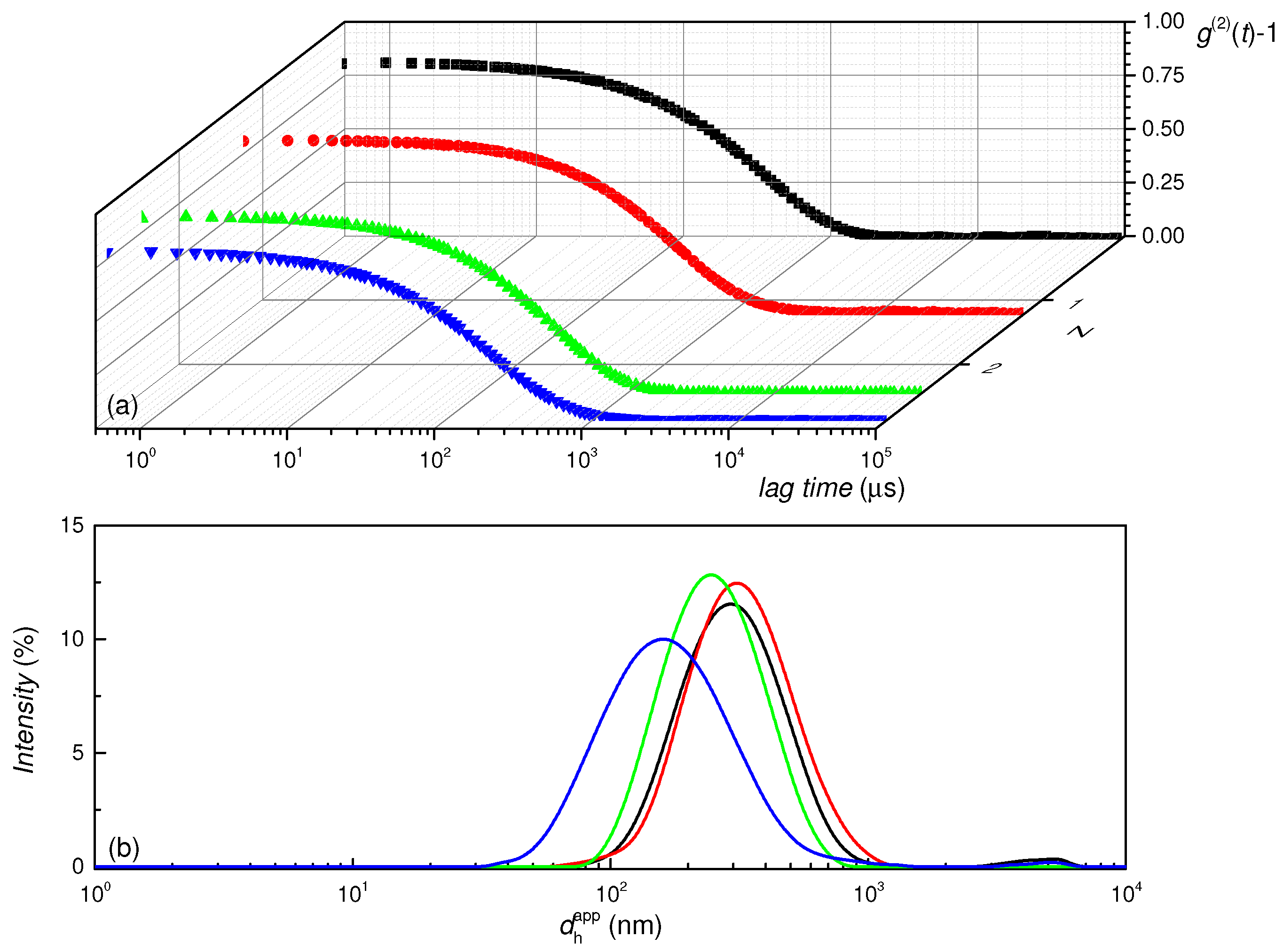
3.1.3. Dynamic Light Scattering Measurements
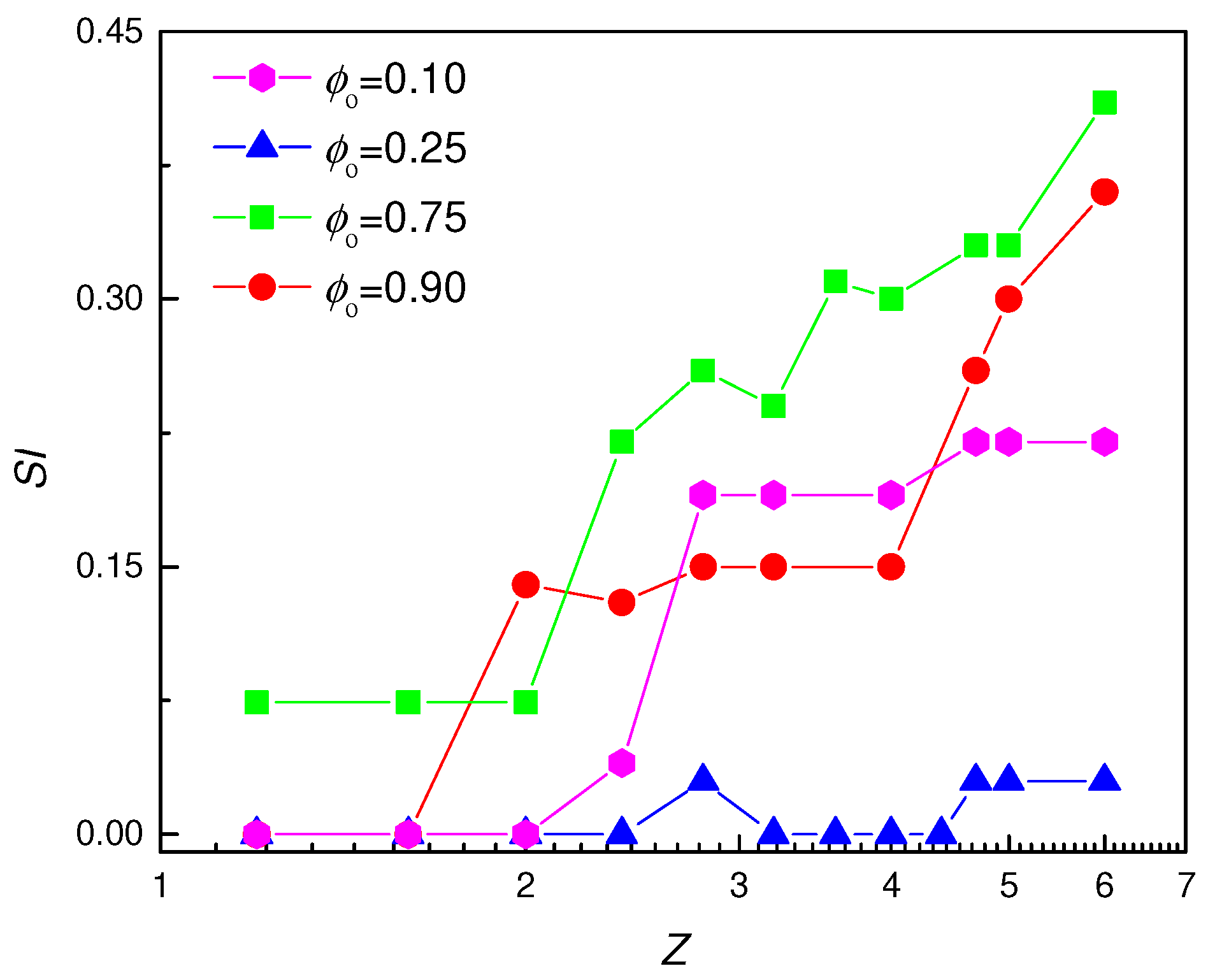
3.2. Phase Diagram of Pickering-like Emulsions Stabilized by IPECs
3.3. Stability of IPECs Pickering-like Emulsions
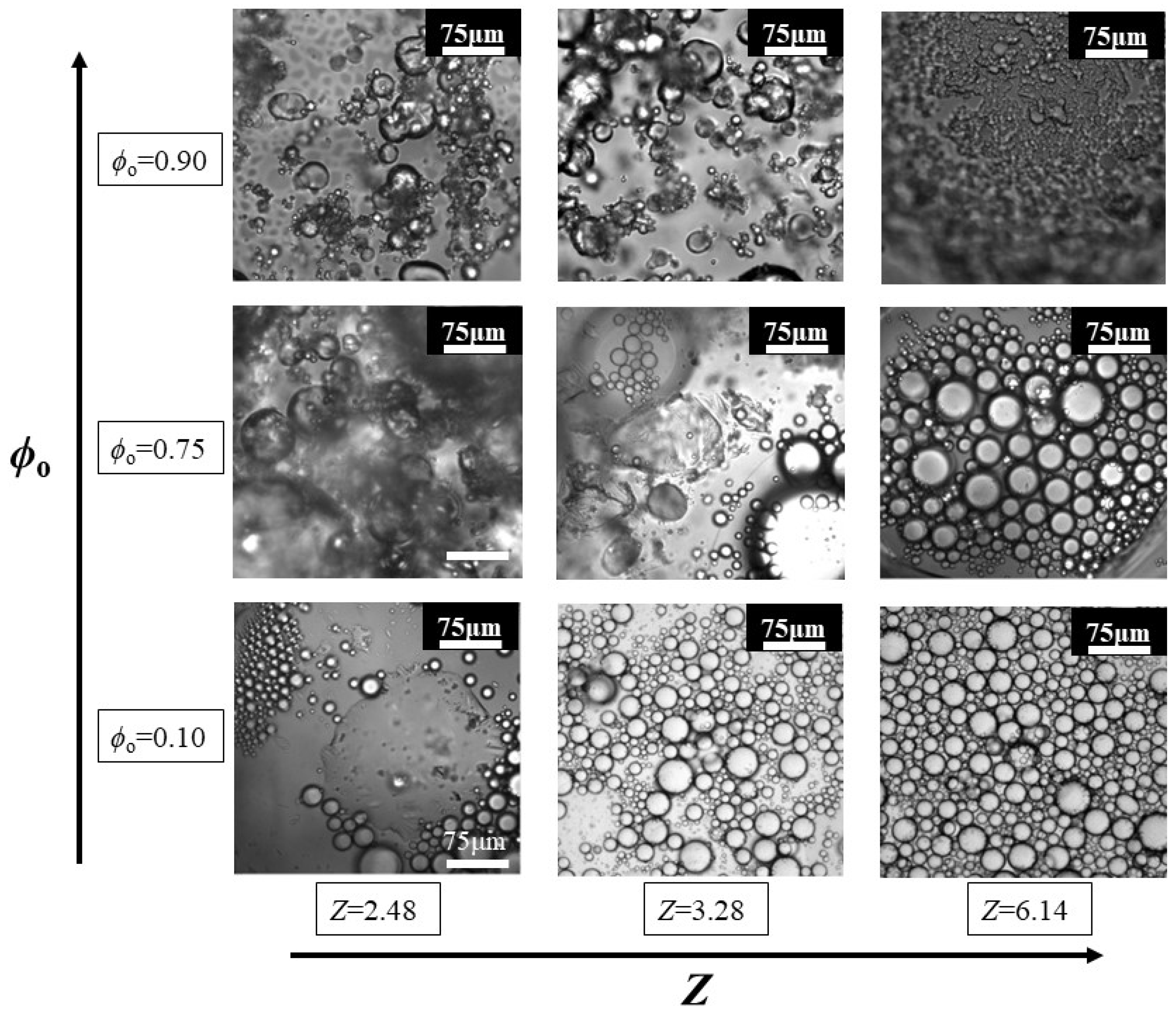
3.4. Morphological Characterization of Pickering-like Emulsions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Babadagli, T. Comprehensive Review on Heavy-Oil Emulsions: Colloid Science and Practical Applications. Chem. Eng. Sci. 2020, 228, 115962. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Rojas, O.J.; McClements, D.J. Recent Innovations in Emulsion Science and Technology for Food Applications. J. Agric. Food Chem. 2021, 69, 8944–8963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Z.; Wang, Q.; Wang, J.; Shang, L. Aqueous Two-Phase Emulsions toward Biologically Relevant Applications. Trends Chem. 2023, 5, 61–75. [Google Scholar] [CrossRef]
- Israelachvili, J. The Science and Applications of Emulsions—An Overview. Colloids Surf. A 1994, 91, 1–8. [Google Scholar] [CrossRef]
- Lucia, A.; Guzmán, E. Emulsions Containing Essential Oils, Their Components or Volatile Semiochemicals as Promising Tools for Insect Pest and Pathogen Management. Adv. Colloid Interface Sci. 2021, 287, 102330. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Pickering Emulsions: A Novel Tool for Cosmetic Formulators. Cosmetics 2022, 9, 68. [Google Scholar] [CrossRef]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and Applications of Particle Stabilized Emulsions in Cosmetic Formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-Stabilized Pickering Emulsions: Formation, Stability, Properties, and Applications in Foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Ramsden, W. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation).—Preliminary Account. Proc. Roy. Soc. Lond. 1904, 72, 156–164. [Google Scholar] [CrossRef]
- Guzmán, E. Pickering Emulsions in Catalytic Processes. ChemCatChem 2024, 16, e202400856. [Google Scholar] [CrossRef]
- Rodriguez, A.M.B.; Binks, B.P. Catalysis in Pickering Emulsions. Soft Matter 2020, 16, 10221–10243. [Google Scholar] [CrossRef]
- de Carvalho-Guimarães, F.B.; Correa, K.L.; de Souza, T.P.; Rodríguez Amado, J.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. A Review of Pickering Emulsions: Perspectives and Applications. Pharmaceuticals 2022, 15, 1413. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yan, X.; Xiao, Y.; Hu, L.; Eggersdorfer, M.; Chen, D.; Yang, Z.; Weitz, D.A. Pickering Emulsions Stabilized by Colloidal Surfactants: Role of Solid Particles. Particuology 2022, 64, 153–163. [Google Scholar] [CrossRef]
- Binks, B.P. Colloidal Particles at a Range of Fluid–Fluid Interfaces. Langmuir 2017, 33, 6947–6963. [Google Scholar] [CrossRef]
- Guzmán, E.; Martínez-Pedrero, F.; Calero, C.; Maestro, A.; Ortega, F.; Rubio, R.G. A Broad Perspective to Particle-Laden Fluid Interfaces Systems: From Chemically Homogeneous Particles to Active Colloids. Adv. Colloid Interface Sci. 2022, 302, 102620. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Guzmán, E.; Ortega, F.; Rubio, R.G. Contact Angle of Micro- and Nanoparticles at Fluid Interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 355–367. [Google Scholar] [CrossRef]
- Maestro, A. Tailoring the Interfacial Assembly of Colloidal Particles by Engineering the Mechanical Properties of the Interface. Curr. Opin. Colloid Interface Sci. 2019, 39, 232–250. [Google Scholar] [CrossRef]
- Liu, L.; Pu, X.; Tao, H.; Chen, K.; Guo, W.; Luo, D.; Ren, Z. Pickering Emulsion Stabilized by Organoclay and Intermediately Hydrophobic Nanosilica for High-Temperature Conditions. Colloids Surf. A 2021, 610, 125694. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Pickering Emulsions Stabilized by Halloysite Nanotubes: From General Aspects to Technological Applications. Adv. Mater. Interfaces 2022, 9, 2102346. [Google Scholar] [CrossRef]
- Lu, T.; Gou, H.; Rao, H.; Zhao, G. Recent Progress in Nanoclay-Based Pickering Emulsion and Applications. J. Environ. Chem. Eng. 2021, 9, 105941. [Google Scholar] [CrossRef]
- Kumar, G.; Mani, E.; Sangwai, J.S. Impact of Surface-Modified Silica Nanoparticle and Surfactant on the Stability and Rheology of Oil-in-Water Pickering and Surfactant-Stabilized Emulsions under High-Pressure and High-Temperature. J. Mol. Liq. 2023, 379, 121620. [Google Scholar] [CrossRef]
- Heidari, F.; Jafari, S.M.; Ziaiifar, A.M.; Anton, N. Preparation of Pickering Emulsions Stabilized by Modified Silica Nanoparticles via the Taguchi Approach. Pharmaceutics 2022, 14, 1561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, G.; Ge, J.; Jiang, P.; Ding, L. PH- and Thermo-Responsive Pickering Emulsion Stabilized by Silica Nanoparticles and Conventional Nonionic Copolymer Surfactants. J. Colloid Interface Sci. 2022, 616, 129–140. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, L.; Li, J. Biopolymer-Based Pickering High Internal Phase Emulsions: Intrinsic Composition of Matrix Components, Fundamental Characteristics and Perspective. Food Res. Int. 2023, 165, 112458. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.F.; Morell, P.; Nicoletti, V.R.; Quiles, A.; Hernando, I. Protein- and Polysaccharide-Based Particles Used for Pickering Emulsion Stabilisation. Food Hydrocoll. 2021, 119, 106839. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, L.; Li, J.; Zhong, S. Pickering Emulsions Stabilized by Biopolymer-Based Nanoparticles or Hybrid Particles for the Development of Food Packaging Films: A Review. Food Hydrocoll. 2024, 146, 109185. [Google Scholar] [CrossRef]
- Bago Rodriguez, A.M.; Binks, B.P.; Sekine, T. Novel Stabilisation of Emulsions by Soft Particles: Polyelectrolyte Complexes. Faraday Discuss. 2016, 191, 255–285. [Google Scholar] [CrossRef] [PubMed]
- Bago Rodriguez, A.M.; Binks, B.P.; Sekine, T. Emulsion Stabilisation by Complexes of Oppositely Charged Synthetic Polyelectrolytes. Soft Matter 2018, 14, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Fay, J.M.; Kabanov, A.V. Interpolyelectrolyte Complexes as an Emerging Technology for Pharmaceutical Delivery of Polypeptides. Rev. Adv. Chem. 2022, 12, 137–162. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Scholten, E.; Moschakis, T.; Biliaderis, C.G. Properties of Emulsions Stabilised by Sodium Caseinate–Chitosan Complexes. Int. Dairy J. 2012, 26, 94–101. [Google Scholar] [CrossRef]
- Stone, A.K.; Nickerson, M.T. Formation and Functionality of Whey Protein Isolate–(Kappa-, Iota-, and Lambda-Type) Carrageenan Electrostatic Complexes. Food Hydrocoll. 2012, 27, 271–277. [Google Scholar] [CrossRef]
- Jourdain, L.; Leser, M.E.; Schmitt, C.; Michel, M.; Dickinson, E. Stability of Emulsions Containing Sodium Caseinate and Dextran Sulfate: Relationship to Complexation in Solution. Food Hydrocoll. 2008, 22, 647–659. [Google Scholar] [CrossRef]
- Sun, F.; Pan, C.; Liu, Y.; Yang, N. Carboxymethyl Tamarind Seed Polysaccharide/Chitosan Complexes through Electrostatic Interaction Stabilize High Internal Phase Emulsions: Roles of the Mass Ratio and Oil-Water Interfacial Activity. LWT 2024, 195, 115833. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Vaseneva, I.N.; Mikhaylov, V.I.; Martakov, I.S.; Legki, P.V.; Sitnikov, P.A. Chitin Nanocrystals/Alginate Complex for Tuning Stability, Rheology and Bioavailability of Cholecalciferol in Pickering Emulsions. Int. J. Biol. Macromol. 2024, 264, 130671. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.-Y.; Shao, J.-Q.; Liu, X.-L.; Wei, J.-T.; Huang, G.-Q.; Xiao, J.-X. Spray Drying the Pickering Emulsions Stabilized by Chitosan/Ovalbumin Polyelectrolyte Complexes for the Production of Oxidation Stable Tuna Oil Microcapsules. Int. J. Biol. Macromol. 2024, 273, 133139. [Google Scholar] [CrossRef] [PubMed]
- Khapre, M.A.; Pandey, S.; Jugade, R.M. Glutaraldehyde-Cross-Linked Chitosan–Alginate Composite for Organic Dyes Removal from Aqueous Solutions. Int. J. Biol. Macormol. 2021, 190, 862–875. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Mojahid, B.Z.E.; Guzmán, E.; Ortega, F.; Rubio, R.G. Performance of Oleic Acid and Soybean Oil in the Preparation of Oil-in-Water Microemulsions for Encapsulating a Highly Hydrophobic Molecule. Colloids Interfaces 2021, 5, 50. [Google Scholar] [CrossRef]
- Song, H.; Taylor, D.C.; Zhang, M. Bioengineering of Soybean Oil and Its Impact on Agronomic Traits. Int. J. Mol. Sci. 2023, 24, 2256. [Google Scholar] [CrossRef] [PubMed]
- Zaaboul, F.; Zhao, Q.; Xu, Y.; Liu, Y. Soybean Oil Bodies: A Review on Composition, Properties, Food Applications, and Future Research Aspects. Food Hydrocoll. 2022, 124, 107296. [Google Scholar] [CrossRef]
- Ormeño-Martínez, M.; Guzmán, E.; Fernández-Peña, L.; Greaves, A.J.; Bureau, L.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Roles of Polymer Concentration and Ionic Strength in the Deposition of Chitosan of Fungal Origin onto Negatively Charged Surfaces. Biomimetics 2024, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Ravera, F.; Santini, E.; Loglio, G.; Ferrari, M.; Liggieri, L. Effect of Nanoparticles on the Interfacial Properties of Liquid/Liquid and Liquid/Air Surface Layers. J. Phys. Chem. B 2006, 110, 19543–19551. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Campbell, R.A. General Physical Description of the Behavior of Oppositely Charged Polyelectrolyte/Surfactant Mixtures at the Air/Water Interface. Langmuir 2017, 33, 5915–5924. [Google Scholar] [CrossRef]
- Guzmán, E.; Maestro, A.; Ortega, F.; Rubio, R.G. Association of Oppositely Charged Polyelectrolyte and Surfactant in Solution: Equilibrium and Nonequilibrium Features. J. Phys. Cond. Matter 2023, 35, 323001. [Google Scholar] [CrossRef] [PubMed]
- Smoluchowski, M. Handbuch der Elektrizität und des Magnetismus; Barth: Leipzig, Germany, 1921. [Google Scholar]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Fernández-Peña, L.; Gutiérrez-Muro, S.; Guzmán, E.; Lucia, A.; Ortega, F.; Rubio, R.G. Oil-In-Water Microemulsions for Thymol Solubilization. Colloids Interfaces 2019, 3, 64. [Google Scholar] [CrossRef]
- Xu, W.; Li, Z.; Li, H.; Sun, H.; Zheng, S.; Luo, D.; Li, Y.; Wang, Y.; Shah, B.R. Stabilization and Microstructural Network of Pickering Emulsion Using Different Xanthan Gum/Lysozyme Nanoparticle Concentrations. LWT 2022, 160, 113298. [Google Scholar] [CrossRef]
- Santini, E.; Guzmán, E.; Ferrari, M.; Liggieri, L. Emulsions Stabilized by the Interaction of Silica Nanoparticles and Palmitic Acid at the Water-Hexane Interface. Colloids Surf. A 2014, 460, 333–341. [Google Scholar] [CrossRef]
- González-González, A.; Sánchez-Arribas, N.; Santini, E.; Rodríguez-Villafuerte, J.L.; Carbone, C.; Ravera, F.; Ortega, F.; Liggieri, L.; Rubio, R.G.; Guzmán, E. Effects of Oil Phase on the Inversion of Pickering Emulsions Stabilized by Palmitic Acid Decorated Silica Nanoparticles. Colloids Interfaces 2022, 6, 27. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, S.; Li, X.; Liang, H.; Li, S. Antioxidant Pickering Emulsions Stabilised by Zein/Tannic Acid Colloidal Particles with Low Concentration. Int. J. Food Sci. Technol. 2020, 55, 1924–1934. [Google Scholar] [CrossRef]
- Luo, S.-Z.; Hu, X.-F.; Pan, L.-H.; Zheng, Z.; Zhao, Y.-Y.; Cao, L.-L.; Pang, M.; Hou, Z.-G.; Jiang, S.-T. Preparation of Camellia Oil-Based W/O Emulsions Stabilized by Tea Polyphenol Palmitate: Structuring Camellia Oil as a Potential Solid Fat Replacer. Food Chem. 2019, 276, 209–217. [Google Scholar] [CrossRef]
- Dong, D.; Hua, Y. Glycinin-Gum Arabic Complex Formation: Turbidity Measurement and Charge Neutralization Analysis. Food Res. Int. 2016, 89, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Nivard, M.; Ortega, F.; Rubio, R.G.; Guzmán, E. Adsorption and Bulk Assembly of Quaternized Hydroxyethylcellulose—Anionic Surfactant Complexes on Negatively Charged Substrates. Polymers 2025, 17, 207. [Google Scholar] [CrossRef]
- Ruano, M.; Mateos-Maroto, A.; Ortega, F.; Ritacco, H.; Rubio, J.E.F.; Guzmán, E.; Rubio, R.G. Fabrication of Robust Capsules by Sequential Assembly of Polyelectrolytes onto Charged Liposomes. Langmuir 2021, 37, 6189–6200. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Transitional Phase Inversion of Solid-Stabilized Emulsions Using Particle Mixtures. Langmuir 2000, 16, 3748–3756. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Catastrophic Phase Inversion of Water-in-Oil Emulsions Stabilized by Hydrophobic Silica. Langmuir 2000, 16, 2539–2547. [Google Scholar] [CrossRef]
- Fernandes Barros, F.M.; Chassenieux, C.; de Souza Lima, M.M.; Benyahia, L. Structure and Rheology during Catastrophic Phase Inversion of Pickering Emulsions Stabilized with Fumed Silica Particles. Colloids Surf. A 2020, 593, 124630. [Google Scholar] [CrossRef]
- Binks, B.P.; Whitby, C.P. Silica Particle-Stabilized Emulsions of Silicone Oil and Water: Aspects of Emulsification. Langmuir 2004, 20, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Lumsdon, S.O. Stability of Oil-in-Water Emulsions Stabilised by Silica Particles. Phys. Chem. Chem. Phys. 1999, 1, 3007–3016. [Google Scholar] [CrossRef]
- Binks, B.P.; Philip, J.; Rodrigues, J.A. Inversion of Silica-Stabilized Emulsions Induced by Particle Concentration. Langmuir 2005, 21, 3296–3302. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, G. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef]
- Zhen Zhou, F.; Swinkels, P.J.M.; Wei Yin, S.; Velikov, K.P.; Schall, P. Pickering Stabilization Mechanism Revealed through Direct Imaging of Particles with Tuneable Contact Angle in a Phase-Separated Binary Solvent. J. Colloid Interface Sci. 2024, 662, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Cao, M. Bicontinuous Interfacially Jammed Emulsion Gels (Bijels): Preparation, Control Strategies, and Derived Porous Materials. Nanomaterials 2024, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.; Stratford, K.; Thijssen, J.H.J. Quantitative Morphological Characterization of Bicontinuous Pickering Emulsions via Interfacial Curvatures. Soft Matter 2016, 12, 4082–4092. [Google Scholar] [CrossRef]




 ) Phase-separated systems, (
) Phase-separated systems, ( ) W/O emulsions and (
) W/O emulsions and ( ) O/W emulsions. Results correspond to emulsions aged for 2 months.
) O/W emulsions. Results correspond to emulsions aged for 2 months.
 ) Phase-separated systems, (
) Phase-separated systems, ( ) W/O emulsions and (
) W/O emulsions and ( ) O/W emulsions. Results correspond to emulsions aged for 2 months.
) O/W emulsions. Results correspond to emulsions aged for 2 months.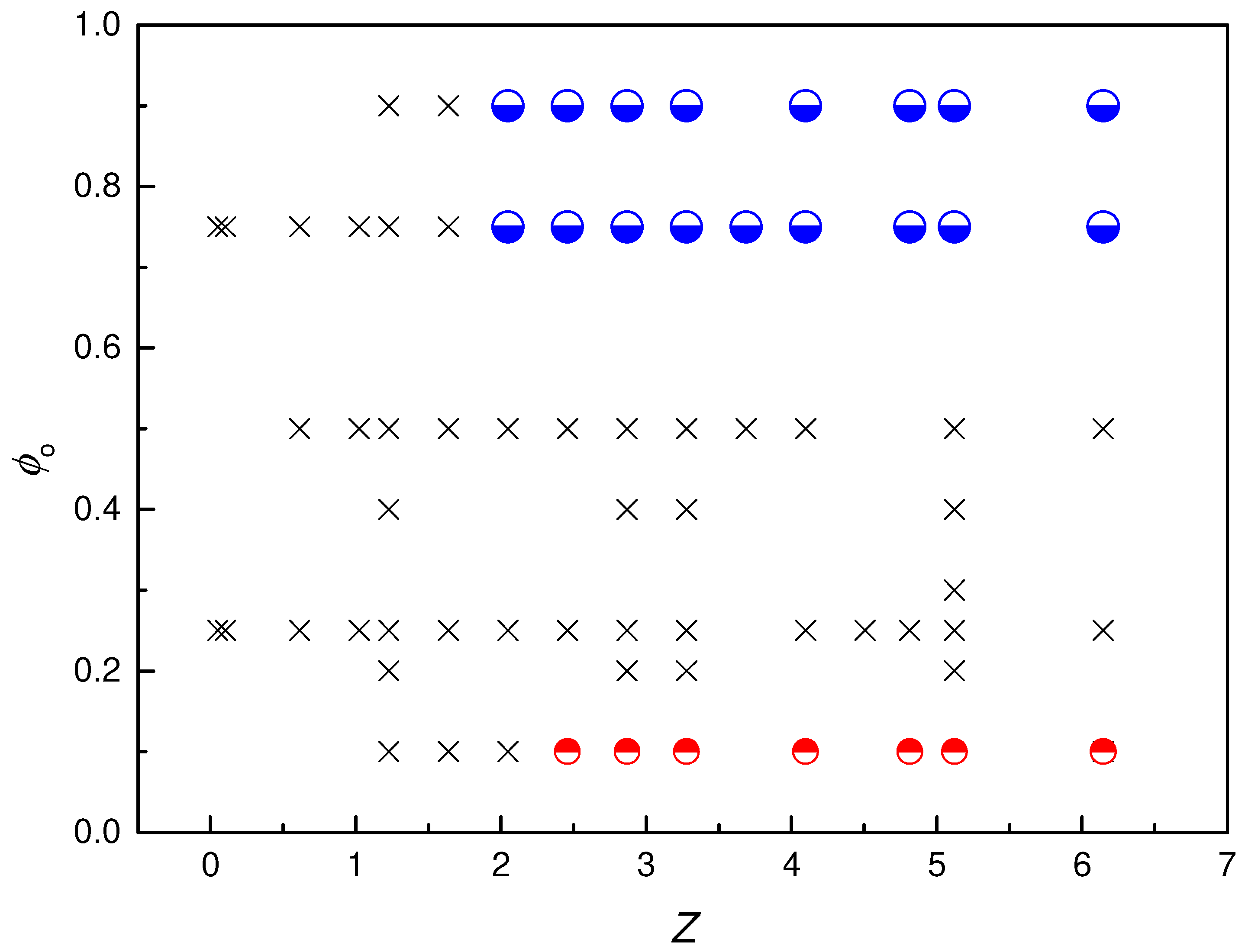
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Vasquez, F.J.; Ortega, F.; Rubio, R.G.; Guzmán, E. Bio-Based Interpolyelectrolyte Complexes for the Stabilization of Pickering-like Emulsions. Colloids Interfaces 2025, 9, 9. https://doi.org/10.3390/colloids9010009
Guerrero-Vasquez FJ, Ortega F, Rubio RG, Guzmán E. Bio-Based Interpolyelectrolyte Complexes for the Stabilization of Pickering-like Emulsions. Colloids and Interfaces. 2025; 9(1):9. https://doi.org/10.3390/colloids9010009
Chicago/Turabian StyleGuerrero-Vasquez, Francisco Joel, Francisco Ortega, Ramón G. Rubio, and Eduardo Guzmán. 2025. "Bio-Based Interpolyelectrolyte Complexes for the Stabilization of Pickering-like Emulsions" Colloids and Interfaces 9, no. 1: 9. https://doi.org/10.3390/colloids9010009
APA StyleGuerrero-Vasquez, F. J., Ortega, F., Rubio, R. G., & Guzmán, E. (2025). Bio-Based Interpolyelectrolyte Complexes for the Stabilization of Pickering-like Emulsions. Colloids and Interfaces, 9(1), 9. https://doi.org/10.3390/colloids9010009








