Flowable Electrodes from Colloidal Suspensions of Thin Multiwall Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
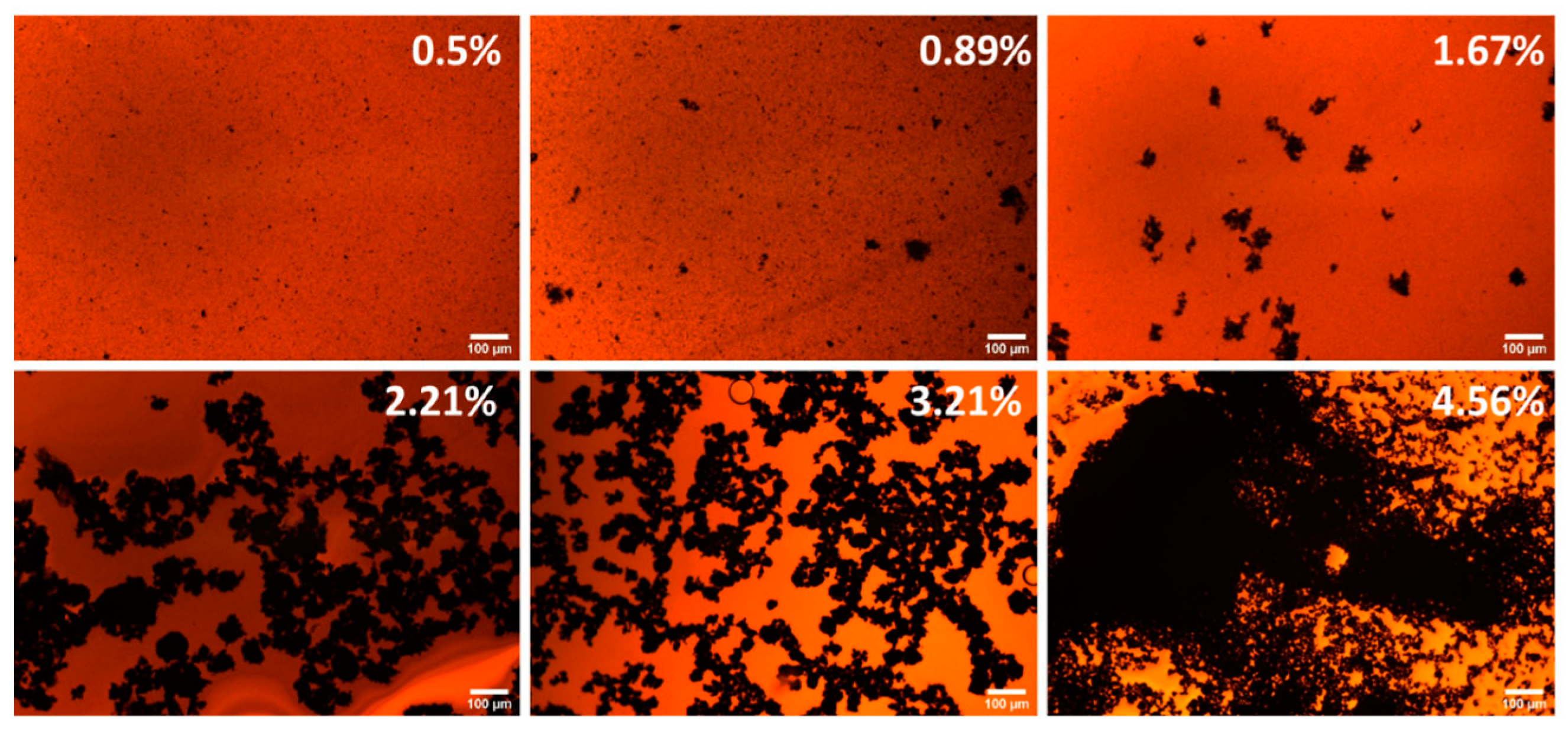
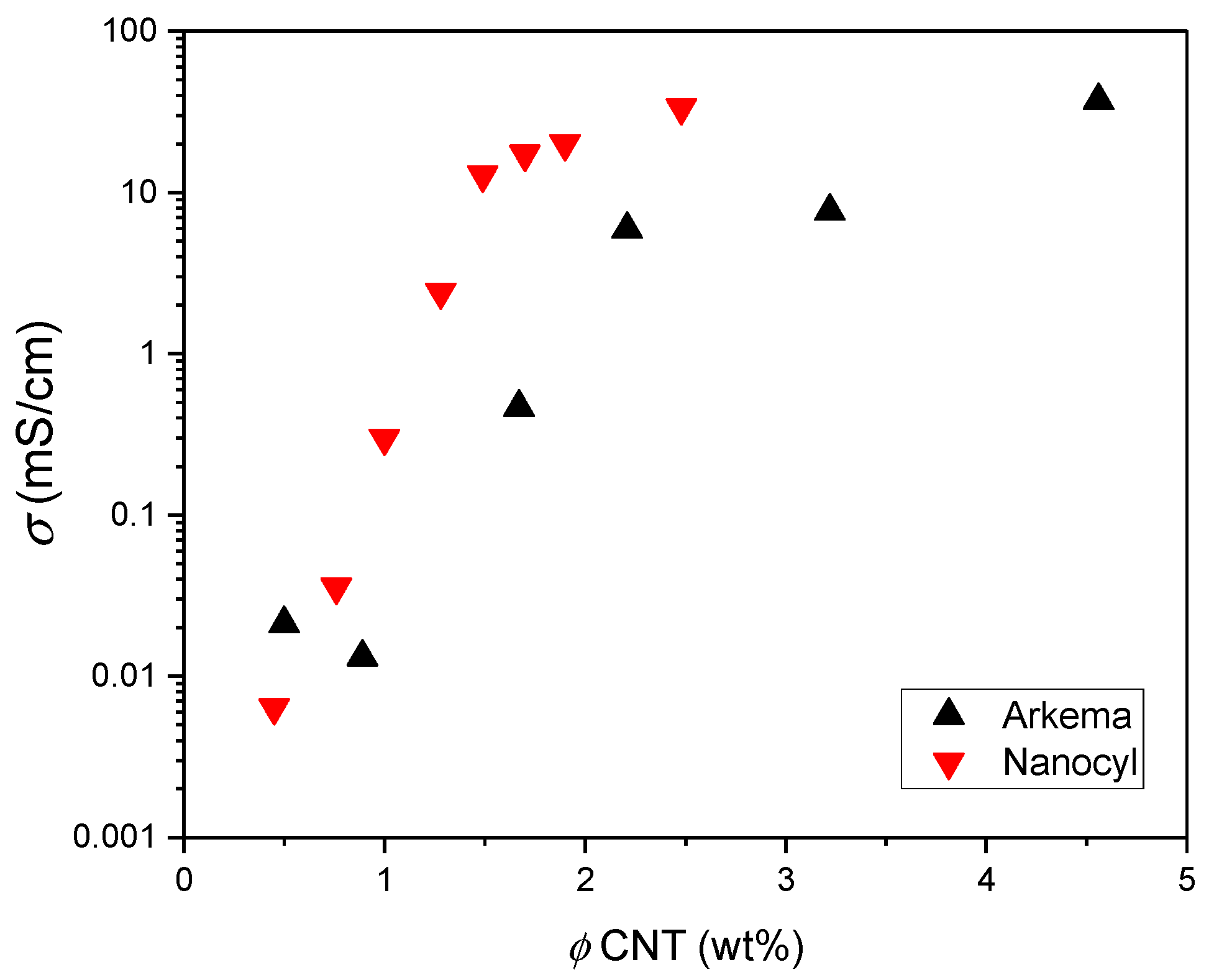
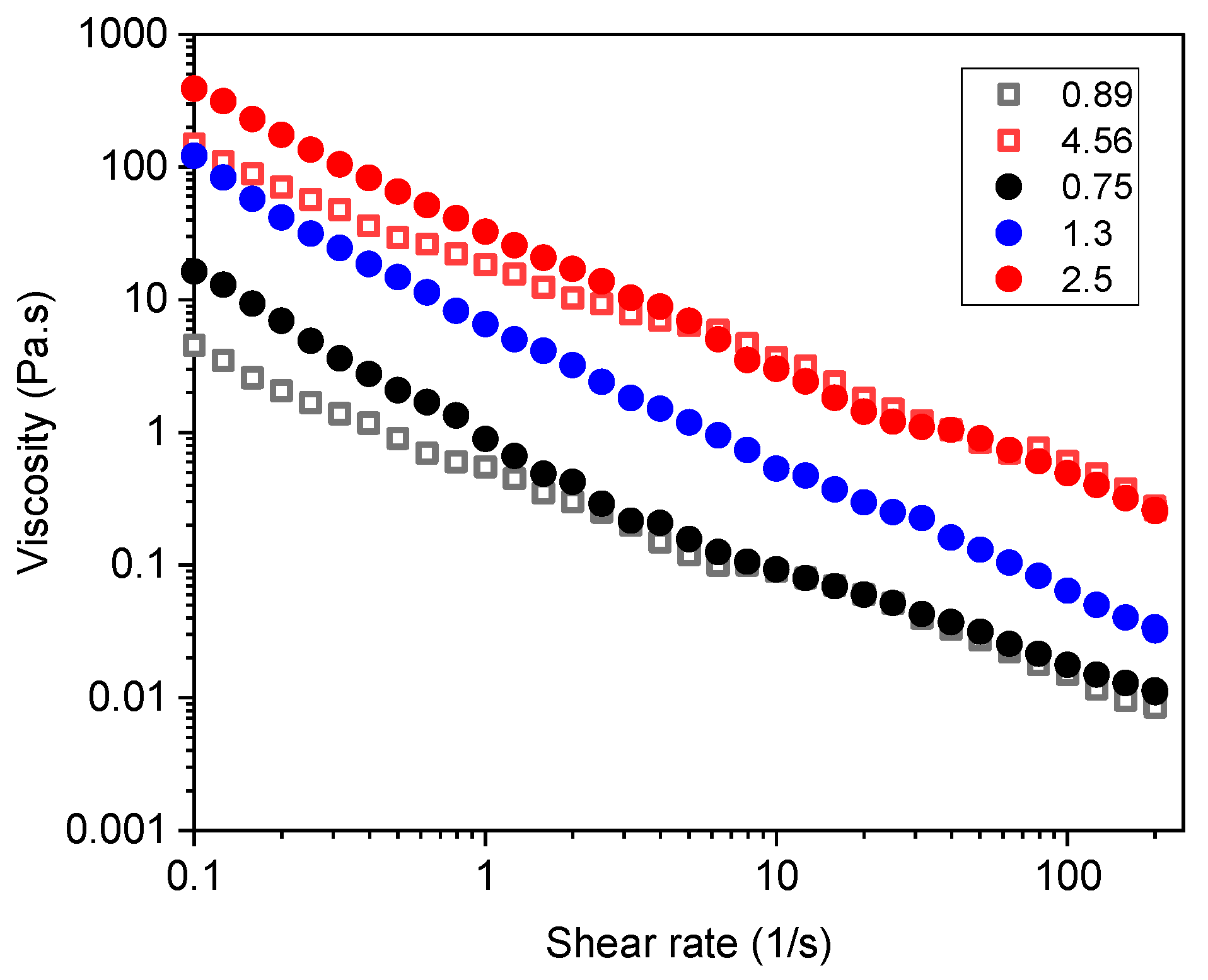
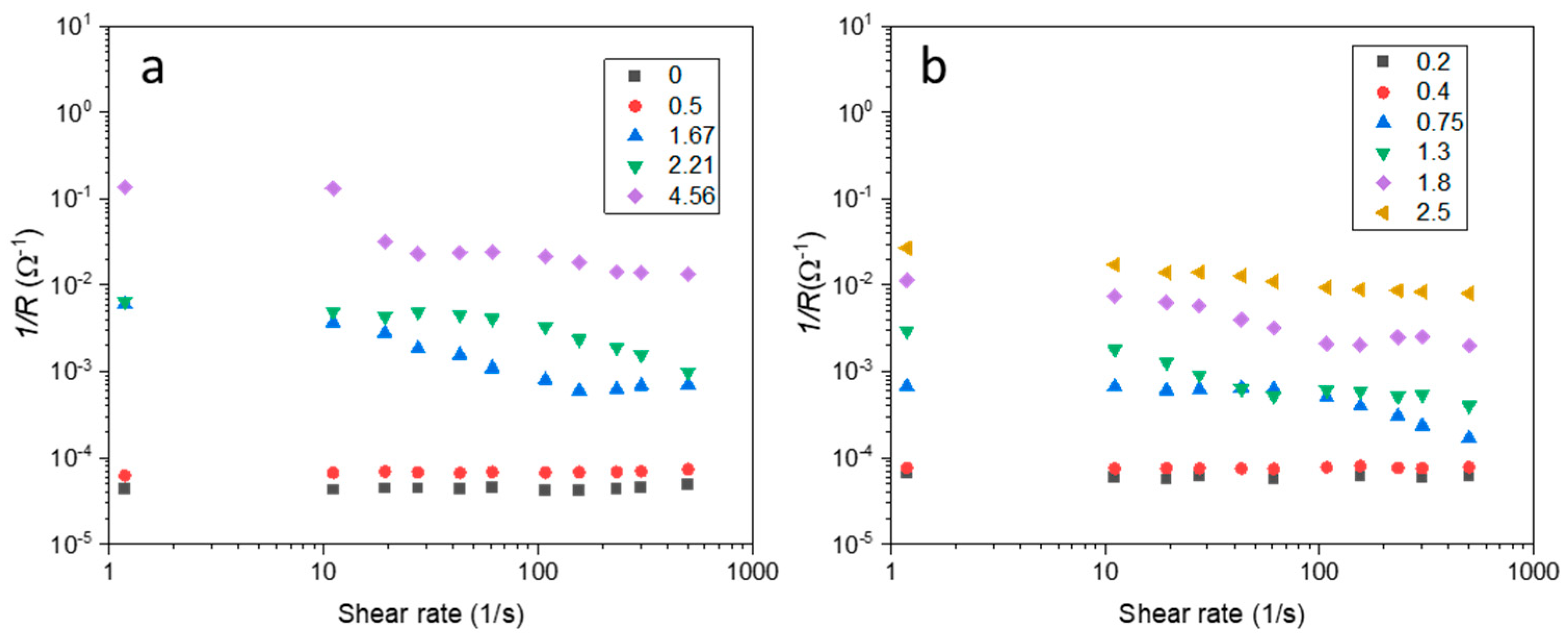
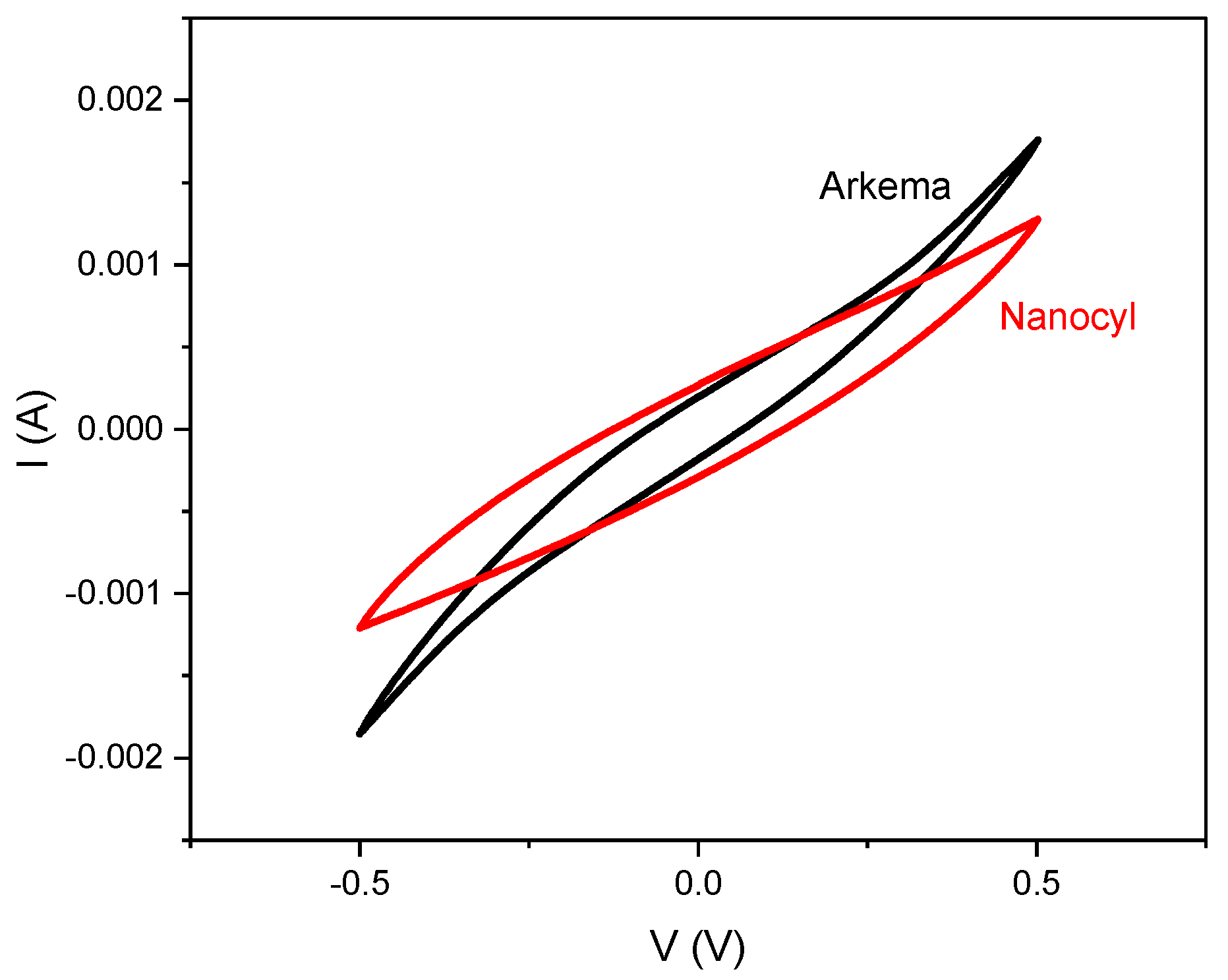
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Presser, V.; Dennison, C.R.; Campos, J.; Knehr, K.W.; Kumbur, E.C.; Gogotsi, Y. The Electrochemical Flow Capacitor: A New Concept for Rapid Energy Storage and Recovery. Adv. Energy Mater. 2012, 2, 895–902. [Google Scholar] [CrossRef]
- Campos, J.W.; Beidaghi, M.; Hatzell, K.B.; Dennison, C.R.; Musci, B.; Presser, V.; Kumbur, E.C.; Gogotsi, Y. Investigation of carbon materials for use as a flowable electrode in electrochemical flow capacitors. Electrochim. Acta 2013, 98, 123–130. [Google Scholar] [CrossRef]
- Alfonso, M.S.; Parant, H.; Yuan, J.K.; Neri, W.; Laurichesse, E.; Kampioti, K.; Colin, A.; Poulin, P. Highly conductive colloidal carbon based suspension for flow-assisted electrochemical systems. Iscience 2021, 24, 102456. [Google Scholar] [CrossRef]
- Yu, F.; Yang, Z.Q.; Cheng, Y.J.; Xing, S.Y.; Wang, Y.Y.; Ma, J. A comprehensive review on flow-electrode capacitive deionization: Design, active material and environmental application. Sep. Purif. Technol. 2022, 281, 119870. [Google Scholar] [CrossRef]
- Ma, J.J.; Zhang, C.Y.; Yang, F.; Zhang, X.D.; Suss, M.E.; Huang, X.; Liang, P. Carbon Black Flow Electrode Enhanced Electrochemical Desalination Using Single-Cycle Operation. Environ. Sci. Technol. 2020, 54, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Ma, J.X.; Wu, L.; Sun, J.Y.; Wang, L.; Li, T.Y.; Waite, T.D. Flow Electrode Capacitive Deionization (FCDI): Recent Developments, Environmental Applications, and Future Perspectives. Environ. Sci. Technol. 2021, 55, 4243–4267. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; He, Y.F.; Rosentsvit, L.; Suss, M.E.; Zhang, X.R.; Gao, T.; Liang, P. Flow-electrode capacitive deionization: A review and new perspectives. Water Res. 2021, 200, 117222. [Google Scholar] [CrossRef]
- Jeon, S.I.; Park, H.R.; Yeo, J.G.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Doornbusch, G.J.; Dykstra, J.E.; Biesheuvel, P.M.; Suss, M.E. Fluidized bed electrodes with high carbon loading for water desalination by capacitive deionization. J. Mater. Chem. A 2016, 4, 3642–3647. [Google Scholar] [CrossRef]
- Yang, S.; Park, H.R.; Yoo, J.; Kim, H.; Choi, J.; Han, M.H.; Kim, D.K. Plate-Shaped Graphite for Improved Performance of Flow-Electrode Capacitive Deionization. J. Electrochem. Soc. 2017, 164, E480–E488. [Google Scholar] [CrossRef]
- Tang, K.X.; Yiacoumi, S.; Li, Y.P.; Tsouris, C. Enhanced Water Desalination by Increasing the Electroconductivity of Carbon Powders for High-Performance Flow-Electrode Capacitive Deionization. ACS Sustain. Chem. Eng. 2019, 7, 1085–1094. [Google Scholar] [CrossRef]
- Rommerskirchen, A.; Linnartz, C.J.; Müller, D.; Willenberg, L.K.; Wessling, M. Energy Recovery and Process Design in Continuous Flow Electrode Capacitive Deionization Processes. ACS Sustain. Chem. Eng. 2018, 6, 13007–13015. [Google Scholar] [CrossRef]
- Lee, J.H.; Weingarth, D.; Grobelsek, I.; Presser, V. Use of Surfactants for Continuous Operation of Aqueous Electrochemical Flow Capacitors. Energy Technol. 2016, 4, 75–84. [Google Scholar] [CrossRef]
- Torop, J.; Summer, F.; Zadin, V.; Koiranen, T.; Jänes, A.; Lust, E.; Aabloo, A. Low concentrated carbonaceous suspensions assisted with carboxymethyl cellulose as electrode for electrochemical flow capacitor. Eur. Phys. J. E 2019, 42, 8. [Google Scholar] [CrossRef] [PubMed]
- Akuzum, B.; Singh, P.; Eichfeld, D.A.; Agartan, L.; Uzun, S.; Gogotsi, Y.; Kumbur, E.C. Percolation Characteristics of Conductive Additives for Capacitive Flowable (Semi-Solid) Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 5866–5875. [Google Scholar] [CrossRef]
- Gloukhovski, R.; Suss, M.E. Measurements of the Electric Conductivity of MWCNT Suspension Electrodes with Varying Potassium Bromide Electrolyte Ionic Strength. J. Electrochem. Soc. 2020, 167, 020528. [Google Scholar] [CrossRef]
- Balmforth, N.J.; Frigaard, I.A.; Ovarlez, G. Yielding to Stress: Recent Developments in Viscoplastic Fluid Mechanics. Annu. Rev. Fluid Mech. 2014, 46, 121–146. [Google Scholar] [CrossRef]
- Ouyang, L.X.; Wu, Z.H.; Wang, J.; Qi, X.P.; Li, Q.; Wang, J.T.; Lu, S.G. The effect of solid content on the rheological properties and microstructures of a Li-ion battery cathode slurry. RSC Adv. 2020, 10, 19360–19370. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Royer, J.R.; Laidlaw, F.H.J.; Poon, W.C.K.; Larsen, T.; Andreasen, S.J.; Christiansen, J.C. Controlling the rheo-electric properties of graphite/carbon black suspensions by ‘flow switching’. arXiv 2023, arXiv:2311.05302. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Shimoyama, H.; Sakai, G.; Kaneto, K. Physical properties of multiwalled carbon nanotubes. Int. J. Inorg. Mater. 1999, 1, 77–82. [Google Scholar] [CrossRef]
- Balberg, I.; Anderson, C.H.; Alexander, S.; Wagner, N. EXCLUDED VOLUME AND ITS RELATION TO THE ONSET OF PERCOLATION. Phys. Rev. B 1984, 30, 3933–3943. [Google Scholar] [CrossRef]
- Cho, Y.; Yoo, C.Y.; Lee, S.W.; Yoon, H.; Lee, K.S.; Yang, S.; Kim, D.K. Flow-electrode capacitive deionization with highly enhanced salt removal performance utilizing high-aspect ratio functionalized carbon nanotubes. Water Res. 2019, 151, 252–259. [Google Scholar] [CrossRef]
- Chen, K.Y.; Shen, Y.Y.; Wang, D.M.; Hou, C.H. Carbon nanotubes/activated carbon hybrid as a high-performance suspension electrode for the electrochemical desalination of wastewater. Desalination 2022, 522, 115440. [Google Scholar] [CrossRef]
- Wang, Z.L.; Hu, Y.D.; Wei, Q.; Li, W.S.; Liu, X.; Chen, F.M. Enhanced Desalination Performance of a Flow-Electrode Capacitive Deionization System by Adding Vanadium Redox Couples and Carbon Nanotubes. J. Phys. Chem. C 2021, 125, 1234–1239. [Google Scholar] [CrossRef]
- Petek, T.J.; Hoyt, N.C.; Savinell, R.F.; Wainright, J.S. Characterizing Slurry Electrodes Using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2016, 163, A5001–A5009. [Google Scholar] [CrossRef]
- Cohen, H.; Eli, S.E.; Jogi, M.; Suss, M.E. Suspension Electrodes Combining Slurries and Upflow Fluidized Beds. ChemSusChem 2016, 9, 3045–3048. [Google Scholar] [CrossRef]
- Vigolo, B.; Coulon, C.; Maugey, M.; Zakri, C.; Poulin, P. An experimental approach to the percolation of sticky nanotubes. Science 2005, 309, 920–923. [Google Scholar] [CrossRef]
- Chiodarelli, N.; Richard, O.; Bender, H.; Heyns, M.; De Gendt, S.; Groeseneken, G.; Vereecken, P.M. Correlation between number of walls and diameter in multiwall carbon nanotubes grown by chemical vapor deposition. Carbon 2012, 50, 1748–1752. [Google Scholar] [CrossRef]
- Lucas, A.; Zakri, C.; Maugey, M.; Pasquali, M.; van der Schoot, P.; Poulin, P. Kinetics of Nanotube and Microfiber Scission under Sonication. J. Phys. Chem. C 2009, 113, 20599–20605. [Google Scholar] [CrossRef]
- Pagani, G.; Green, M.J.; Poulin, P.; Pasquali, M. Competing mechanisms and scaling laws for carbon nanotube scission by ultrasonication. Proc. Natl. Acad. Sci. USA 2012, 109, 11599–11604. [Google Scholar] [CrossRef] [PubMed]
- Parant, H.; Muller, G.; Le Mercier, T.; Tarascon, J.M.; Poulin, P.; Colin, A. Flowing suspensions of carbon black with high electronic conductivity for flow applications: Comparison between carbons black and exhibition of specific aggregation of carbon particles. Carbon 2017, 119, 10–20. [Google Scholar] [CrossRef]
- Simsek, Y.; Ozyuzer, L.; Seyhan, A.; Tanoglu, M.; Schulte, K. Temperature dependence of electrical conductivity in double-wall and multi-wall carbon nanotube/polyester nanocomposites. J. Mater. Sci. 2007, 42, 9689–9695. [Google Scholar] [CrossRef]
- Kyrylyuk, A.V.; van der Schoot, P. Continuum percolation of carbon nanotubes in polymeric and colloidal media. Proc. Natl. Acad. Sci. USA 2008, 105, 8221–8226. [Google Scholar] [CrossRef]
- Mao, Y.; Cates, M.E.; Lekkerkerker, H.N.W. Depletion force in colloidal systems. Phys. A 1995, 222, 10–24. [Google Scholar] [CrossRef]
- Nadiv, R.; Fernandes, R.M.F.; Ochbaum, G.; Dai, J.; Buzaglo, M.; Varenik, M.; Biton, R.; Furó, I.; Regev, O. Polymer nanocomposites: Insights on rheology, percolation and molecular mobility. Polymer 2018, 153, 52–60. [Google Scholar] [CrossRef]
- Fan, Z.; Advani, S.G. Rheology of multiwall carbon nanotube suspensions. J. Rheol. 2007, 51, 585–604. [Google Scholar] [CrossRef]
- Faulkner, L.R.; Bard, A.J. (Eds.) Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000; ISBN 978-0-471-04372-0. [Google Scholar]
- Hatzell, K.B.; Beidaghi, M.; Campos, J.W.; Dennison, C.R.; Kumbur, E.C.; Gogotsi, Y. A high performance pseudocapacitive suspension electrode for the electrochemical flow capacitor. Electrochim. Acta 2013, 111, 888–897. [Google Scholar] [CrossRef]
- Hou, S.J.; Wang, M.; Xu, X.T.; Li, Y.D.; Li, Y.J.; Lu, T.; Pan, L.K. Nitrogen-doped carbon spheres: A new high-energy-density and long life pseudo-capacitive electrode material for electrochemical flow capacitor. J. Colloid Interface Sci. 2017, 491, 161–166. [Google Scholar] [CrossRef]
- Hunt, C.; Mattejat, M.; Anderson, C.; Sepunaru, L.; Ménard, G. Symmetric Phthalocyanine Charge Carrier for Dual Redox Flow Battery/Capacitor Applications. ACS Appl. Energy Mater. 2019, 2, 5391–5396. [Google Scholar] [CrossRef]
- Tomai, T.; Saito, H.; Honma, I. High-energy-density electrochemical flow capacitors containing quinone derivatives impregnated in nanoporous carbon beads. J. Mater. Chem. A 2017, 5, 2188–2194. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, H.J.; Yoo, J.J.; Yoo, C.Y.; Park, J.H.; Lee, Y.A.; Cho, W.K.; Han, Y.K.; Kim, D.H. Pseudocapacitive slurry electrodes using redox-active quinone for high-performance flow capacitors: An atomic-level understanding of pore texture and capacitance enhancement. J. Mater. Chem. A 2015, 3, 23323–23332. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamouma, M.; Neri, W.; Bril, X.; Yuan, J.; Colin, A.; Brémond, N.; Poulin, P. Flowable Electrodes from Colloidal Suspensions of Thin Multiwall Carbon Nanotubes. Colloids Interfaces 2024, 8, 32. https://doi.org/10.3390/colloids8030032
Hamouma M, Neri W, Bril X, Yuan J, Colin A, Brémond N, Poulin P. Flowable Electrodes from Colloidal Suspensions of Thin Multiwall Carbon Nanotubes. Colloids and Interfaces. 2024; 8(3):32. https://doi.org/10.3390/colloids8030032
Chicago/Turabian StyleHamouma, Massinissa, Wilfrid Neri, Xavier Bril, Jinkai Yuan, Annie Colin, Nicolas Brémond, and Philippe Poulin. 2024. "Flowable Electrodes from Colloidal Suspensions of Thin Multiwall Carbon Nanotubes" Colloids and Interfaces 8, no. 3: 32. https://doi.org/10.3390/colloids8030032
APA StyleHamouma, M., Neri, W., Bril, X., Yuan, J., Colin, A., Brémond, N., & Poulin, P. (2024). Flowable Electrodes from Colloidal Suspensions of Thin Multiwall Carbon Nanotubes. Colloids and Interfaces, 8(3), 32. https://doi.org/10.3390/colloids8030032





