Abstract
In this study, nanocomposite active films were fabricated containing silver nanoparticles (SNPs) embedded within soy protein isolate (SPI)/Persian gum (PG) matrices. The physical, mechanical, and antibacterial properties of these composite films were then characterized. In addition, scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD) were used to provide information about the microstructure, interactions, and crystallinity of the films. Pure SPI films had poor physicochemical attributes but the addition of PG (0.25, 0.5, or 1 wt%) improved their water vapor permeability, mechanical properties, and water solubility (WS). The moisture content (MC) of the films decreased after the introduction of PG, which was attributed to fewer free hydroxyl groups to bind to the water molecules. Our results suggest there was a strong interaction between the SPI and the PG and SNPs in the films, suggesting these additives behaved like active fillers. Optimum film properties were obtained at 0.25% PG in the SPI films. The addition of PG (0.25%) and SNPs (1%) led to a considerable increase in tensile strength (TS) and a decrease in elongation at break (EB). Furthermore, the incorporation of the SNPs into the SPI/PG composite films increased their antibacterial activity against pathogenic bacteria (Escherichia coli and Staphylococcus aureus), with the effects being more prominent for S. aureus. Spectroscopy analyses provided insights into the nature of the molecular interactions between the different components in the films. Overall, the biodegradable active films developed in this study may be suitable for utilization as eco-friendly packaging materials in the food industry.
1. Introduction
Plastic pollution is an acute environmental problem, which is mainly caused by mismanagement, inadequate disposal, lack of recycling facilities, and accumulation of waste. Nearly 50% of plastics produced are disposable, much of which is used in the food packaging segment [1]. In recent decades, environmental and health concerns associated with the use of petrochemical-based plastics have motivated extensive efforts towards the development of biodegradable packaging materials from renewable biopolymers [2]. Nanocomposite materials containing antimicrobial, antioxidant, and sensing compounds are being used to create active and smart packaging materials [2,3]. Likewise, hybrid nanocomposite materials are a great and ideal platform for the introduction of innovative materials such as metals with advanced characteristics for food packaging applications. These nanocomposite packaging materials may be a suitable alternative to commercial plastic packaging materials [4,5,6]. For example, smart biodegradable packaging materials are being created containing pH-sensitive pigments that can provide information about the freshness and spoilage conditions of the product [2,7]. In addition, nanocomposite packaging can be designed that contain phytochemicals and barrier materials that reduce oxidation and spoilage of food products due to their antimicrobial activity and ability to reduce vapor and gas transfer [8]. Polymers can be physically or chemically modified to improve the performance of nanocomposite films [9,10].
A variety of eco-friendly materials, including those from animal, plant, and microbial sources, can be used to replace plastics in fabricating films [11]. Soy proteins are plant-based film-forming materials that are a by-product of soybean oil refining [12,13]. Soy protein isolate (SPI) is an amphiphilic molecule with a high protein content that contains >90% globulins, with the two major fractions being β-conglycinin (7S) and glycine (11S). Since SPI is abundant, non-toxic, affordable, biocompatible, and biodegradable, it has been widely explored for its potential in food packaging [12,14]. However, their commercial application is limited by their poor mechanical strength, barrier properties, thermal tolerance, and water resistance [13,15]. Efforts have been taken to improve these poor properties of SPI films by incorporating barrier-enhancers and mechanical modulators [16]. The antimicrobial efficacy of innovative nanocomposites depends on the stability of nanoparticles on film structure/surfaces [17]. Functional groups in the SPI structure play an important role in SPI interactions with metal ions/or metal nanoparticles. More importantly, the main amine and hydroxyl groups in the SPI and PG network interact with metal surfaces and act as adsorb sites for the stabilization of nanoparticles [17,18]. Accordingly, SPI has the ideal potential to form composite films with metals in their ionic or metallic forms through the coordination of metal ions via the amino groups on the SPI chain and the creation of chemical bonds.
Persian gum (PG), also known as Zedu, Shirazi, or Angum gum, is an anionic hydrocolloid with a trace amount of protein exudate naturally produced by almond trees (Amygdalus scoparia Spach) growing wild in the forests of Iran. PG is composed of 30% water-soluble and 70% water-insoluble fractions. Arabinose and galactose are the major components of PG, so it is known as arabinogalactan. Due to the cheap, good water absorption capacity, and emulsifying properties of this gum, it has found various food applications as a gelling agent, fat replacer, and film-forming agent [19].
In addition, biodegradable packaging materials can act as supporters of various bioactive compounds such as antimicrobials, antioxidants, colors, and flavors. Silver nanoparticles (SNPs) have good stability, non/or low toxicity, and biocompatibility properties. Moreover, SNPs have excellent antimicrobial activities against a broad spectrum of microorganisms (microbes, fungi, and viruses), which has been ascribed to their unspecific mechanism of action and its ability to penetrate the cell membrane and cause DNA damage [20].
Considering that packaging films belong to the materials in contact with food products, therefore, having antimicrobial activity is necessary for the packaging films. Therefore, antimicrobial packaging films loaded with SNPs have developed as a successful technology to prevent microbial growth on food contact surfaces in the food industry, as SNPs are used as a coating on mainly stainless steel surfaces [21]. The selection of SNP is owing to its multiple functions in various fields, and the larger surface-to-volume ratio which caused higher surface exposure to the microorganisms resulting in better antimicrobial activity [22]. On the other hand, nanohybrid improvement of biopolymers is considered a rapidly developing technique being implemented in the composite films modification, as well as reinforcing their thermal stability, physicochemical/mechanical, light-blocking, and barrier characteristics via the cross-linking system, and formation of hydrogen bonds. As a result, composite films containing nanoparticles such as SNPs as a promising improving filler can be a suitable alternative to synthetic packaging. In this study, biodegradable films were prepared by blending soy protein isolate, Persian gum, and Ag nanoparticles, and evaluated the morphological, structural, physicomechanical parameters, and antibacterial activities of the resulting films.
2. Material and Methods
2.1. Materials
Soy protein isolate (SPI, protein ≥ 90%) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), and silver nanoparticles (SNPs, 20–60 nm, purity > 99%) were acquired from ARMINANO Co., (Tehran, Iran). The Persian gum (PG) was purchased from Freer Co. (Esfahan, Iran). Mueller Hinton agar was provided by Quelab (Montreal, Canada) and bacterial strains Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 33591) were obtained from the Biological and Genetic Resources Center (Tehran, Iran). All the chemicals were analytical grade.
2.2. Preparation of Films
To prepare films, firstly, the SPI solution (5%, w/v) was prepared by dissolving SPI powder in distilled water at 80 °C for 30 min. The Persian gum solution (0.25, 0.5, 1% w/v) was prepared by dissolving PG powder in distilled water at 50 °C for 1 h. Next, the SPI, PG solutions, and glycerol (30% w/v) were mixed and stirred for 1 h at 60 °C with pH adjustment (9 ± 0.1) by adding 2 M NaOH. After testing the properties of the SPI-PG films, 0.25% of the PG was chosen as the optimal concentration of the film. Next, SNPs (1 and 2%) were added to SPI/0.25% PG film solutions. After sonication for 15 min, the equal aliquots of film solutions were poured into plates and dried at 25 °C for 24 h. Table 1. illustrates the codes of the prepared films.

Table 1.
Formulations of SPI/PG/SNPs active films.
2.3. Characterization of Films
2.3.1. Material Characterization
The surface morphology of the active films was analyzed by scanning electron microscopy (FE-SEM, TeScan-Mira3 xmu, Brno-Kohoutovice, Czech Republic). The film samples were coated with gold before SEM measurement at 10 kV. Interactions among the functional groups in the films were observed using an FTIR spectrometer (Tensor 27, Bruker, Billerica, MA, USA) with KBr pellets in the range of 4000–400 cm−1. The film samples were scanned 24 times with a resolution of 4 cm−1. The crystalline structure of active films was investigated by X-ray diffraction (PANalytical X’pert pro-MRD diffractometer, Malvern Panalytical, Malvern, UK) with nickel-filtered Cu Kα radiation under 40 kV and 30 mA at room temperature. The 2θ range was 10–75°.
2.3.2. Thickness and Mechanical Properties
The thickness of the film samples was randomly determined at 5 positions, using a digital hand-held micrometer (Mitutoyo Corporation, Kawasaki, Japan) with an accuracy of 0.001 mm. The auto tensile tester (DBBP-20, Bongshin, Gyeonggi-do, Korea) was used for the evaluation of tensile strength (TS, MPa) and elongation at break (EB, %). Before the analysis, the samples were cut into 10 × 1 cm2 strips and equilibrated at 25 °C and RH = 50 ± 2%. Initial gauge length and crosshead speed were set at 50 mm, and 10 mm/min, respectively.
2.3.3. Water Vapor Permeability (WVP)
Gravimetric measurements were used to determine the films’ WVP. A circular disc was cut from the film and then used to seal glass vials containing CaCl2 granules as a desiccant (0% RH) in a desiccator containing distilled water (100% RH). In a two-day period, the vials were weighed every 3 h. A plot of the weight change of the cup versus time was then used to calculate the water vapor transmission rate (WVTR, g/s). The WVP was calculated by using the given equation [2].
WVTR—water vapor transmission rate (g/h)
X—film thickness (m)
S—film area (m2)
ΔP—Saturated water vapor pressure (3.165 kPa) at 25 °C.
2.3.4. Transparency
Transparency of film samples (1 × 4 cm) was recorded at the wavelength of 600 nm using a UV–vis spectrophotometer (Ultrospec 2000, Scinteck, Cambridge, UK). The formula used to measure the transparency of films was given below.
2.3.5. Moisture Content and Water Solubility
The moisture content (MC) of each film was obtained by the weight loss of films before and after drying at 110 °C for 24 h. The film pieces (2 × 2 cm2) were accurately weighed (Wa) after drying in the oven at 105 °C for 5 h. Then, they were immersed in distilled water (25 mL) with periodic shaking at 25 °C for 24 h. Finally, the undissolved samples were removed and their weight was measured after drying at 105 °C (Wb). The WS of the films was computed as follows:
where W1 and W2 are the weight of the film before and after drying, respectively.
2.3.6. Antibacterial Activity
The antibacterial activity of the active films was tested against Staphylococcus aureus and Escherichia coli according to the previously published study [2]. Mueller Hinton agar plates were inoculated with standardized inoculums (~1.5 × 106 CFU/mL) of S. aureus and E. coli. After that, the film samples of 10 mm in diameter were placed on the surface of the inoculated plates and were incubated at 37 °C for 24 h. For each sample, inhibition zone diameter (mm) was recorded by a caliper.
2.4. Statistical Analysis
Statistical analysis has been performed using SPSS® 16. The data were represented as the average ± standard deviation of at least three observations and analyzed by one-way analysis of variance (ANOVA). Statistical significance was regarded as p < 0.05.
3. Results and Discussion
3.1. Physical Characteristics of Films
3.1.1. Thickness and Density Degree
The thickness of food packaging films affects several important attributes, including strength, light transmission, and WVP. Therefore, films must have the desired thickness [2]. The film thickness data are reported in Table 2. The results showed that the films varied between 0.152 and 0.182 mm. As can be seen from the data, the highest thickness was for the SPI/PG/2%SNPs (0.182 mm). The biopolymers must have a thickness that provides appropriate mechanical and barrier properties. The increase in film thickness with SPI incorporation can be attributed to the higher solid content of film matrix, as well as interruption in the structure of the film matrix [23]. The addition of PG and SNPs also increased the film thickness, which can mainly be attributed to their ability to interrupt the biopolymer network structure [24].

Table 2.
Physico-mechanical properties of SPI/PG/SNPs active films.
3.1.2. Solubility in Water and Moisture Content
The solubility of packaging films in water is one of the most important factors impacting their utilization and environmental fate. Due to the watery nature of most foods, less solubility of films is preferred. In this study, pure SPI films had the highest WS value (37.15%) compared to SPI films containing PG/SNPs (Table 2). The addition of PG to the SPI matrix slightly reduced the water solubility of the films (34.5% to 35%). In the composite SPI/PG matrix, the interactions between amino groups on the soy proteins and carboxyl groups on the PG may have created a more coherent network structure, which reduced water penetration [25]. Adding SNPs also reduced WS values of composite films ranging from 32.15% to 30.75% due to their insolubility. Similarly, Ortega et al. (2017) showed that solubility in water of starch-based films decreased from 39.2% to 29.0%, as the concentration of silver nanoparticles increased [26]. In general, the use of SPI alone can not be used as packaging films for many foods because they are hydrophilic proteins and have more solubility in water, and the use of polysaccharide compounds such as gums in a mixture with SPI can strengthen and reduce their solubility [27]. Previous similar studies have shown that the use of PG can reduce the solubility of films. For example, in one study, the effect of different percentages of PG on the properties of polyvinyl alcohol (PVA) films by casting method was investigated, and the results showed that the addition of PG reduces the solubility of PVA-based films [19].
Likewise, increasing the amounts of other materials (nanoparticles, fillers, additives, etc.) in the film’s matrix reduced their MC. Packaging material is composed of a network of polymers or particles with pores between them that trap water molecules and other substances. Table 2 shows MC values for different film samples. For example, SPI film had high moisture (13.04%) and with the addition of PG to the film, this value decreased (from 12.98% to 12.50%). Furthermore, the presence of SNPs in the film matrix reduced the MC between 11.66% and 11.23%, and there was no significant decrease in MC between different concentrations of SNPs. Thus, the addition of PG led to the formation of hydrogen bonding between the PG and SPI molecules, which inhibited the interaction between SPI and water, thereby reducing the water-solubility of the films. Our results show that incorporating SNPs into the SPI matrix reduced the water-solubility of the films. Other researchers have also shown that adding PG and halloysite nanotubes into PVA and SPI films reduced the MC, respectively [19,28].
3.1.3. Water Vapor Permeability
One of the most important factors in the stability properties of edible films is their low WVP, otherwise, films with high WVP values will cause food spoilage and reduce the shelf life of the product. The interaction of the functional groups of the compounds participating in the film and the crystallinity are important factors in reducing WVP in the films. As can be seen in Table 2, the amount of WVP for SPI (3.25 × 10−10 g Pa−1 s−1 m−1) film was higher compared to SPI/PG (3.10 × 10−10 g Pa−1 s−1 m−1) and SPI/PG/SNPs (2.60 × 10−10 g Pa−1 s−1 m−1) films. In general, WVP is associated with solubility in water of compounds. For this purpose, the highest water vapor resistance was observed in SPI/PG/2%SNPs films, which could prevent the transfer of water and water vapor molecules, and the lowest stability was shown in pure SPI films due to the hydrophilic nature of the SPI protein. Xiao et al. (2021) showed that the addition of cellulose nanocrystals to the SPI matrix reduced the amount of WVP compared to pure SPI [29].
3.1.4. Transparency
In this study, the UV-vis spectrum at 600 nm was used to measure the transparency of films. The transparency and opacity of films are directly related to how much light transmits through them. Furthermore, consumer perceptions are affected by the transparency or opacity of a film. Furthermore, food packaging materials can reduce the degradation of light-sensitive food components [29]. The transparency of films is determined by polymer blend miscibility and compatibility. Consequently, measurements of film transparency can provide valuable information about film microstructure [14]. As shown in Table 2 and Figure 1, the neat SPI films exhibited the highest transparency (68.6%), while SPI films containing PG had a lower transparency (65.4% to 28.7%). By adding SNPs to the SPI/PG film, we observed cloudiness in the films and reduced light transmission with transparency of 18.6% and 10.3%, which could be due to nanoparticles absorbing and scattering light. Similarly, Kanmani and Rhim (2013) found that adding SNPs to gelatin films affected their transmittance and reduced their transparency [30]. In general, a linear decrease in transmittance of the composite films was observed with increasing SNPs concentration.
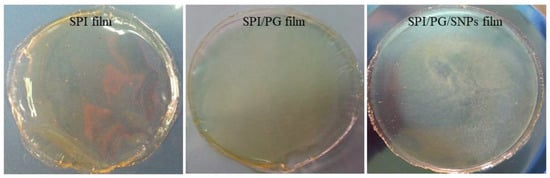
Figure 1.
Appearance of SPI-based films containing persian gum (PG) and silver nanoparticles (SNPs).
3.2. Mechanical Characteristics
Mechanical properties of films such as elongation at break, and tensile strength, are important characteristics that influence their suitability as packaging materials [30]. In this research, nanoparticles were added to SPI-based films to determine how they affect EB (flexibility), and TS (strength) values. A pure SPI film had a strength of 3.15 MPa, and flexibility of 140.5% (Table 2). When the PG and SNPs were added to the SPI film, the strength of the film increased from 3.15 MPa to 3.65 MPa except for SPI/1% PG film (2.78 MPa), whereas the flexibility of the films decreased significantly from 140.5% to 58.1% (p ≤ 0.05). The addition of nanoparticles improved the mechanical strength and stiffness of the films due to an enhancement of intermolecular bonding. Additionally, due to the large surface area of nanoparticles, SNPs act as effective fillers in the SPI/PG matrix, establishing strong interfacial interactions with the matrix. One study showed that the addition of zinc oxide nanoparticles to the SPI matrix had similar mechanical properties to our results [31].
3.3. Instrumental and Spectroscopy Characteristics of Films
3.3.1. FE-SEM
In Figure 2, SEM images show the morphology and surface properties of the films. The neat SPI film indicated a slightly non-smooth surface with protrusions. The structure of the films became compacted with the addition of PG and SNPs. PG deposition in the SPI matrix showed an even distribution of PG with low aggregation on the film with a relatively rough surface. The SEM results showed that the surface roughness of the films increased with increasing PG concentration, which adversely affected film appearance. This effect was greater at 2% SNPs than at 1% SNPs, and so this latter concentration was used in the remainder of the experiments. The SEM images showed that 1% SNPs were distributed fairly uniformly throughout the SPI matrix, which suggested that they had favorable interactions with the biopolymer network [32]. As a result, the mechanical and barrier properties of the films were improved after SNPs addition.

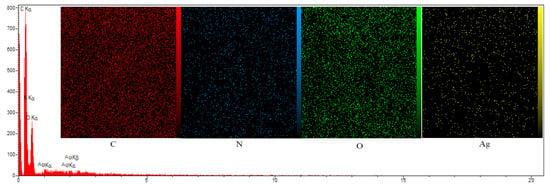
Figure 2.
SEM-EDX image of (a) SPI, (b) SPI/0.25%PG, (c) SPI/1%PG, (d) SPI/PG/1%SNPs, (e) SPI/PG/2%SNPs films.
3.3.2. ATR-FTIR
The ATR-FTIR spectroscopy was used to determine the molecular interactions within the different types of films. Based on the analysis of the FTIR spectra, we found that a number of characteristic peaks corresponded to specific functional groups within the films, which indicates compatibility and interaction as well as good dispersion of components in the film matrix. According to Figure 3, the SPI, SPI/PG, and SPI/PG/SNPs films exhibited different characteristic peaks between 3500 and 660 cm−1. All three films did not exhibit any additional peak formation, indicating that SPI, SPI/PG, and SPI/PG/SNPs did not cross-link chemically. However, slight changes are seen in some situations that could be due to the molecular interaction of components. The IR peaks at around 3500–3300 cm−1, 2900 cm−1, and 1700–1650 cm−1 in the spectrum are typically owing to the bending vibration and stretching of OH, CH2, C=O, C=N, and C=C groups. There was a strong and broad absorption peak at 3400 cm−1 corresponding to SPI film, which indicates the stretching vibration of the hydroxyl groups (O-H). However, the intensity of the spectra decreased in the wavelength around 3500 cm−1 by adding PG, which reveals an increase in hydrogen bonds between SPI and PG. However, with the addition of SNPs, the peak intensity of the functional groups was somewhat increased, which may be due to the free of functional groups owing to the bonding of nanoparticles with molecules [30,33]. A study by Pandey et al. (2012) showed that silver ions can efficiently coordinate with polymer O-H groups [34].
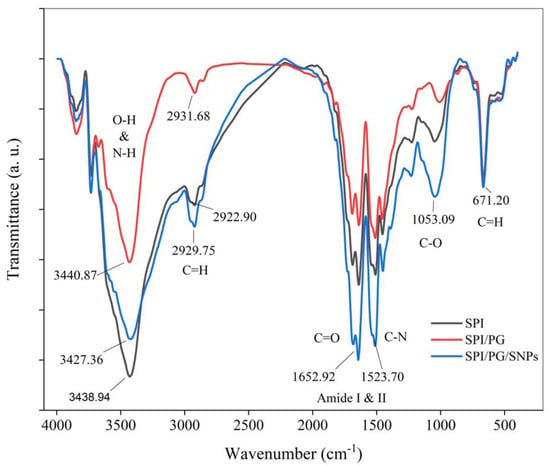
Figure 3.
FTIR spectra of SPI, SPI/PG, and SPI/PG/SNPs films.
3.3.3. XRD
Packaging materials’ mechanical and barrier properties are affected by the crystallinity of the film, and XRD is performed in order to determine both the crystal structure as well as the affinity of the SNPs and SPI/PG matrix [35]. The XRD patterns of composite films formed from SPI, SPI/PG, and SPI/PG/SNPs are shown in Figure 4. All films exhibited similar spectral patterns: there were peaks at around 2θ = 7° and 18°, and two peaks around 7° and 18°. These peaks are associated with the α-helix and β-sheet structures of the SPI secondary conformation, respectively [36]. The SPI/PG film did not contain any other peaks associated with crystalline regions. This indicates that PG had a good compatibility with SPI and therefore no distinct other peak appeared. The addition of PG and SNPs to the SPI film did not cause a major change in the XRD pattern, indicating that these nanoparticles had good compatibility with SPI/PG matrix [37]. Accordingly, the control film did not show any diffraction peaks for silver, while the SPI/SNPs film showed three distinct peaks at 2θ = 38.5°, 46.4°, and 69°, which corresponded to the (111), (200), and (220) planes of silver, respectively. It can be concluded from these typical XRD peaks that the crystalline SNPs are formed in a face-centered cubic structure (fcc). Furthermore, the (111) plane exhibited a higher intensity than all the other planes, which could be the result of its predominant orientation [30,33].
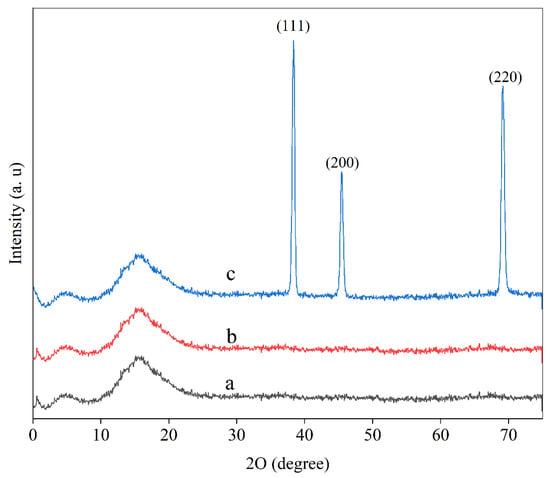
Figure 4.
XRD spectra of (a) SPI, (b) SPI/PG, (c) SPI/PG/SNPs films.
3.4. Antibacterial Activity
In active packaging films, the main goal is to increase the shelf life of foods, one of which is inhibiting the growth and elimination of microorganisms that cause food spoilage [38]. In this study, the antibacterial activity of the films against E. coli and S. aureus was determined by the disk diffusion method (Figure 5). As expected, SPI and SPI/PG films were not able to inhibit bacterial growth because SPI and PG did not have an inhibitory effect on microorganisms. The addition of SNPs to the films inhibited bacterial growth and the SPI/PG/SNPs film at both concentrations of SNPs was able to overcome both bacteria, owing to positively charged SNPs ions and interaction with negatively charged macromolecular components and nucleic acids, causing permeability to the bacterial cell wall membrane, structural changes, and deformation and finally cell death [14,39]. It is possible that the positive surface charge on SNPs is caused by protonated amino groups (-NH3+) within the SPI molecules. This above hypothesis is supported by this result, i.e., the SNP surfaces are coated by a layer of SPI. A similar mechanism of action has also been demonstrated in several studies investigating the antimicrobial properties of SNPs. For instance, a study conducted by Sui et al. (2006) and Mofidfar et al. (2019) found that SNPs exhibited antimicrobial activity because of the positive charges present on their surfaces [40,41]. It was also observed that the SPI/PG/2%SNPs film had an inhibitory effect on S. aureus bacteria (13.8 mm) more inhibitory than E. coli, (12.7 mm) which may be due to the difference in the cell wall of Gram-positive and Gram-negative bacteria in having a peptidoglycan layer. However, the results showed that SPI/PG/SNPs packaging film was able to inhibit bacterial growth and could be used as an antibacterial food packaging for various types of food. Previous similar studies have shown that the use of Ag nanoparticles is effective in inhibiting bacterial growth and increases the inhibitory effect on the film matrix [33].
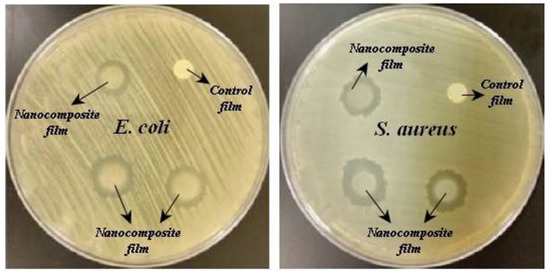
Figure 5.
Antimicrobial activity of the SPI/PG/SNPs nanocomposite film against E. coli and S. aureus.
4. Conclusions
The purpose of this study was to develop active packaging materials by incorporating PG and SNPs into a SPI film. The nanocomposite films had properties that suggested they may be suitable as alternatives to conventional plastics for some applications. The addition of PG decreased the MC of the film by inhibiting the hydrogen bonds between SPI and water molecules. Physical, mechanical, and barrier properties of SPI film were improved by adding gum and nanoparticles. By adding PG and SNPs as a filler agent to the film, it was observed that EB (flexibility) decreased and TS (strength) increased due to strong interaction with SPI polypeptide chains. Therefore, the addition of gums and nanoparticles improved the limitations and challenges of protein films due to their hydrophilic nature. On the other hand, nanocomposite films containing SNPs exhibited acceptable antibacterial effects against food pathogens. To conclude, this study indicates that loading SPI films with PG and SNPs can improve their functional characteristics, which may increase their potential application as active packaging in the food industry. However, more study is still needed to confirm they can be produced economically on industry scales and that they will continue to reveal their acceptable functional properties under real-life situations.
Author Contributions
M.A.S., M.T., A.K., K.M. and S.H.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, and Writing—original draft. A.E.: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, and Visualization. D.J.M.: Investigation, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was approved & supported by Tabriz University of Medical Sciences, Tabriz, Iran (Project No. 64595).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was approved & supported by Tabriz University of Medical Sciences, Tabriz, Iran (Project No. 64595).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An overview of plastic waste generation and management in food packaging industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Tavassoli, M.; Salim, S.A.; Azizi-lalabadi, M.; McClements, D.J. Development of green halochromic smart and active packaging materials: TiO2 nanoparticle- and anthocyanin-loaded gelatin/κ-carrageenan films. Food Hydrocoll. 2022, 124, 107324. [Google Scholar] [CrossRef]
- Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Fetisova, O.Y.; Antonov, A.V.; Bondarenko, G.N.; Sychev, V.V. Production and description the characterization of guar gum galactomannan butyl ether. Iraqi J. Agric. Sci. 2022, 53, 198–206. [Google Scholar] [CrossRef]
- Golachowski, A.; Drożdż, W.; Golachowska, M.; Kapelko-Żeberska, M.; Raszewski, B. Production and properties of starch citrates—Current research. Foods 2020, 9, 1311. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Buksa, K.; Szumny, A.; Zięba, T.; Gryszkin, A. Analysis of molecular structure of starch citrate obtained by a well-stablished method. LWT-Food Sci. Technol. 2016, 69, 334–341. [Google Scholar] [CrossRef]
- Tavassoli, M.; Alizadeh Sani, M.; Khezerlou, A.; Ehsani, A.; Jahed-Khaniki, G.; McClements, D.J. Smart Biopolymer-Based Nanocomposite Materials Containing pH-Sensing Colorimetric Indicators for Food Freshness Monitoring. Molecules 2022, 27, 3168. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Maleki, M.; Eghbaljoo-Gharehgheshlaghi, H.; Khezerlou, A.; Mohammadian, E.; Liu, Q.; Jafari, S.M. Titanium dioxide nanoparticles as multifunctional surface-active materials for smart/active nanocomposite packaging films. Adv. Colloid Interface Sci. 2022, 300, 102593. [Google Scholar] [CrossRef]
- Zhao, N.; Chai, Y.; Wang, T.; Wang, K.; Jiang, J.; Yang, H.-y. Preparation and physical/chemical modification of galactomannan film for food packaging. Int. J. Biol. Macromol. 2019, 137, 1060–1067. [Google Scholar] [CrossRef]
- Ganie, S.A.; Ali, A.; Mir, T.A.; Li, Q. Physical and chemical modification of biopolymers and biocomposites. In Advanced Green Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 359–377. [Google Scholar]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-Y.; Chen, M.; Wang, Z.-W. Release of Thymol, Cinnamaldehyde and Vanillin from Soy Protein Isolate Films into Olive Oil. Packag. Technol. Sci. 2012, 25, 97–106. [Google Scholar] [CrossRef]
- Ye, Q.; Han, Y.; Zhang, J.; Zhang, W.; Xia, C.; Li, J. Bio-based films with improved water resistance derived from soy protein isolate and stearic acid via bioconjugation. J. Clean. Prod. 2019, 214, 125–131. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Q.; Huang, H.; Duan, Y.; Xiao, G.; Le, T. Enhanced physico-mechanical, barrier and antifungal properties of soy protein isolate film by incorporating both plant-sourced cinnamaldehyde and facile synthesized zinc oxide nanosheets. Colloids Surf. B Biointerfaces 2019, 180, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Martelli-Tosi, M.; Masson, M.M.; Silva, N.C.; Esposto, B.S.; Barros, T.T.; Assis, O.B.G.; Tapia-Blácido, D.R. Soybean straw nanocellulose produced by enzymatic or acid treatment as a reinforcing filler in soy protein isolate films. Carbohydr. Polym. 2018, 198, 61–68. [Google Scholar] [CrossRef]
- Thakur, M.K.; Thakur, V.K.; Gupta, R.K.; Pappu, A. Synthesis and applications of biodegradable soy based graft copolymers: A review. ACS Sustain. Chem. Eng. 2016, 4, 1–17. [Google Scholar] [CrossRef]
- Zienkiewicz-Strzałka, M.; Deryło-Marczewska, A.; Skorik, Y.A.; Petrova, V.A.; Choma, A.; Komaniecka, I. Silver nanoparticles on chitosan/silica nanofibers: Characterization and antibacterial activity. Int. J. Mol. Sci. 2019, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Kumar, R. A Review on Material and Antimicrobial Properties of Soy Protein Isolate Film. J. Polym. Environ. 2019, 27, 1613–1628. [Google Scholar] [CrossRef]
- Razmjoo, F.; Sadeghi, E.; Rouhi, M.; Mohammadi, R.; Noroozi, R.; Safajoo, S. Polyvinyl alcohol–Zedo gum edible film: Physical, mechanical and thermal properties. J. Appl. Polym. Sci. 2021, 138, 49875. [Google Scholar] [CrossRef]
- Braga, L.R.; Pérez, L.M.; Soazo, M.d.V.; Machado, F. Evaluation of the antimicrobial, antioxidant and physicochemical properties of Poly(Vinyl chloride) films containing quercetin and silver nanoparticles. LWT 2019, 101, 491–498. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Lagaron, J.M.; Ocio, M.J. Development and characterization of silver-based antimicrobial ethylene–vinyl alcohol copolymer (EVOH) films for food-packaging applications. J. Agric. Food Chem. 2012, 60, 5350–5359. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Dhital, R.; Wang, W.; Sun, L.; Zeng, W.; Mustapha, A.; Lin, M. Development of multifunctional nanocomposites containing cellulose nanofibrils and soy proteins as food packaging materials. Food Packag. Shelf Life 2019, 21, 100366. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticle-loaded chitosan–starch based films: Fabrication and evaluation of tensile, barrier and antimicrobial properties. Mater. Sci. Eng. C 2010, 30, 891–897. [Google Scholar] [CrossRef]
- Moghaddas Kia, E.; Ghasempour, Z.; Alizadeh, M. Fabrication of an eco-friendly antioxidant biocomposite: Zedo gum/sodium caseinate film by incorporating microalgae (Spirulina platensis). J. Appl. Polym. Sci. 2018, 135, 46024. [Google Scholar] [CrossRef]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active composite starch films containing green synthetized silver nanoparticles. Food Hydrocoll. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- González, A.; Igarzabal, C.I.A. Soy protein–Poly (lactic acid) bilayer films as biodegradable material for active food packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Liu, X.; Song, R.; Zhang, W.; Qi, C.; Zhang, S.; Li, J. Development of eco-friendly soy protein isolate films with high mechanical properties through HNTs, PVA, and PTGE synergism effect. Sci. Rep. 2017, 7, 44289. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Wang, K.; Xu, H. Development and evaluation of soy protein isolate-based antibacterial nanocomposite films containing cellulose nanocrystals and zinc oxide nanoparticles. Food Hydrocoll. 2020, 106, 105898. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Li, W.; Li, M.; Deng, Q.; Wang, Y.; Li, C.; Chu, P.K. Ecofriendly and biodegradable soybean protein isolate films incorporated with zno nanoparticles for food packaging. ACS Appl. Bio Mater. 2019, 2, 2202–2207. [Google Scholar] [CrossRef]
- Han, Y.; Wang, L. Improved water barrier and mechanical properties of soy protein isolate films by incorporation of SiO 2 nanoparticles. RSC Adv. 2016, 6, 112317–112324. [Google Scholar] [CrossRef]
- Rhim, J.; Wang, L.; Hong, S. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocoll. 2013, 33, 327–335. [Google Scholar] [CrossRef]
- Pandey, S.; Goswami, G.K.; Nanda, K.K. Green synthesis of biopolymer–silver nanoparticle nanocomposite: An optical sensor for ammonia detection. Int. J. Biol. Macromol. 2012, 51, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Beak, S.; Kim, H.; Song, K.B. Characterization of an olive flounder bone gelatin-Zinc oxide nanocomposite film and evaluation of its potential application in spinach packaging. J. Food Sci. 2017, 82, 2643–2649. [Google Scholar] [CrossRef]
- Uranga, J.; Llamas, M.G.; Agirrezabala, Z.; Dueñas, M.T.; Etxebeste, O.; Guerrero, P.; de la Caba, K. Compression Molded Soy Protein Films with Exopolysaccharides Produced by Cider Lactic Acid Bacteria. Polymers 2020, 12, 2106. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Li, P.; Li, W.; Li, C.; Wang, Y.; Chu, P.K. Degradable and photocatalytic antibacterial Au-TiO2/sodium alginate nanocomposite films for active food packaging. Nanomaterials 2018, 8, 930. [Google Scholar] [CrossRef]
- Assis, R.Q.; Pagno, C.H.; Stoll, L.; Rios, P.D.A.; de Oliveira Rios, A.; Olivera, F.C. Active food packaging of cellulose acetate: Storage stability, protective effect on oxidation of riboflavin and release in food simulants. Food Chem. 2021, 349, 129140. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Alizadeh-Sani, M.; Khezerlou, A.; Mirzanajafi-Zanjani, M.; Zolfaghari, H.; Bagheri, V.; Divband, B.; Ehsani, A. Nanoparticles and zeolites: Antibacterial effects and their mechanism against pathogens. Curr. Pharm. Biotechnol. 2019, 20, 1074–1086. [Google Scholar] [CrossRef]
- Sui, Z.; Chen, X.; Wang, L.; Xu, L.; Zhuang, W.; Chai, Y.; Yang, C. Capping effect of CTAB on positively charged Ag nanoparticles. Phys. E Low-Dimens. Syst. Nanostructures 2006, 33, 308–314. [Google Scholar] [CrossRef]
- Mofidfar, M.; Kim, E.S.; Larkin, E.L.; Long, L.; Jennings, W.D.; Ahadian, S.; Ghannoum, M.A.; Wnek, G.E. Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers. Micromachines 2019, 10, 829. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).