Abstract
New hexadecylpiperidinium surfactants, containing one or two butylcarbamate fragments, were synthesized. The antimicrobial activity, toxicity, aggregation behavior in aqueous solutions, and solubilization capacity of these surfactants towards the hydrophobic drug ibuprofen were characterized. These surfactants demonstrated a high antimicrobial activity against a wide range of pathogenic bacteria, including both Gram-positive and Gram-negative strains, as well as fungi. By forming mixed-micellar compositions of the cationic surfactant 1-CB(Bu)-P-16 and the nonionic surfactant Brij®35, highly functional and low-toxic formulations were obtained. Furthermore, the transition from mixed micelles to niosomes was accomplished, enhancing their potential as drug delivery systems. Niosomes were found to be less toxic compared to mixed micelles, while also increasing the solubility of ibuprofen in water. The modification of niosomes with cationic surfactants made it possible to increase the stability of the system and improve the solubility of the drug. The data obtained indicate that these new carbamate-containing hexadecylpiperidinium surfactants have significant potential in biomedical applications, particularly in the formulation of advanced drug delivery systems.
1. Introduction
The use of cationic surfactants in biomedicine has become more common in recent years. First of all, these compounds exhibit strong antimicrobial activity, which makes it possible to include them in compositions with a bactericidal effect. Unlike traditional antibiotics, which are based on the “lock–key” mechanism, cationic surfactants exhibit biological activity, mainly due to integration into bacterial membranes through electrostatic and hydrophobic interactions. This entails a violation of their permeability, the disordering of membranes, and, in some cases, leads to their destruction [1,2,3,4,5]. Importantly, cationic amphiphiles form complexes with a variety of biomolecules, changing their properties and facilitating their delivery to the specific targets. For example, these compounds are able to form a complex with DNA, acting as a non-viral vector for genetic engineering [6,7,8,9]. Cationic surfactants are highly effective as modifying additives in liposomal systems, since imparting a positive charge increases their stability, in some cases increases the efficiency of drug encapsulation, and provides a better affinity for biological membranes, thereby increasing the cellular uptake and therapeutic effect of the proposed formulations [10,11,12]. The nature and charge of the head group of a cationic surfactant molecule, as well as the length of the hydrophobic tail, are two of the most significant structural features that can contribute to practical applications [6,13]. Although cationic surfactants can be effective, it is important to note that they can also have toxic effects, especially when used in high concentrations or for prolonged contact with cells. One approach to reducing the toxicity of these compounds is to functionalize them with various biodegradable or natural fragments [14,15]. For example, replacing the alkyl groups in the molecule of a cationic surfactant with a sugar or amino acid moiety can significantly reduce its toxicity without losing its antimicrobial activity [16,17].
Despite the advances in cationic surfactants, there remains a critical need for the development of new surfactants that balance low toxicity with a high efficacy. In addition, efforts are being undertaken to explore new ways to reduce the toxicity of cationic surfactants by developing mixed formulations with nonionic amphiphilic compounds [18,19]. Such compositions can combine the advantages of both cationic and nonionic surfactants, providing a balance between efficacy and safety. Nonionic surfactants typically have a low toxicity and a good biocompatibility, which makes them attractive components for use in mixed systems [18,20]. The interaction between cationic and nonionic surfactants can result in the formation of stable micelles or vesicles that exhibit high solubilization properties and stability [21,22]. These features make them appealing for use in biomedical and pharmaceutical applications. In recent years, there has been increasing interest in the development of niosomes, which are nanoscale systems that have the potential to be used as efficient carriers for delivering therapeutic agents [23,24,25]. Niosomes, which are composed of nonionic surfactants and cholesterol, exhibit a high biocompatibility and stability, as well as the ability to encapsulate both hydrophobic and hydrophilic therapeutic agents. The combination of cationic surfactants with niosomes creates systems with improved properties, as follows: an increased stability, targeted delivery, and a reduced toxicity [23,26,27,28].
In this study, derivatives of hexadecylpiperidinium bromide, functionalized with hydroxyl groups at different positions on the molecule, were used as a basis for designing and synthesizing new carbamate-containing surfactants. Previously, we synthesized and investigated acyclic carbamate-containing surfactants, which demonstrated lower critical micelle concentrations (CMCs), a reduced toxicity, the ability to penetrate cell membranes, and significant antimicrobial activity compared to their trimethylammonium analogs [29,30]. These properties suggest promising potential for the use of this class of surfactants in various biomedical applications. Notably, hexadecyl derivatives outperformed their lower homologues in terms of efficacy. Additionally, the transition to surfactants with cyclic head groups provides an opportunity to vary both the position and number of carbamate substituents, thus influencing the properties of the resulting compounds. As a result, cationic surfactants of the CB(Bu)-P-16 series, which contain one or two butylcarbamate fragments, were first synthesized (Figure 1). The work aimed to systematically investigate these compounds, making it possible to characterize their antimicrobial activity, toxicity, and aggregation behavior in aqueous solutions, as well as their solubilization effect towards the hydrophobic non-steroidal anti-inflammatory drug ibuprofen (IBF).
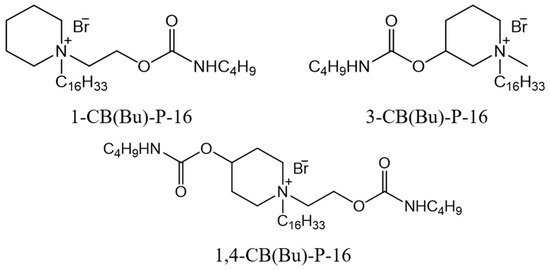
Figure 1.
Structural formulas of hexadecylpiperidinium surfactants containing butylcarbamate fragments.
To obtain compositions with a reduced toxicity and high functional properties, it was planned to form mixed micellar systems based on these cationic surfactants and the nonionic amphiphile Brij®35, as well as to develop niosomes that could serve as drug carriers.
2. Materials and Methods
2.1. Materials
Commercially available nonionic surfactant Brij®35 (Sigma Aldrich, Madrid, Spain), N-methyl-3-hydroxypiperidine (Sigma Aldrich, Darmstadt, Germany, 98%), 1-(2-hydroxyethyl)piperidine (Sigma Aldrich, Darmstadt, Germany, 99%), 4-hydroxy-1-piperidineethanol (ChemCruz, Dallas, TX, USA, 98%), 1-bromohexadecane (Sigma Aldrich, Chengdu, China, 97%), butyl isocyanate (Sigma Aldrich, Chengdu, China, 98%), 1,4-diazabicyclo [2.2.2]octane (DABCO) (Acros Organic, Newark, NJ, USA, 97%), cholesterol (Sigma Aldrich, St. Louis, MO, USA, ≥99%), and ibuprofen (Sigma Aldrich, Chengdu, China, ≥98%) were used without preliminary purification. All other reagents and solvents, acetonitrile (EKOS-1, analytical grade, Moscow, Russia), ethyl acetate (Component-reaktiv, reagent grade, Moscow, Russia), and diethyl ether (Kuzbassorghim, analytical grade, Kemerovo, Russia), were purified prior to use according to [31].
General Procedure for Synthesis of Carbamate-Containing Hexadecylpiperidinium Surfactants
The starting hydroxyl-containing hexadecylpiperidinium bromides were synthesized by reacting the corresponding piperidine with bromohexadecane in acetonitrile, then recrystallizing from acetone or ethyl acetate, as described in [32]. At the next stage, butyl isocyanate (2 eq. per 1 hydroxyl group) was added to a solution of hydroxyl-containing hexadecylpiperidinium bromide (1 eq.), DABCO (0.1 eq.), and 30 mL od dry acetonitrile. The reaction mixture was stirred for 16 h and heated under reflux conditions. The solvent was removed under vacuum (20 mm Hg) after reaction, and the solid residue was recrystallized from ethyl acetate. The precipitate was filtered, washed with diethyl ether, and dried in a water bath (40 °C) under vacuum (15 mm Hg). The final products were obtained as white powders with good yields (79–90%). The structure of the compounds was confirmed using IR and NMR spectroscopy, ESI mass spectrometry, and elemental analysis data. The data obtained are presented in the Supplementary Materials (Figures S1–S9).
As an example, Figure 2 shows a scheme of the synthesis of the cationic surfactant 1,4-CB(Bu)-P-16.
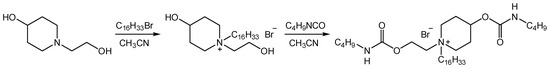
Figure 2.
Synthesis scheme of piperidinium surfactants containing a butylcarbamate fragment.
2.2. Methods
2.2.1. In Vitro Biological Studies
The antimicrobial activity was determined using the serial dilution method in Mueller–Hinton broth for bacterial cultures and Sabouraud broth for fungal pathogens. The bacterial cultures included Staphylococcus aureus ATCC 6538 P FDA 209P, Bacillus cereus ATCC 10702 NCTC 8035, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 9027, as well as methicillin-resistant strains of Staphylococcus aureus (MRSA). The fungal cultures included Candida albicans ATCC 10231. The bacterial and fungal concentrations in the experiment were 3.0 × 105 and 2.0 × 103 CFU·mL−1, respectively. The bacterial and fungal cultures were incubated at 37 °C and 25 °C, respectively. The results were recorded every 24 h for five days. This experiment was repeated three times.
The hemolytic activity of the cationic surfactants was evaluated according to the method [33]. Briefly, heparinized red blood cells (10% suspension) were washed three times with saline (0.9% NaCl), centrifuged for 10 min at 800 rpm, and resuspended to a final concentration of 10%. Surfactant samples were prepared in saline and added to 0.5 mL of the erythrocyte suspension. After 1 h of incubation at 37 °C and centrifugation (10 min at 2000 rpm), hemoglobin release was measured by determining the optical density of the supernatant using a microplate reader (InVitroLogic, Novosibirsk, Russia) at λmax = 540 nm. Control samples for 0% and 100% hemolysis were prepared using saline and distilled water, respectively.
Aqueous solutions of cationic surfactants were tested for cytotoxicity on human normal (Chang liver) cells. The Chang liver cell line was procured from the D.I. Ivanovskiy Institute of Virology (N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health, Moscow, Russia). The cells were cultured in standard Eagle’s nutrient medium (PanEco, Moscow, Russia) supplemented with 10% fetal bovine serum and 1% essential amino acid solution (PanEco, Moscow, Russia) at 37 °C in an atmosphere containing 5% CO2. The cytotoxicity of the tested surfactants against conditionally normal human cells was assessed using the MTT assay. The cells were seeded onto a 96-well Nunc plate with 100 µL of standard Eagle’s medium at a density of 5000 cells per well. After 24 h of incubation, the culture medium in the wells was replaced with a medium containing a solution of the tested compounds. Control wells without test samples were used as a reference. After another 24 h, the cells were stained with an MTT solution (1 mg·mL−1). The resulting insoluble formazan crystals were then dissolved in DMSO. The optical density of the medium was measured at λmax = 540 nm using an InVitroLogic microplate reader (LLC “Medico-Biological Union”, Novosibirsk, Russia). The half-maximal inhibition concentration (IC50) was calculated using the MLA—“Quest Graph™ IC50 Calculator” (AAT Bioquest, Inc., Pleasanton, CA, USA).
2.2.2. Physicochemical Studies of Cationic Surfactants
The aggregation properties of the surfactants were investigated under conditions of maintaining the solutions at 25 °C. The surface tension of the solutions was studied using the anchor-ring method with a KRUSS 6 tensiometer. The specific electrical conductivity was determined using an Inolab Cond 720 conductometer. The CMC was determined from the inflection point on graphs of surface tension and specific conductivity vs. surfactant concentration (Figure 3A,B).
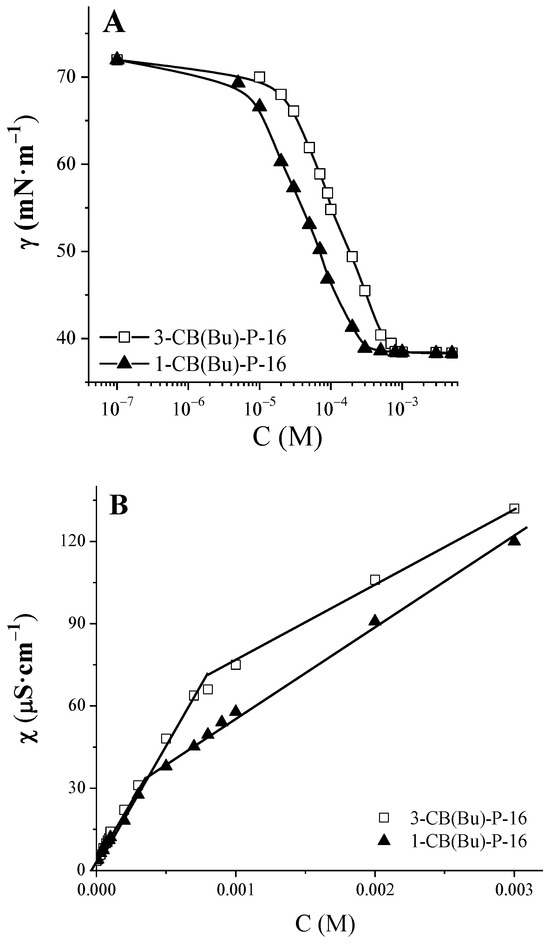
Figure 3.
Surface tension isotherms (A) and concentration dependencies of specific conductivity (B) of carbamate-containing piperidinium surfactants, 25 °C.
The surface potential of micelles was determined by using p-nitrophenol and studying the influence of surfactant solutions on the pKa of this compound at 25 °C. This was performed using a procedure similar to that described in [29]. The absorption spectra of p-nitrophenol were recorded using a Specord 250 spectrophotometer (Analytik Jena, Jena, Germany) in quartz cuvettes with a thickness of 1 cm at various pH values. The measurements were taken in the range from 250 to 600 nm. The concentration of p-nitrophenolate ions at a given pH was determined from the relationship Cphenolate = ε/DL. The molar absorption coefficient (ε) of the phenolate form of p-nitrophenol at its absorption maximum at 400 nm was 18,000 L·mol−1·cm−1. The pKa value of p-nitrophenol was calculated using the Henderson–Hasselbalch Equation (1) as follows:
The average values of the observed pKa,obs were obtained from three to five independent experiments conducted at different pH values.
The solubilization effect of the micellar solutions on ibuprofen (IBF) was evaluated using a spectrophotometric method [34]. The maximum absorption of this compound at 264 nm was used as the analytical signal. The solutions were prepared using a 0.1 M acetate buffer with a pH of 4.5.
2.2.3. Preparation of Niosomes
The formation of niosomes was performed using the thin-film hydration method, followed by sonication, as described in [28]. The hydrated film was prepared using a 0.05 M phosphate buffer solution (PBS) at pH 6.86. Unmodified niosomes were produced at various molar ratios of Brij®35 and cholesterol (Chol). In the case of positively charged niosomes, 0.5 molar equivalents of cationic surfactant were added to the initial formulation. IBF was encapsulated into niosomes during the formation of a thin film. After that, the IBF-encapsulated niosomes were separated from the unencapsulated drug by centrifugation at a speed of 13,000 rpm for 15 min. The samples were stored at 4 °C.
The encapsulation efficiency (EE) was calculated using Equation (2) as follows:
2.2.4. Investigation of the Physicochemical Characteristics of Niosomes
The sizes and zeta potentials of the obtained niosomes were determined using a photon correlation spectrometer for dynamic and electrophoretic light scattering, specifically, the Malvern ZetaSizer Nano (Malvern Instruments, Malvern, UK). The angle of light scattering was set at 173°.
The release of IBF from the niosomes was carried out using the dialysis method. To achieve this, 5 mL of IBF-encapsulated niosomes was placed in a dialysis bag with a pore size of 3.5 kDa. A receiving medium of 0.025 M phosphate buffer solution (PBS) at a pH of 7.4 and a volume of 50 mL was used.
The process was conducted with constant stirring at 37 °C. Aliquots were sampled at intervals, and the concentration of IBF was measured using a spectrophotometric method. The plateau in the dependency curve, which reflects the change in the drug concentration over time, corresponds to the completion of the drug release process from the niosomes.
3. Results and Discussions
3.1. The Antimicrobial Activity of Carbamate-Containing Piperidinium Surfactants
The antimicrobial activity of synthesized carbamate-containing piperidinium surfactants was tested against various pathogenic bacteria and fungi. The bacteria included Gram-positive strains (Staphylococcus aureus (Sa), Bacillus cereus (Bc), and Enterococcus faecalis (Ef)) and Gram-negative strains (Escherichia coli (Ec) and Pseudomonas aeruginosa (Pa)). In addition, resistant forms of Sa were tested, specifically MRSA-1 and MRSA-2. These strains have developed resistance to commonly used antibiotics, making them difficult to treat and posing a significant threat to public health [35,36]. MRSA-1 has developed a high resistance to fluoroquinolone and β-lactam antibiotics, while MRSA-2 is only resistant to β-lactams. The data on the antimicrobial activity of the synthesized surfactants are presented in Table 1.

Table 1.
Antimicrobial activity of butylcarbamate-containing piperidinium surfactants and the mixed system 1-CB(Bu)-P-16/Brij®35 at equimolar ratio.
Based on these data, it can be concluded that the piperidinium surfactants with the butylcarbamate fragment exhibit significant antimicrobial activity. They not only demonstrate inhibitory activity, characterized by the value of their minimum inhibitory concentration (MIC), but also possess pronounced bactericidal and fungicidal properties, as evidenced by the values of their minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC). The tested compounds show a particularly high activity against Gram-positive bacteria. In this case, their bacteriostatic effect is comparable to that of commonly used antibiotics, such as ciprofloxacin. The compounds 1-CB(Bu)-P-16 and 3-CB(But)-P16 show little difference in their antimicrobial activity. However, the introduction of a second butylcarbamate group results in a decrease in activity (see Table 1). It should be noted that the synthesized carbamate-containing piperidinium surfactants are slightly more effective than their analogues that do not contain a cyclic group, namely N-(2-((butylcarbamoyl)oxy)ethyl)-N,N-dimethylhexadecylammonium bromide (CB(Bu)-16) [30]. It was found out that the tested surfactants showed a high activity against resistant strains of Sa, MRSA-1 and MRSA-2. The activity of these surfactants exceeded that of the reference drugs, as shown in Table 1. To eliminate the influence of the different molecular weights of the compounds and ensure an accurate comparison of their antimicrobial activity, the data were converted and are expressed in units of μmol·dm−3 (see Table S1). It should be noted that the MIC and MBC values remained comparable.
The investigated compounds exhibit antimicrobial activity not only against bacteria, but also against fungi. An analysis of their effect on Candida albicans (Ca) strains showed that their fungistatic activity was comparable to that of the commercial antifungal drug ketoconazole.
Thus, the synthesized carbamate-containing piperidinium surfactants demonstrate a high activity against a wide variety of pathogenic strains. However, recommendation for the use of biocides in therapy requires testing their safety in mammals. Therefore, the cytotoxic effect of the synthesized surfactants on blood erythrocytes and the Chang liver (human hepatocyte cell line) was investigated. The data obtained are presented in the form of HC50 and IC50 values, which indicate the concentration of the compound that caused a 50% hemolysis of red blood cells or 50% cell death, respectively. They are also presented as a selectivity index (SI), which is the ratio between the concentration leading to a 50% lysis of human erythrocytes and the minimum concentration that inhibits bacterial growth [37,38]. These values are shown in Table 2.

Table 2.
Evaluation of the cytotoxicity of carbamate-containing piperidinium surfactants and the mixed system 1-CB(Bu)-P-16/Brij®35 at equimolar ratio on human red blood and Chang liver cells.
The selectivity index values obtained for the test strain of Sa indicate the low toxicity of the surfactants studied towards healthy human cells. This finding is important in the development of antimicrobial agents that could be safely used in mammals. It should be noted that the hemolytic activity of the carbamate-containing piperidinium surfactants and their methylpiperidinium analog PM-16 are similar, but their selectivity is significantly higher than that of the reference compound, as shown by the SI values in Table 2.
3.2. Aggregation Behavior of Carbamate-Containing Piperidinium Surfactants
Aqueous solutions of the synthesized surfactants were analyzed using tensiometry and conductometry. Figure 3A,B show the surface tension isotherms and concentration dependences of specific electrical conductivity for the compounds 1-CB(Bu)-P-16 and 3-CB(Bu)-P-16, respectively. The values of the CMC determined on the basis of these data are given in Table 3. It should be noted that there is a good agreement between the values obtained using the two methods, indicating their high reliability. Due to the low water solubility of 1,4-CB(Bu)-P-16, it was not possible to conduct a reliable study on its aggregation behavior.

Table 3.
Aggregation characteristics of carbamate-containing piperidinium surfactants and the maximum solubility of IBF a.
Based on conductometric measurements, the degree of counterion binding was evaluated by comparing the slopes of the curves before and after the CMC. For carbamate-containing piperidinium surfactants, this value was found to be lower than the β of their non-functionalized analogue PM-16, as shown in Table 3. This suggests a decrease in the compensation of the surface charge of micelles by bromide ions, which was supported by the values of the surface potential (ψ) of the micelles, as shown in Table 3. These values were determined using spectrophotometry with p-nitrophenol as a spectral probe, similar to that described in [34,39]. This method is based on the change in the pKa value of the spectral probe in the presence of surfactants. This change is associated with the different bindings of the neutral and anionic forms of p-nitrophenol by micelles. The magnitude of the shift depends on the surface potential of micelles and is determined by Equation (3), as follows:
where pKa,0 is the non-electrostatic component, determined as the pKa in micellar solutions based on nonionic surfactants (pKa of p-nitrophenol in Brij®35 is 7.6); F = 96,486 C·mol−1 is the Faraday constant; and R = 8.314 J⋅K−1·mol−1 is the molar gas constant. The results obtained are presented in Table 3. The value of ψ for compounds of the CB(Bu)-P-16 series is lower than that of PM-16.
pKa,m = pKa,0 − Fψ/2.303RT,
At concentrations of the surfactants studied above the CMC, their ability to increase the solubility of the hydrophobic drug IBF was tested using a spectrophotometric method. IBF is a non-steroidal anti-inflammatory drug with strong analgesic and antipyretic effects, commonly administered orally [40]. However, the poor water solubility of IBF limits its absorption and bioavailability, often requiring higher doses and increasing the risk of side effects such as gastritis, ulcers, and gastrointestinal bleeding [41,42,43]. Several strategies have been suggested to improve the solubility and bioavailability of IBF by using nanocarriers. For example, the use of the nonionic surfactant Tween 80 and a polymeric stabilizer has been shown to increase the oral bioavailability of IBF by two times compared to commercial formulations [41]. The encapsulation of IBF into mixed micelles not only enhances its absorption through cell membranes, but also reduces its toxicity to cells [44]. An effective strategy to improve the oral bioavailability of a drug is encapsulating it in solid lipid nanoparticles. This approach has been shown to increase bioavailability ninefold, as well as providing slow release and prolonged therapeutic effects [45].
The absorption of IBF in the ultraviolet region, with a maximum at 264 nm (the molar absorption coefficient at pH 4.5 is 282 L·mol−1·cm−1 [34]), allows us to determine its solubility in solutions of carbamate-containing piperidinium surfactants (Table 3).
Based on the data presented in Table 3, it can be concluded that the addition of 1 mM surfactant increased the solubility of IBF in water by 3–3.5 times. However, further increasing the surfactant concentration in the solution did not enhance the solubility of IBF. Instead, precipitation began to form in the system when the surfactant concentration exceeded approximately three times the CMC. It is possible that the anionic form of IBF forms a complex with a cationic surfactant through electrostatic interactions, which can lead to a reduced solubility in water.
3.3. Mixed Systems Based on Carbamate-Containing Piperidinium Surfactants and Nonionic Surfactant Brij®35
One approach to creating drug delivery systems is to form mixed compositions that contain cationic and nonionic surfactants in specific ratios. This approach allows for an increase in system safety by using non-toxic nonionic surfactants, while maintaining a high efficiency through electrostatic interactions between the cationic surfactant and charged components of biological targets. The successful application of binary systems of ionic and nonionic surfactants has been demonstrated in various examples, including tetracycline family antibiotics [46] and antimicrobial drugs such as norfloxacin and cyclosporin [47,48].
In this study, mixed micellar solutions of the cationic surfactant 1-CB(Bu)-P-16 and the nonionic surfactant Brij®35 were formed and studied. The formation of mixed micelles involves the incorporation of the hydrophilic parts of the nonionic surfactant between the charged headgroups of the cationic amphiphiles. This reduces the electrostatic repulsion between them and facilitates the formation of micelles in solution. It also reduces the overall charge density on the surfaces of the aggregates. The interaction between surfactant molecules can lead to a synergistic effect, the magnitude of which is determined by the structure of each component in the binary composition [49,50,51].
The CMC values of the systems 1-CB(Bu)-P-16 with the nonionic surfactant Brij®35 were determined using tensiometry (Figure S10). The values obtained from the surface tension isotherms under different conditions, where the ratio of their components varied, are shown in Figure 4. These values are compared with those calculated using the Clint’s model [49], which allows us to estimate the CMC of an ideal mixed system using Equation (4), as follows:
where α1 and α2 are the molar fractions of the ionic and nonionic surfactants in the solution, respectively, and C*, C1, and C2 are the CMC values for the mixed system and its ionic and nonionic surfactants, respectively.
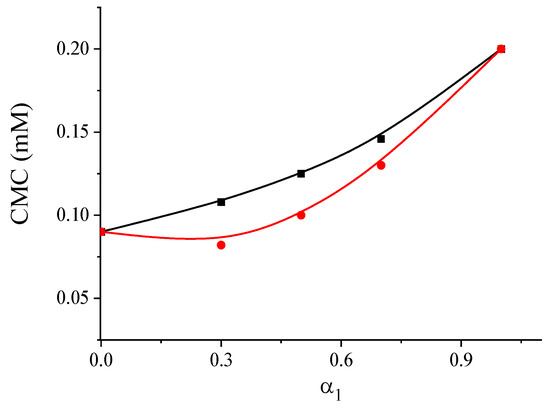
Figure 4.
The dependence of calculated (black line) and experimental (red line) CMC values on the composition of the solution for mixtures of 1-CB(Bu)-P-16/Brij®35 (α1—mole fraction of cationic surfactant).
The experimental CMC values for the system were found to be lower than the calculated values, indicating a mutual attraction between the different types of surfactants in the micelles and suggesting a synergistic effect. This effect was most pronounced when the molar fraction of the cationic surfactant in the binary composition was in the range from 0.3 to 0.5. For the system 1-CB(Bu)-P-16/Brij®35 (α1 = 0.5), the surface potential was determined to be 47 mV, and its ability to increase the solubility of IBF was evaluated. The observed decrease in micellar charge probably reduced their electrostatic interaction with the anionic form of IBF. As a result, the concentration of the mixed system could be increased over a wide range without precipitate formation. It was found that, for a mixed system with α1 = 0.5 at pH 4.5 and a total surfactant concentration of 1 mM, the solubility of IBF was 0.25 g L−1. Doubling the total concentration of the surfactant allowed for achieving a concentration of this drug of 0.67 g L−1, thus increasing its solubility in water by seven times. For comparison, the solubility limit of IBF in an individual solution of Brij®35 is 0.13 g·L−1. This value is almost two times lower than the solubility in the mixed system of 1-CB(Bu)-P-16/Brij®35 at an equimolar ratio, and more than two times lower compared to an individual solution of the cationic surfactant 1-CB(Bu)-P-16 (see Table 3). A comparison of the solubility limits of IBF in micellar solutions was conducted at a surfactant concentration of 1 mM.
Additionally, the antimicrobial properties of the 1-CB(Bu)-P-16/Brij®35 system were tested with α1 = 0.5. Based on the data presented in Table 1, it follows that the antimicrobial activity of the system was lower than that of the individual cationic surfactant. Furthermore, the obtained effective concentration values did not indicate a synergistic effect. However, the mixed composition showed a quite high activity and can be considered as a potential basis for antimicrobial formulations. An additional benefit of using mixed formulations is their reduced toxicity due to the inclusion of safe nonionic surfactants. This is indicated by the hemolytic activity index value of the system 1-CB(Bu)-P-16/Brij®35 at an equimolar ratio (see Table 2).
3.4. Niosomes Modified with Cationic Surfactants
The next step in the development of systems based on Brij®35 and carbamate-containing piperidinium surfactants is the transition from mixed systems to modified niosomes. Niosomes are nano-sized vesicular structures composed of nonionic surfactants and cholesterol (Chol). Cholesterol enhances the stability of the lipid bilayer, while also influencing its fluidity and permeability [52,53]. In recent years, niosomes have been intensively studied as potential drug delivery systems for antigens, hormones, and other biologically active agents [54,55,56]. Incorporating cationic surfactant additives into the niosome structure imparts it with a positive charge, which enhances its ability to penetrate biological barriers. This allows for increased drug loading through the use of electrostatic interactions. In this study, the thin-film hydration method was used to produce niosomes based on Brij®35 and cholesterol, as well as their modified analogs containing the cationic surfactant 1-CB(Bu)-P-16. The niosomes were prepared at different molar ratios of components (see Table 4).

Table 4.
Physicochemical characteristics and stability of empty niosomes Brij®35/Chol/1-CB(Bu)-P-16 at various molar ratios of components.
Using the dynamic light scattering method, it was found that the hydrodynamic diameter (Dh) of the Brij®35/Chol niosomes was 70–80 nm. As shown in Table 4, variations in the molar ratios directly affected the system stability. For example, increasing the Brij®35 content to a 7:3 ratio led to a higher polydispersity index (PdI) and a decrease in zeta potential (ZP), which was likely due to increased membrane fluidity. However, when a 0.5 mole fraction of a cationic surfactant was added to the niosomes, there was a slight decrease in particle size and an increase in ZP. These results are summarized in Table 4.
It should be noted that all the obtained niosomes remained quite stable for at least two months. The stability of the niosomes was assessed using dynamic and electrophoretic light scattering methods over a two-month period. The results showed that niosomes composed of Brij®35 and cholesterol at ratios of 5:5 and 6:4 demonstrated the highest storage stability (Table 4). Throughout the storage period, the hydrodynamic diameter of the particles ranged from 72 to 78 nm. The PdI remained low, between 0.22 and 0.26, indicating minimal aggregation and confirming both the high stability and uniformity of the system. The modification of niosomes with cationic surfactants resulted in a positive surface charge of up to 40 mV, which further enhanced their stability. Similar results were obtained for niosomes containing ibuprofen, where particle size changes were minimal and a high uniformity was maintained throughout the two-month storage period (Table 5).

Table 5.
Physicochemical properties and stability of IBF-loaded niosomes.
Using the example of niosomes containing an equimolar ratio of Brij®35 and cholesterol, as well as those modified with a cationic surfactant, their cytotoxicity was investigated. The study showed that these systems had a lower toxicity to human blood cells and healthy liver cells (Chang liver) than mixed compositions. The HC50 values ranged from 117 to 120 µg·mL−1, while the IC50 was more than 3900 µg·mL−1. These data highlight the potential safety and efficacy of niosomal systems for the development of novel dosage forms.
Therefore, niosomes with equimolar amounts of Brij®35 and cholesterol, including those containing 0.5 M of 1-CB(Bu)-P-16, were selected for drug loading. The characteristics of the particles obtained are presented in Table 5.
Analysis showed that the presence of IBF had a minor effect on the size of the niosomes, but it slightly reduced their ZP. The calculated values of the encapsulation efficiency indicate that the optimal concentration for loading IBF was its initial concentration of 2 g·L−1. At this concentration, the particles were more uniform in size, as indicated by the PdI. This would also help to avoid unnecessary drug costs. It should be noted that the presence of the cationic surfactant significantly enhanced the encapsulation efficiency of the IBF into the niosomes. Monitoring the size and charge of IBF-loaded niosomes for two months showed reproducible results, indicating their stability (see Table 5).
An important property of drug delivery systems is their ability to slowly release encapsulated substances. The release of IBF from the niosomal formulation was monitored using a dialysis method, followed by a spectrophotometric determination of the drug concentration (Figure 5).
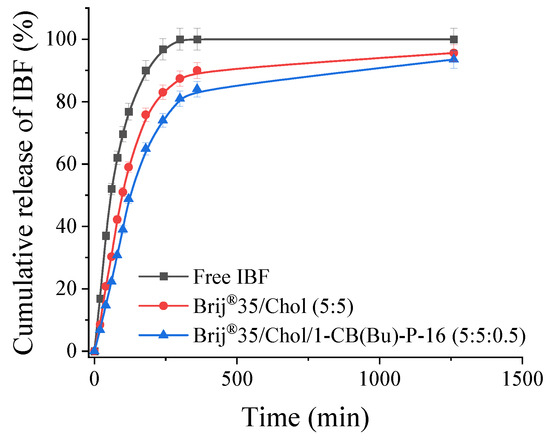
Figure 5.
IBF release profile from niosomes at pH 7.4, 37 °C.
It was found that, when free IBF was placed in a dialysis bag as an aqueous ethanol solution, it diffused through the pores (3.5 kDa) into the external solution over a period of approximately 4 h. The release rate of IBF encapsulated into niosomes was slightly lower, with around 70% of the drug being released into the bulk medium. Niosomes modified with a cationic surfactant reduced the release of IBF compared to systems with a neutral charge. This fact determines the potential of niosomes for creating dosage forms with prolonged release. In addition, one of the main advantages of niosomes modified with carbamate-containing piperidinium surfactants is that they allow for the concentration of the drug to be about 20 times higher than its maximum solubility in its free form.
4. Conclusions
Thus, a systematic investigation of new butylcarbamate-containing hexadecylpiperidinium surfactants of CB(Bu)-P-16 was carried out. Their high antimicrobial activity against a wide range of fungi and pathogenic bacteria, both Gram-positive and Gram-negative, was demonstrated. This indicates their potential as promising antimicrobial agents. The cytotoxic effect of the compounds on human blood erythrocytes and liver cells was also evaluated. It was shown that the presence of a carbamate fragment affected the aggregation behavior of the surfactants studied, as follows: the concentration threshold for micelle formation decreased compared to the unsubstituted analogs. To improve the safety of the system, binary compositions based on CB(Bu)-P-16 and the non-toxic nonionic surfactant Brij®35 were formulated. The formation of mixed micelles showed a synergistic effect, which was manifested in lower values of the critical micelle concentration compared to those calculated based on an ideal mixing model. Further, a transition was made from mixed micelles to niosomes, making them suitable for application as drug delivery systems. Using the example of the anti-inflammatory drug ibuprofen, an increase in solubility was demonstrated upon the transition from single 1-CB(Bu)-P-16 solutions to mixed micelles and niosomes composed of a nonionic surfactant, cholesterol, and the cationic surfactant. The mixed micelles allowed for an increase in ibuprofen solubility by up to two times, compared to the individual nonionic surfactant Brij®35. Additionally, the modification of niosomes with cationic surfactants doubled the encapsulation efficiency of ibuprofen, potentially enhancing its bioavailability and prolonging its therapeutic effect. This suggests that the inclusion of cationic surfactants in both mixed micelles and niosomes consistently improves their functional activity, leading to more efficient and versatile delivery systems. Furthermore, all the studied systems demonstrated a low cytotoxicity, further confirming their safety and suitability for biomedical applications. The data obtained indicate a significant nanomedicine potential for new carbamate-containing hexadecylpiperidinium surfactants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/colloids8050057/s1, Description of the synthesis and spectral characteristics of carbamate-containing hexadecylpiperidinium surfactants; Figures S1, S4 and S7: NMR 1H spectrum of 1-CB(Bu)-P-16, 3-CB(Bu)-P-16, 1,4-CB(Bu)-P-16, respectively; Figures S2, S5 and S8: ESI mass spectrum of 1-CB(Bu)-P-16, 3-CB(Bu)-P-16, 1,4-CB(Bu)-P-16, respectively; Figures S3, S6 and S9: IR spectrum of 1-CB(Bu)-P-16, 3-CB(Bu)-P-16, 1,4-CB(Bu)-P-16, respectively; Figure S10: Surface tension isotherms of micellar solutions, Table S1: Antimicrobial activity of cationic surfactants, expressed in µmol·dm−3.
Author Contributions
Conceptualization, A.M. and L.Z.; methodology, R.K., A.M. and A.V.; formal analysis, D.B., D.K. and A.L.; investigation, R.K., D.B., D.K. and A.L.; writing–original draft preparation, R.K. and A.M.; writing–review and editing, R.K., A.M., A.V. and L.Z.; supervision, L.Z.; project administration, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the government assignment for the FRC Kazan Scientific Center of RAS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author: Alla Mirgorodskaya.
Acknowledgments
The authors thank Assigned Spectral-Analytical Center of FRC Kazan Scientific Center of RAS and Distributed Spectral-Analytical Center of Shared Facilities for Study of Structure, Composition and Properties of Substances and Materials of FRC Kazan Scientific Center of RAS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nunes, B.; Cagide, F.; Fernandes, C.; Borges, A.; Borges, F.; Simões, M. Efficacy of Novel Quaternary Ammonium and Phosphonium Salts Differing in Cation Type and Alkyl Chain Length against Antibiotic-Resistant Staphylococcus Aureus. Int. J. Mol. Sci. 2023, 25, 504. [Google Scholar] [CrossRef]
- Boyce, J.M. Quaternary Ammonium Disinfectants and Antiseptics: Tolerance, Resistance and Potential Impact on Antibiotic Resistance. Antimicrob. Resist. Infect. Control 2023, 12, 32. [Google Scholar] [CrossRef]
- Lu, Z.; Mahony, A.K.; Arnold, W.A.; Marshall, C.W.; McNamara, P.J. Quaternary Ammonia Compounds in Disinfectant Products: Evaluating the Potential for Promoting Antibiotic Resistance and Disrupting Wastewater Treatment Plant Performance. Environ. Sci. Adv. 2024, 3, 208–226. [Google Scholar] [CrossRef]
- Mai, Y.; Wang, Z.; Zhou, Y.; Wang, G.; Chen, J.; Lin, Y.; Ji, P.; Zhang, W.; Jing, Q.; Chen, L.; et al. From Disinfectants to Antibiotics: Enhanced Biosafety of Quaternary Ammonium Compounds by Chemical Modification. J. Hazard. Mater. 2023, 460, 132454. [Google Scholar] [CrossRef]
- Rambo, M.K.D.; Lins, R.F.; Silva, F.L.N.; Alonso, A.; Rambo, M.C.D.; Leal, J.E.C.; Sousa-Neto, D. de Effect of Cationic Surfactant on the Physicochemical and Antibacterial Properties of Colloidal Systems (Emulsions and Microemulsions). Braz. J. Biol. 2024, 84, e278013. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Holmberg, K.; Lindman, B. Cationic Surfactants: A Review. J. Mol. Liq. 2023, 375, 121335. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- Radhakrishnan, A.V.; Madhukar, S.; Chowdhury, A.; Raghunathan, V.A. Influence of Micellar Size on the Structure of Surfactant-DNA Complexes. Phys. Rev. E 2022, 105, 064504. [Google Scholar] [CrossRef]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for mRNA Vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Maganova, F.I.; Sinyashin, K.O.; Gaynanova, G.A.; Mirgorodskaya, A.B.; Vasilieva, E.A.; Sinyashin, O.G. Supramolecular Strategy for the Design of Nanocarriers for Drugs and Natural Bioactives: Current State of the Art (A Review). Russ. J. Gen. Chem. 2023, 93, 1867–1899. [Google Scholar] [CrossRef]
- Muthuprasanna, P.; Suriaprabha, K.; Rao, T.S.; Mandava, V.; Sindhu, B. Analgesic and Antiinflammatory Study of Surfactant Modified Liposomes. Biosci. Biotechnol. Res. Asia 2016, 5, 255–260. [Google Scholar]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Obata, Y.; Takayama, K.; Ngawhirunpat, T.; Pamornpathomkul, B. Role of the Charge, Carbon Chain Length, and Content of Surfactant on the Skin Penetration of Meloxicam-Loaded Liposomes. Int. J. Nanomed. 2014, 9, 2005–2017. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure–Activity Relationship of Cationic Surfactants as Antimicrobial Agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between Surfactants and the Skin—Theory and Practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Matteo Jörgensen, A.; Knoll, P.; Haddadzadegan, S.; Fabian, H.; Hupfauf, A.; Gust, R.; Georg Jörgensen, R.; Bernkop-Schnürch, A. Biodegradable Arginine Based Steroid-Surfactants: Cationic Green Agents for Hydrophobic Ion-Pairing. Int. J. Pharm. 2023, 630, 122438. [Google Scholar] [CrossRef]
- Jesus, C.F.; Alves, A.A.S.; Fiuza, S.M.; Murtinho, D.; Antunes, F.E. Mini-Review: Synthetic Methods for the Production of Cationic Sugar-Based Surfactants. J. Mol. Liq. 2021, 342, 117389. [Google Scholar] [CrossRef]
- Olutas, E.B.; Kartal, N.B.; Birinci Yildirim, A. Self-Assembly, Surface, Antibacterial, and Solubilization Properties of Phenylglycine Type Amino Acid-Based Cationic Surfactants. J. Mol. Liq. 2022, 367, 120528. [Google Scholar] [CrossRef]
- Han, W.; Long, W.; Peng, L.; Zhang, W.; Shi, B. Effect of Nonionic and Anionic Surfactant on Ecotoxicity and Micellization Behaviors of Dodecyl Trimethyl Ammonium Bromide (DTAB). Colloids Surf. Physicochem. Eng. Asp. 2023, 671, 131588. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Voloshina, A.D.; Amerhanova, S.K.; Lenina, O.A.; Petrov, K.A.; Zakharova, L.Y. Improvement of Aggregation Behavior, Toxicity and Antimicrobial Properties of Hydroxypiperidinium Surfactants by the Formation of Mixed Micelles with Tween 80. J. Mol. Liq. 2023, 384, 122289. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E.; Miksch, K.; Malachowska-Jutsz, A.; Kalka, J. Acute Toxicity and Genotoxicity of Five Selected Anionic and Nonionic Surfactants. Chemosphere 2005, 58, 1249–1253. [Google Scholar] [CrossRef]
- Öztürk, K.; Arslan, F.B.; Öztürk, S.C.; Çalış, S. Mixed Micelles Formulation for Carvedilol Delivery: In-Vitro Characterization and in-Vivo Evaluation. Int. J. Pharm. 2022, 611, 121294. [Google Scholar] [CrossRef]
- Dehghan, S.; Naghipour, A.; Anbaji, F.Z.; Golshanrad, P.; Mirazi, H.; Adelnia, H.; Bodaghi, M.; Far, B.F. Enhanced in Vitro and in Vivo Anticancer Activity through the Development of Sunitinib-Loaded Nanoniosomes with Controlled Release and Improved Uptake. Int. J. Pharm. 2023, 640, 122977. [Google Scholar] [CrossRef]
- Poustforoosh, A. Investigation on the Structural and Dynamical Properties of Cationic, Anionic, and Catanionic Niosomes as Multifunctional Controlled Drug Delivery System for Cabozantinib. Colloids Surf. Physicochem. Eng. Asp. 2024, 687, 133547. [Google Scholar] [CrossRef]
- Faddah, H.; Nsairat, H.; Shalan, N.M.; El-Tanani, M.; Alqudah, D.A.; Alshaer, W. Preparation, Optimization and In Vitro Evaluation of Doxorubicin-loaded into Hyaluronic Acid Coated Niosomes Against Breast Cancer. Chem. Biodivers. 2024, 21, e202301470. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Vyas, S.P. Non-Ionic Surfactant Based Vesicles (Niosomes) in Drug Delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Roque, L.; Fernández, M.; Benito, J.M.; Escudero, I. Stability and Characterization Studies of Span 80 Niosomes Modified with CTAB in the Presence of NaCl. Colloids Surf. Physicochem. Eng. Asp. 2020, 601, 124999. [Google Scholar] [CrossRef]
- Grijalvo, S.; Puras, G.; Zárate, J.; Sainz-Ramos, M.; Qtaish, N.A.L.; López, T.; Mashal, M.; Attia, N.; Díaz Díaz, D.; Pons, R.; et al. Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery. Pharmaceutics 2019, 11, 50. [Google Scholar] [CrossRef]
- Kushnazarova, R.A.; Mirgorodskaya, A.B.; Zakharova, L.Y. Niosomes Modified with Cationic Surfactants to Increase the Bioavailability and Stability of Indomethacin. Russ. Chem. Bull. 2021, 70, 585–591. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Kuznetsov, D.M.; Tyryshkina, A.A.; Zakharova, L.Y. Aggregation Behavior and Catalytic Action of Carbamate-Bearing Surfactants in Aqueous Solutions. Kinet. Catal. 2022, 63, 261–269. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Voloshina, A.D.; Lenina, O.A.; Zakharova, L.Y.; Sinyashin, O.G. Carbamate-Bearing Surfactants: Micellization, Solubilization, and Biological Activity. J. Mol. Liq. 2018, 269, 203–210. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2024; Available online: https://www.academia.edu/8067506/W_L_F_Armarego_D_D_Perrin_Purification_of_Laboratory_Chemicals_4th_edition (accessed on 4 October 2024).
- Kushnazarova, R.A.; Mirgorodskaya, A.B.; Kuznetsov, D.M.; Tyryshkina, A.A.; Voloshina, A.D.; Gumerova, S.K.; Lenina, O.A.; Nikitin, E.N.; Zakharova, L.Y. Modulation of Aggregation Behavior, Antimicrobial Properties and Catalytic Activity of Piperidinium Surfactants by Modifying Their Head Group with a Polar Fragment. J. Mol. Liq. 2021, 336, 116318. [Google Scholar] [CrossRef]
- Voloshina, A.D.; Gumerova, S.K.; Sapunova, A.S.; Kulik, N.V.; Mirgorodskaya, A.B.; Kotenko, A.A.; Prokopyeva, T.M.; Mikhailov, V.A.; Zakharova, L.Y.; Sinyashin, O.G. The Structure – Activity Correlation in the Family of Dicationic Imidazolium Surfactants: Antimicrobial Properties and Cytotoxic Effect. Biochim. Biophys. Acta BBA Gen. Subj. 2020, 1864, 129728. [Google Scholar] [CrossRef]
- Kushnazarova, R.A.; Mirgorodskaya, A.B.; Bekrenev, D.D.; Lyubina, A.P.; Lenina, O.A.; Petrov, K.A.; Voloshina, A.D.; Zakharova, L.Y. Supramolecular Systems Based on 2-Hydroxyethylpiperidinium Surfactants and Brij® 35: Aggregation Behavior, Solubilization Properties, and Antimicrobial Activity. Russ. Chem. Bull. 2024, 73, 536–545. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental Factors Influencing the Development and Spread of Antibiotic Resistance. FEMS Microbiol. Rev. 2018, 42, 68–80. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of In-Vitro Bioassay Methods: Application in Herbal Drug Research. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 46, pp. 273–307. ISBN 978-0-12-824127-1. [Google Scholar]
- Ilić, N.; Novković, M.; Guida, F.; Xhindoli, D.; Benincasa, M.; Tossi, A.; Juretić, D. Selective Antimicrobial Activity and Mode of Action of Adepantins, Glycine-Rich Peptide Antibiotics Based on Anuran Antimicrobial Peptide Sequences. Biochim. Biophys. Acta Biomembr. 2013, 1828, 1004–1012. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O. Protolytic Equilibrium in Lyophilic Nanosized Dispersions: Differentiating Influence of the Pseudophase and Salt Effects. Pure Appl. Chem. 2008, 80, 1459–1510. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Theken, K.N.; Gong, L.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Ibuprofen Pathways. Pharmacogenet. Genom. 2015, 25, 96–106. [Google Scholar] [CrossRef]
- Hedaya, M.; Bandarkar, F.; Nada, A. In Vitro and in Vivo Evaluation of Ibuprofen Nanosuspensions for Enhanced Oral Bioavailability. Med. Princ. Pract. 2021, 30, 361–368. [Google Scholar] [CrossRef]
- Stoyanova, K.; Vinarov, Z.; Tcholakova, S. Improving Ibuprofen Solubility by Surfactant-Facilitated Self-Assembly into Mixed Micelles. J. Drug Deliv. Sci. Technol. 2016, 36, 208–215. [Google Scholar] [CrossRef]
- Álvarez, C.; Núñez, I.; Torrado, J.J.; Gordon, J.; Potthast, H.; García-Arieta, A. Investigation on the Possibility of Biowaivers for Ibuprofen. J. Pharm. Sci. 2011, 100, 2343–2349. [Google Scholar] [CrossRef]
- Kumar, M.; Khushi, K.; Bhardwaj, A.; Deb, D.K.; Singh, N.; Elahi, D.; Sharma, S.; Bajpai, G.; Srivastava, A. In-Vitro Study for Ibuprofen Encapsulation, Controlled Release and Cytotoxicity Improvement Using Excipient-Drugs Mixed Micelle. Colloids Surf. Physicochem. Eng. Asp. 2022, 654, 130057. [Google Scholar] [CrossRef]
- Imran, B.; Din, F.U.; Ali, Z.; Fatima, A.; Khan, M.W.; Kim, D.W.; Malik, M.; Sohail, S.; Batool, S.; Jawad, M.; et al. Statistically Designed Dexibuprofen Loaded Solid Lipid Nanoparticles for Enhanced Oral Bioavailability. J. Drug Deliv. Sci. Technol. 2022, 77, 103904. [Google Scholar] [CrossRef]
- Tinku; Choudhary, S. A Thermodynamic Approach to Understand the Partitioning of Tetracycline Family Antibiotics in Individual and Mixed Micellar Systems. J. Chem. Thermodyn. 2022, 165, 106664. [Google Scholar] [CrossRef]
- Tănase, M.A.; Raducan, A.; Oancea, P.; Diţu, L.M.; Stan, M.; Petcu, C.; Scomoroşcenco, C.; Ninciuleanu, C.M.; Nistor, C.L.; Cinteza, L.O. Mixed Pluronic—Cremophor Polymeric Micelles as Nanocarriers for Poorly Soluble Antibiotics—The Influence on the Antibacterial Activity. Pharmaceutics 2021, 13, 435. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Poloxamer 407/TPGS Mixed Micelles as Promising Carriers for Cyclosporine Ocular Delivery. Mol. Pharm. 2018, 15, 571–584. [Google Scholar] [CrossRef]
- Clint, J.H. Micellization of Mixed Nonionic Surface Active Agents. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 1327. [Google Scholar] [CrossRef]
- Rosen, M.J.; Hua, X.Y. Surface Concentrations and Molecular Interactions in Binary Mixtures of Surfactants. J. Colloid Interface Sci. 1982, 86, 164–172. [Google Scholar] [CrossRef]
- Kumar Shah, S.; Chakraborty, G.; Bhattarai, A.; De, R. Synergistic and Antagonistic Effects in Micellization of Mixed Surfactants. J. Mol. Liq. 2022, 368, 120678. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Moacă, E.-A.; Péter, F. Niosomes: Composition, Formulation Techniques, and Recent Progress as Delivery Systems in Cancer Therapy. Pharmaceutics 2024, 16, 223. [Google Scholar] [CrossRef]
- Nasseri, B. Effect of Cholesterol and Temperature on the Elastic Properties of Niosomal Membranes. Int. J. Pharm. 2005, 300, 95–101. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent Advances in Non-Ionic Surfactant Vesicles (Niosomes): Fabrication, Characterization, Pharmaceutical and Cosmetic Applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Pires, P.C.; Paiva-Santos, A.C.; Veiga, F. Liposome-Derived Nanosystems for the Treatment of Behavioral and Neurodegenerative Diseases: The Promise of Niosomes, Transfersomes, and Ethosomes for Increased Brain Drug Bioavailability. Pharmaceuticals 2023, 16, 1424. [Google Scholar] [CrossRef]
- Barani, M.; Paknia, F.; Roostaee, M.; Kavyani, B.; Kalantar-Neyestanaki, D.; Ajalli, N.; Amirbeigi, A. Niosome as an Effective Nanoscale Solution for the Treatment of Microbial Infections. BioMed Res. Int. 2023, 2023, 9933283. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).